Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganisms
2.2. Growth Medium
2.3. Batch Reactors
2.4. Trace Elements, Plackett-Burman Method and Bottle Batch Experiments
2.5. Analytical Methods
2.6. Kinetic Analysis
3. Results
3.1. Bacterial Growth
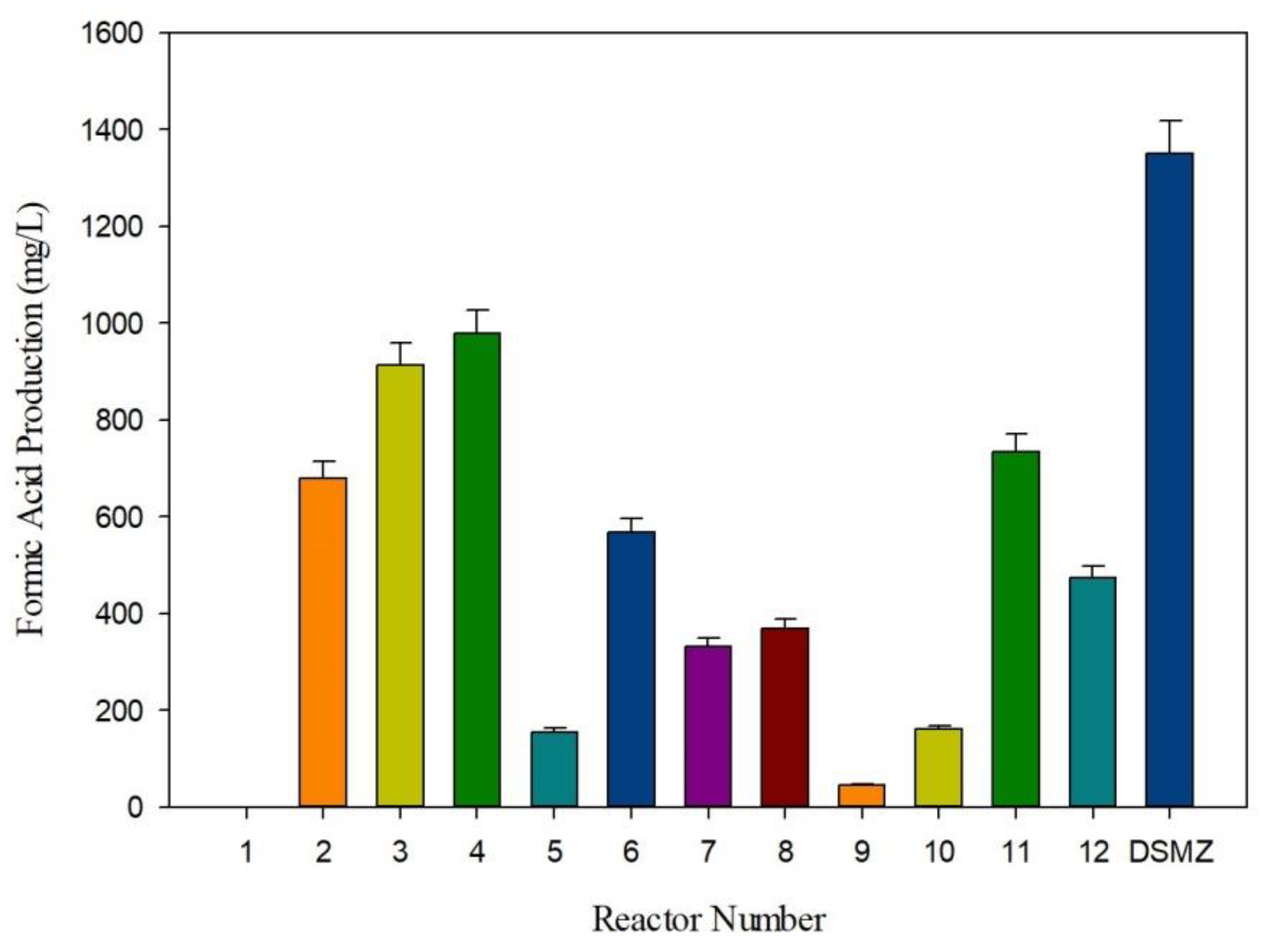
3.2. Formic Acid Production
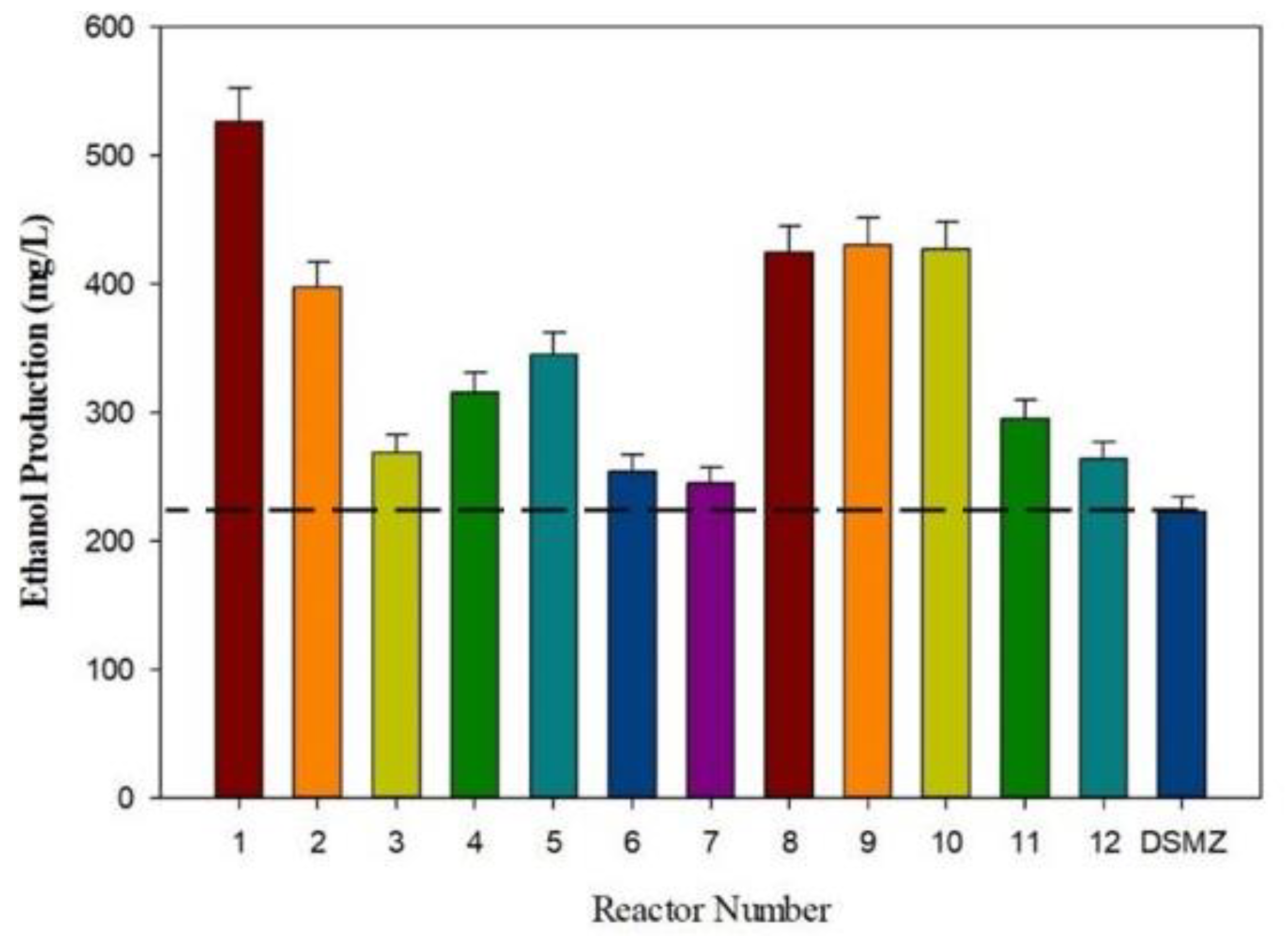
3.3. Ethanol Production
3.4. Statistical Analysis
3.4.1. Pareto Chart
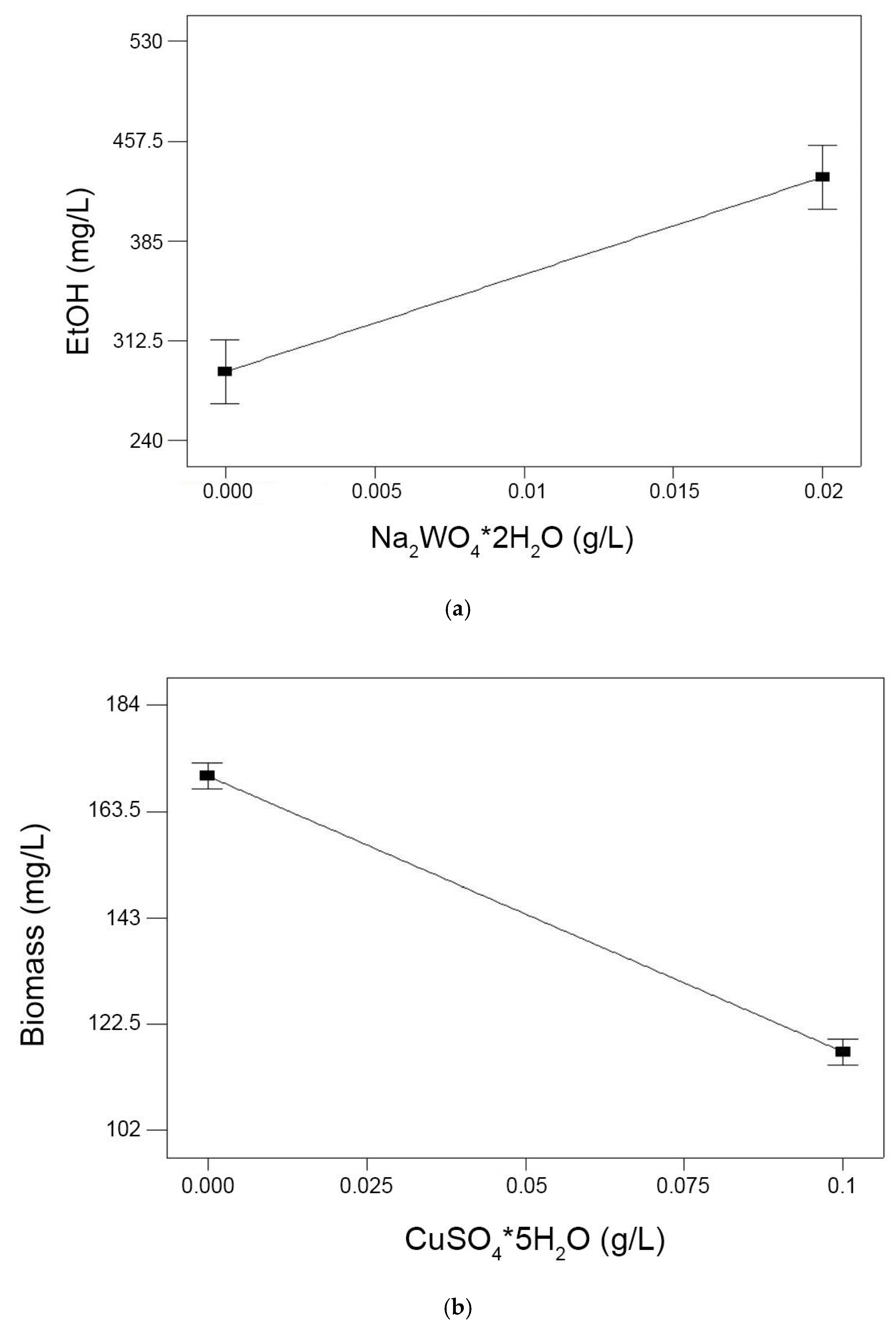
3.4.2. Main Effects Plot
3.4.3. Optimized Trace Metal Composition for Ethanol
3.5. Kinetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karmee, S.K. Liquid biofuels from food waste: Current trends, prospect and limitation. Renew. Sustain. Energy Rev. 2016, 53, 945–953. [Google Scholar] [CrossRef]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.J.; Du, W.; Qian, F. Transformation technologies for CO2 utilisation: Current status, challenges and future prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- Kennes, D.; Abubackar, H.N.; Diaz, M.; Veiga, M.C.; Kennes, C. Bioethanol production from biomass: Carbohydrate vs syngas fermentation. J. Chem. Technol. Biotechnol. 2016, 91, 304–317. [Google Scholar] [CrossRef]
- Bengelsdorf, F.R.; Beck, M.H.; Erz, C.; Hoffmeiste, S.; Karl, M.M.; Riegler, P.; Wirth, S.; Poehlein, A.; Botz, D.W.; Dürre, P. Bacterial Anaerobic Synthesis Gas (Syngas) and CO2 + H2 Fermentation. Adv. Appl. Microbiol. 2018, 103, 143–221. [Google Scholar] [CrossRef] [PubMed]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N., Jr.; Ferreira, T. Clostridium sp. as Bio-Catalyst for Fuels and Chemicals Production in a Biorefinery Context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.F.; Xie, B.T.; Wu, G.X.; Guo, Y.Q.; Li, D.M.; Huang, Z.Y. Combination of Trace Metal to Improve Solventogenesis of Clostridium carboxidivorans P7 in Syngas Fermentation. Front. Microbiol. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Richter, H.; Martin, M.E.; Angenent, L.T. A Two-Stage Continuous Fermentation System for Conversion of Syngas into Ethanol. Energies 2013, 6, 3987–4000. [Google Scholar] [CrossRef] [Green Version]
- Ragsdale, S.W.; Pierce, E. Acetogenesis and the Wood-Ljungdahl pathway of CO2 fixation. Biochim. Biophys. Acta-Proteins Proteom. 2008, 1784, 1873–1898. [Google Scholar] [CrossRef] [Green Version]
- Appel, A.M.; Bercaw, J.E.; Bocarsly, A.B.; Dobbe, H.; DuBois, D.L.; Dupuis, K.; Ferry, J.G.; Fujita, E.; Hille, R.; Kenis, P.J.A.; et al. Frontiers, opportunities, and challenges in biochemical and chemical catalysis of CO2 fixation. Chem. Rev. 2013, 113, 6621–6658. [Google Scholar] [CrossRef] [Green Version]
- Korkhin, Y.; Kalb, A.J.; Peretz, M.; Bogin, O.; Burstein, Y.; Frolow, F. NADP-dependent bacterial alcohol dehydrogenases: Crystal structure, cofactor-binding and cofactor specificity of the ADHs of Clostridium beijerinckii and Thermoanaerobacter brockii. J. Mol. Biol. 1998, 278, 967–981. [Google Scholar] [CrossRef]
- Saxena, J.; Tanner, R.S. Effect of trace metals on ethanol production from synthesis gas by the ethanologenic acetogen, Clostridium ragsdalei. J. Ind. Microbiol. Biotechnol. 2011, 38, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.R.; Atiyeh, H.K.; Tanner, R.S.; Torres, J.R.; Saxena, J.; Wilkins, M.R.; Huhnke, R.L. Butanol and hexanol production in Clostridium carboxidivorans syngas fermentation: Medium development and culture techniques. Bioresour. Technol. 2015, 190, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Carbon monoxide fermentation to ethanol by Clostridium autoethanogenum in a bioreactor with no accumulation of acetic acid. Bioresour. Technol. 2015, 186, 122–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanner, R.S.; Miller, L.M.; Yang, D. Clostridial rRNA Homology Group I. Int. J. Syst. Bacteriol. 1993, 43, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Köpke, M.; Mihalcea, C.; Liew, F.M.; Tizard, J.H.; Ali, M.S.; Conolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [Green Version]
- Benevenuti, C.; Botelho, A.; Ribeiro, R.; Branco, M.; Pereira, A.; Vieira, A.C.; Ferreira, T.; Amaral, P. Experimental Design to Improve Cell Growth and Ethanol Production in Syngas Fermentation by Clostridium carboxidivorans. Catalysts 2020, 10, 59. [Google Scholar] [CrossRef] [Green Version]
- Gundogdu, T.K.; Deniz, I.; Caliskan, G.; Sahin, S.; Azbar, N. Experimental design methods for bioengineering applications. Crit. Rev. Biotechnol. 2014, 36, 368–388. [Google Scholar] [CrossRef]
- Najafpour, G.; Younesi, H. Ethanol and acetate synthesis from waste gas using batch culture of Clostridium ljungdahlii. Enzyme Microb. Technol. 2006, 38, 223–228. [Google Scholar] [CrossRef]
- Cotter, J.L.; Chinn, M.S.; Grunden, A.M. Influence of process parameters on growth of Clostridium ljungdahlii and Clostridium autoethanogenum on synthesis gas. Enzyme Microb. Technol. 2009, 44, 281–288. [Google Scholar] [CrossRef]
- Park, S.; Ahn, B.; Kim, Y.K. Growth enhancement of bioethanol-producing microbe Clostridium autoethanogenum by changing culture medium composition. Bioresour. Technol. Rep. 2019, 6, 237–240. [Google Scholar] [CrossRef]
- Aghbashlo, M.; Tabatabaei, M.; Hosseini, S.S.; Younesi, H.; Najafpour, G. Performance analysis of a continuous bioreactor for ethanol and acetate synthesis from syngas via Clostridium ljungdahlii using exergy concept. Clean Technol. Environ. Policy 2016, 18, 853–865. [Google Scholar] [CrossRef]
- Xie, B.T.; Liu, Z.Y.; Tian, L.; Li, F.L.; Chen, X.H. Physiological response of Clostridium ljungdahlii DSM 13528 of ethanol production under different fermentation conditions. Bioresour. Technol. 2015, 177, 302–307. [Google Scholar] [CrossRef]
- Gunay, B.; Azbar, N.; Keskin, T. The effect of corn syrup and whey on the conversion process of CO to ethanol using Clostridium ljungdahlii. Chemosphere 2020, 261, 127734. [Google Scholar] [CrossRef] [PubMed]
- Phukoetphim, N.; Salakkam, A.; Laopaiboon, P.; Laopaiboon, L. Kinetic models for batch ethanol production from sweet sorghum juice under normal and high gravity fermentations: Logistic and modified Gompertz models. J. Biotechnol. 2017, 243, 6975. [Google Scholar] [CrossRef] [PubMed]
- Har, C.L.; Hii, S.L.; Yong, C.K.; Siew, S.P. Statistical Screening of Factors Affecting Production of Fermentable Sugars from Sugarcane Bagasse under Solid-state Conditions. BioResources 2013, 8, 4546–4562. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Atiyeh, H.K.; Zhang, H.; Tanner, R.S.; Huhnke, R.L. Enhanced ethanol production from syngas by Clostridium ragsdalei in continuous stirred tank reactor using medium with poultry litter biochar. Appl. Energy 2019, 236, 1269–1279. [Google Scholar] [CrossRef]
- Jack, J.; Lo, J.; Maness, P.C.; Ren, Z.J. Directing Clostridium ljungdahlii fermentation products via hydrogen to carbon monoxide ratio in syngas. Biomass Bioenergy 2019, 124, 95–101. [Google Scholar] [CrossRef]
- Daniell, J.; Köpke, M.; Simpson, S.D. Commercial Biomass Syngas Fermentation. Energies 2012, 5, 5372–5417. [Google Scholar] [CrossRef] [Green Version]
- Shen, S.; Gu, Y.; Chai, C.; Jiang, W.; Zhuang, Y.; Wang, Y. Enhanced alcohol titre and ratio in carbon monoxide-rich off-gas fermentation of Clostridium carboxidivorans through combination of trace metals optimization with variable-temperature cultivation. Bioresour. Technol. 2017, 239, 236–243. [Google Scholar] [CrossRef]
- Seravalli, J.; Xiao, Y.; Gu, W.; Cramer, S.P.; Antholine, W.E.; Krymov, V.; Gerfen, G.J.; Ragsdale, S.W. Evidence that NiNi acetyl-CoA synthase is active and that the CuNi enzyme is not. Biochemistry 2004, 43, 3944–3955. [Google Scholar] [CrossRef] [PubMed]
- Drennan, C.L.; Doukov, T.I.; Ragsdale, S.W. The metalloclusters of carbon monoxide dehydrogenase/acetyl-CoA synthase: A story in pictures. J. Biol. Inorg. Chem. 2004, 9, 511–515. [Google Scholar] [CrossRef]
- Naomichi, K.; Nagai, N.S. Effects of trace metal ions on the growth, homoacetogenesis and corrinoid production by Clostridium thermoaceticum. J. Ferment. Bioeng. 1991, 71, 181–185. [Google Scholar] [CrossRef]
- Hu, S.I.; Pezacka, E.; Wood, H.G. Acetate synthesis from carbon monoxide by Clostridium thermoaceticum: Purification of the corrinoid protein. J. Biol. Chem. 1984, 259, 8892–8897. [Google Scholar] [CrossRef]
- Bramlett, M.R.; Tan, X.; Lindahl, P.A. Inactivation of acetyl CoA synthase/carbon monoxide dehydrogenase by copper. J. Am. Chem. Soc. 2003, 125, 9316–9317. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Mortenson, L.E. Formate dehydrogenase of Clostridium pasteurianum. J. Bacteriol. 1984, 159, 375–380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, R.; Andreesen, J.R. Accumulation and incorporation of 185W-tungsten into proteins of Clostridium acidiurici and Clostridium cylindrosporum. Arch. Microbiol. 1987, 147, 295–299. [Google Scholar] [CrossRef]
- Yamamoto, I.; Saiki, T.; Liu, S.M.; Ljungdahl, L.G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J. Biol. Chem. 1983, 258, 1826–1832. [Google Scholar] [CrossRef]
- Chen, J.S. Alcohol dehydrogenase: Multiplicity and relatedness in the solvent-producing clostridia. FEMS Microbiol. Rev. 1995, 17, 263–273. [Google Scholar] [CrossRef]
- Scopes, R.K. An iron-activated alcohol dehydrogenase. FEBS Lett. 1983, 156, 303–306. [Google Scholar] [CrossRef] [Green Version]
- Drake, H.L.; Kusel, K.; Matthies, C. Acetogenic prokaryotes. The prokaryotes. In A Handbook on the Biology of Bacteria; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, K.H., Stackebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 2, pp. 354–420. [Google Scholar]
- Abubackar, H.N.; Veiga, M.C.; Kennes, C. Ethanol and acetic acid production from carbon monoxide in a clostridium strain in batch and continuous gas-fed bioreactors. Int. J. Environ. Res. Public Health 2015, 12, 1029–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Meng, C.; Wu, G.; Xie, B.; Han, Y.; Guo, Y.; Song, C.; Gao, Z.; Huang, Z. Efects of zinc on the production of alcohol by Clostridium carboxidivorans P7 using model syngas. J. Ind. Microbiol. Biotechnol. 2018, 45, 61–69. [Google Scholar] [CrossRef] [PubMed]


| Trace Element Present in Inoculum | Mo | W | Fe | Zn | Ni | Mg | Mn | Co | Ca | Cu | B |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (g/L) | 1.3 × 10−5 | - | 1.3 × 10−5 | 1.35 × 10−4 | 7 × 10−6 | 2.17 × 10−2 | 2.66 × 10−4 | 5.6 × 10−5 | 2.752 × 10−3 | 0 | 3.7 × 10−5 |
| Reactor No. | Na2MoO4 × 2H2O | Na2WO4 × 2H2O | FeSO4 × 7H2O | ZnSO4 × 7H2O | NiCl2 × 6H2O | MgSO4 × 7H2O | MnSO4 × H2O | CoSO4 × 7H2O | CaCl2 × 2H2O | CuSO4 × 5H2O | H3BO3 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.02003 | 0.02 | 0.00055 | 0.20059 | 0.02003 | 3.21996 | 0.00082 | 0.00027 | 0.01009 | 0.10000 | 0.00021 |
| 2 | 0.00003 | 0.02 | 0.80055 | 0.00059 | 0.02003 | 3.21996 | 1.00082 | 0.00027 | 0.01009 | 0.00000 | 0.01021 |
| 3 | 0.02003 | 0.00 | 0.80055 | 0.20059 | 0.00003 | 3.21996 | 1.00082 | 0.20027 | 0.01009 | 0.00000 | 0.00021 |
| 4 | 0.00003 | 0.02 | 0.00055 | 0.20059 | 0.02003 | 0.21996 | 1.00082 | 0.20027 | 0.11009 | 0.00000 | 0.00021 |
| 5 | 0.00003 | 0.00 | 0.80055 | 0.00059 | 0.02003 | 3.21996 | 0.00082 | 0.20027 | 0.11009 | 0.10000 | 0.00021 |
| 6 | 0.00003 | 0.00 | 0.00055 | 0.20059 | 0.00003 | 3.21996 | 1.00082 | 0.00027 | 0.11009 | 0.10000 | 0.01021 |
| 7 | 0.02003 | 0.00 | 0.00055 | 0.00059 | 0.02003 | 0.21996 | 1.00082 | 0.20027 | 0.01009 | 0.10000 | 0.01021 |
| 8 | 0.02003 | 0.02 | 0.00055 | 0.00059 | 0.00003 | 3.21996 | 0.00082 | 0.20027 | 0.11009 | 0.00000 | 0.01021 |
| 9 | 0.02003 | 0.02 | 0.80055 | 0.00059 | 0.00003 | 0.21996 | 1.00082 | 0.00027 | 0.11009 | 0.10000 | 0.00021 |
| 10 | 0.00003 | 0.02 | 0.80055 | 0.20059 | 0.00003 | 0.21996 | 0.00082 | 0.20027 | 0.01009 | 0.10000 | 0.01021 |
| 11 | 0.02003 | 0.00 | 0.80055 | 0.20059 | 0.02003 | 0.21996 | 0.00082 | 0.00027 | 0.11009 | 0.00000 | 0.01021 |
| Source | Sum of Squares | df | Main Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 89,600.67 | 10 | 8960.07 | 331.85 | 0.0427 | significant |
| A-Na2MoO4 × H2O | 2945.33 | 1 | 2945.33 | 109.09 | 0.0608 | |
| B-Na2WO4 × 2H2O | 59,925.34 | 1 | 59,925.34 | 2219.44 | 0.0135 | |
| C-FeSO4 × 7H2O | 1541.33 | 1 | 1541.33 | 57.09 | 0.0838 | |
| E-NiCl2 × 6H2O | 261.33 | 1 | 261.33 | 9.68 | 0.1980 | |
| F-MgSO4 × H2O | 4800.00 | 1 | 4800.00 | 177.78 | 0.0477 | |
| G-MnSO4 × H2O | 11,408.33 | 1 | 11,408.33 | 422.53 | 0.0309 | |
| H-CoCl2 × 6H2O | 1633.33 | 1 | 1633.33 | 60.49 | 0.0814 | |
| J-CaCl2 × 2H2O | 341.33 | 1 | 341.33 | 12.64 | 0.1745 | |
| K- CuSO4 × 5H2O | 5808.00 | 1 | 5808.00 | 215.11 | 0.0433 | |
| L-H3BO3 | 936.33 | 1 | 936.33 | 34.68 | 0.1071 | |
| Residual | 27.00 | 1 | 27.00 | |||
| Cor Total | 89,627.67 | 11 |
| Trace Metal Solutions | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Solution No | CaCl2 × 2H2O | Na2WO4 × 2H2O | FeSO4 × 7H2O | Na2MoO4 × 2H2O | NiCl2 × 6H2O | MgSO4 × 7H2O | MnSO4 × H2O | CoSO4 × 7H2O | ZnSO4 × 7H2O | CuSO4 × 5H2O | H3BO3 |
| #1 | 0.027738 | 0.019619 | 0.79914 | 0.015796 | 0.019969 | 2.9978 | 0.16116 | 0.001311 | 0.074341 | 0.099975 | 0.00000373 |
| #2 | 0.024065 | 0.02 | 0.75617 | 0.019999 | 0.0090496 | 2.7713 | 0.00002252 | 0.0069195 | 0.16108 | 0.088160 | 0.0026510 |
| #3 | 0.023996 | 0.01999 | 0.63063 | 0.019998 | 0.010224 | 2.9999 | 0.00000227 | 0.071353 | 0.043956 | 0.1 | 0.00733 |
| DSMZ * | 0.10 | 0.004 | 0.10 | 0.01 | 0.03 | 3.00 | 0.5 | 0.18 | 0.18 | 0.01 | 0.01 |
| EtOHmax (mg EtOH/L) | Rate (mg EtOH/L.h) | Lag Time (h) | R2 | |
|---|---|---|---|---|
| Reactor 1 | 504.92 | 18.5 | 4.74 | 0.9415 |
| Reactor 2 | 308.21 | 14.10 | 4.72 | 0.9242 |
| Reactor 3 | 248.64 | 12.58 | 5.38 | 0.9877 |
| Reactor 4 | 282.39 | 14.99 | 5.36 | 0.9802 |
| Reactor 5 | 296.77 | 9.92 | 3.67 | 0.9549 |
| Reactor 6 | 245.04 | 13.51 | 5.41 | 0.9854 |
| Reactor 7 | 229.10 | 12.62 | 5.27 | 0.9826 |
| Reactor 8 | 290.68 | 11.94 | 4.34 | 0.8573 |
| Reactor 9 | 360.77 | 18.55 | 5.41 | 0.9663 |
| Reactor 10 | 301.71 | 8.92 | 2.58 | 0.8857 |
| Reactor 11 | 257.15 | 13.56 | 5.55 | 0.9811 |
| Reactor 12 | 242.07 | 16.68 | 5.66 | 0.9913 |
| Element | Role in Acetogens | Reference |
|---|---|---|
| Nickel | Cell growth, CODH, H2ase, ACS | [31,32] |
| Cobalt | Cell growth | [33,34] |
| Copper | CODH/ACS | [31,32,35] |
| Tungsten | FDH, AOR | [36,37,38] |
| Zinc | Cell growth, ADH, acetyl CoA | [10,39,40] |
| Iron | FDH, CODH, H2ase, ACS, ADH, acetyl CoA | [10,36,39] |
| Molybdenum | FDH, acetyl Co-A | [41] |
| Microorganisms | Gas Composition | Ni (µM) | Co (µM) | W (µM) | Mn (µM) | Fe (µM) | Cu (µM) | Zn (µM) | Mo (µM) | Se (µM) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. ragsdalei | 100% CO | 0.84 | 8.40 | 0.68 | 59.17 | 20.40 | 1.17 | 34.8 | 0.83 | 1.06 | [11] |
| C. carboxidivorans | %56 CO, %20 CO2, %15 N2, %9 H2, | 4.02 | 42.05 | 3.05 | 59.16 | 54.52 | 4.07 | 3.48 | 0.46 | 5.3 | [30] |
| C. carboxidivorans | %50 CO,%35 CO2, %15 H2 | 0.84 | 8.41 | 0.61 | 0 | 20.4 | 1.49 | 6.96 | 0 | 1.06 | [6] |
| C. carboxidivorans | %70 CO, %10 CO2, %20 H2 | 1.98 | 8.4 | 1.21 | 59.17 | 20.51 | 0 | 1.4 | 2.25 | 1.06 | [12] |
| C. autoethanogenum | %20 CO, %20 CO2, %10 H2, %50 N2 | 0.23 | 0.64 | 0.00121 | 2.95 | 0.36 | 0.04 | 0.63 | 0.048 | 0.0011 | [21] |
| C. ljungdahlii | %60 CO, %10 CO2, %10 N2, %10 CH4, %10 H2 | 0.84 | 0.05 | 0.59 | 9.54 | 28.76 | 4.01 | 2.59 | 0.65 | 0 | This Study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sertkaya, S.; Azbar, N.; Abubackar, H.N.; Gundogdu, T.K. Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii. Energies 2021, 14, 6981. https://doi.org/10.3390/en14216981
Sertkaya S, Azbar N, Abubackar HN, Gundogdu TK. Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii. Energies. 2021; 14(21):6981. https://doi.org/10.3390/en14216981
Chicago/Turabian StyleSertkaya, Simge, Nuri Azbar, Haris Nalakath Abubackar, and Tugba Keskin Gundogdu. 2021. "Design of Low-Cost Ethanol Production Medium from Syngas: An Optimization of Trace Metals for Clostridium ljungdahlii" Energies 14, no. 21: 6981. https://doi.org/10.3390/en14216981







