Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation
Abstract
:1. Introduction
2. Zero-Dimension (0D) Photocatalysts
| SCs | Modification on SCs | Irradiation Source | Scavenger | Findings | Ref. |
|---|---|---|---|---|---|
| TiO2 | Cu loading | Xe lamp | CH3OH | 18× enhancement | [55] |
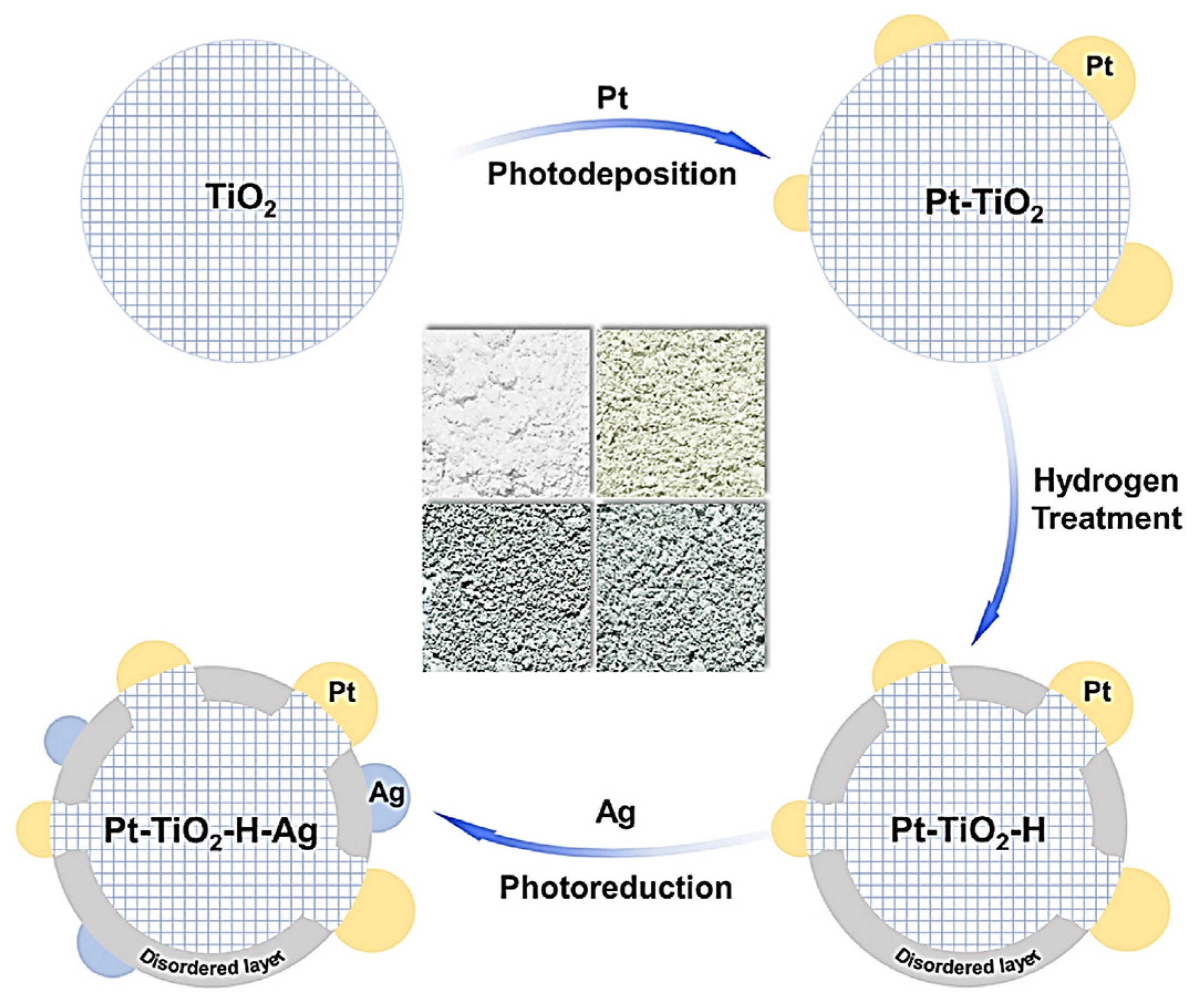
| TiO2 | Pt-H-Ag deposition | Xe lamp (>420 nm) | CH3OH | 17.2× enhancement | [68] |
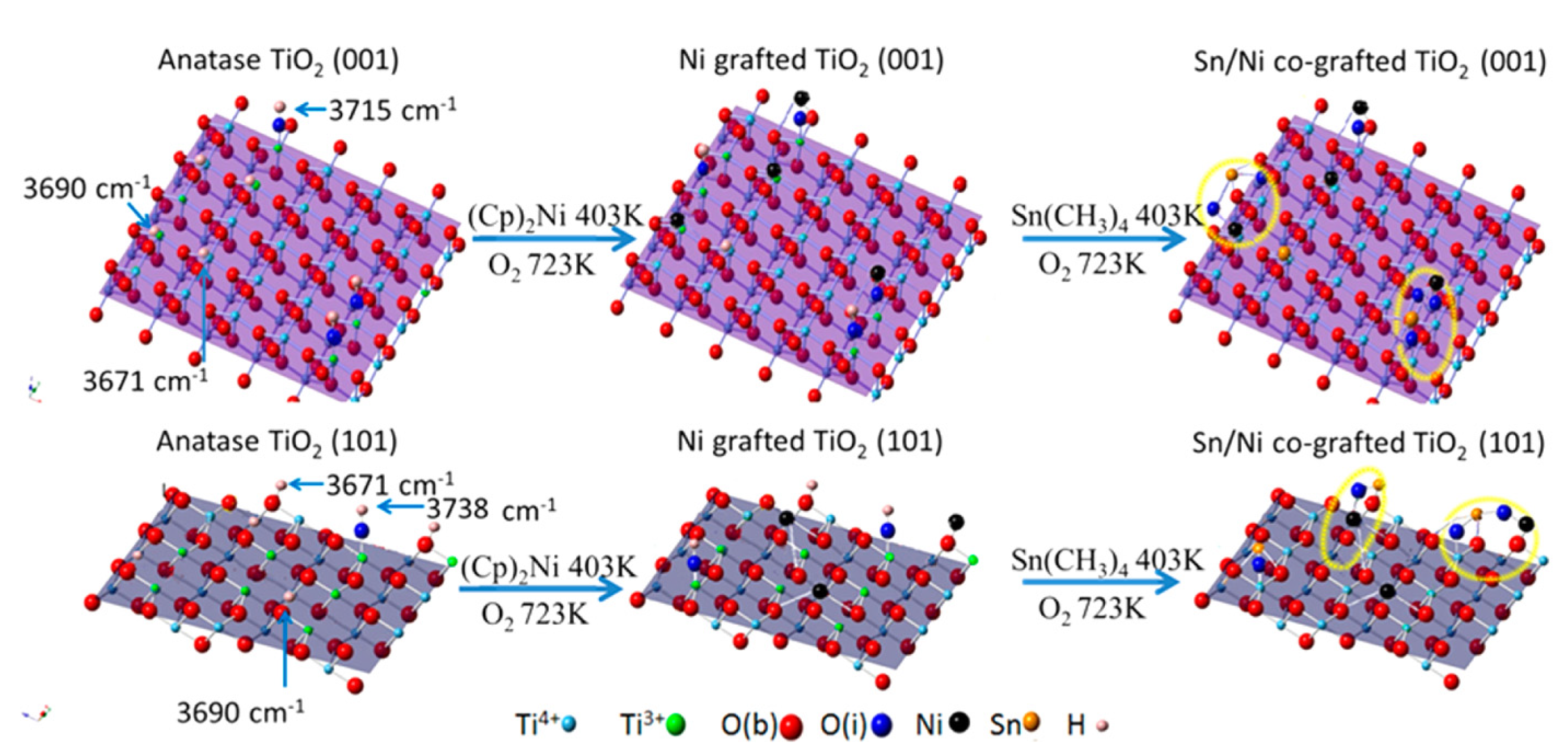
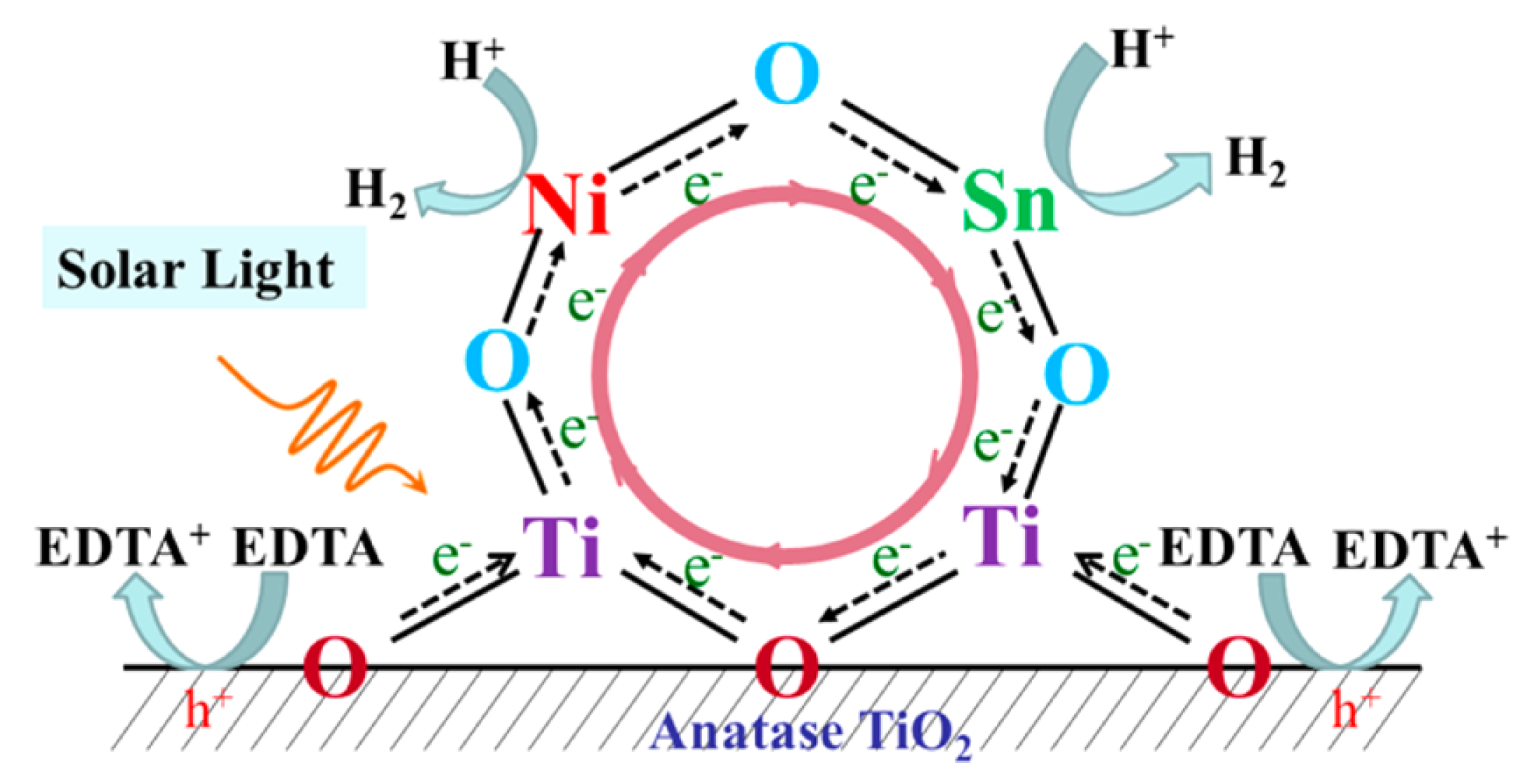
| TiO2 | Ni/Sn loading | Xe lamp (>200 nm) | EDTA | 20× enhancement | [71] |
| TiO2 | octahedral | Hg lamp (290 nm) | CH3OH | 1.5× enhancement | [73] |
| TiO2 | Ag loading | Xe lamp | CH3CHOHCH3 | improved activity | [76] |
| TiO2 | Au loading | Xe lamp (>420 nm) | CH3OH | 8.3× enhancement | [77] |
| TiO2 | In2O3 loading | LED lamp/solar simulator | CH3OH | high activity | [78] |
| TiO2-CdS | core-shell | Xe lamp | Na2S/Na2SO3 | 1.4× enhancement | [88] |
| graphene | CeO2-CuO QDs loading | Xe lamp | CH3OH | 2481 µmol g−1·h−1 | [97] |
| CdSe | TiO2 coating | AM 1.5 light | Na2S/Na2SO3 | 2.7× enhancement | [98] |
3. One-Dimension (1D) Photocatalysts
| SCs | Modification on SCs | Irradiation Source | Scavenger | Findings | Ref. |
|---|---|---|---|---|---|
| TiO2 NTs | TiO2/Ti2O3 | Xe lamp (full-sunlight filter) | CH3OH | 1440 μmol g−1 h−1 | [112] |
| TiO2 NTs | Pt loading | Xe lamp(AM 1.5 G) | CH3OH | 9.8 mmol h−1 g−1, 2.5× enhancement | [113] |
| TiO2 | Ti3+ | solar simulator (AM 1.5) | CH3OH | enhanced H2 rate | [114] |
| TiO2 NTs | N-doped | halogen lamp (5% UV light) | CH3OH | 3.77 mmol cm−2 h−1 | [115] |
| TiO2 nanorod | Pd/Au/Pd-Au | 100 P/F lamp (365 nm) | alcohols | 45.6 mmol g−1 h−1 | [116] |
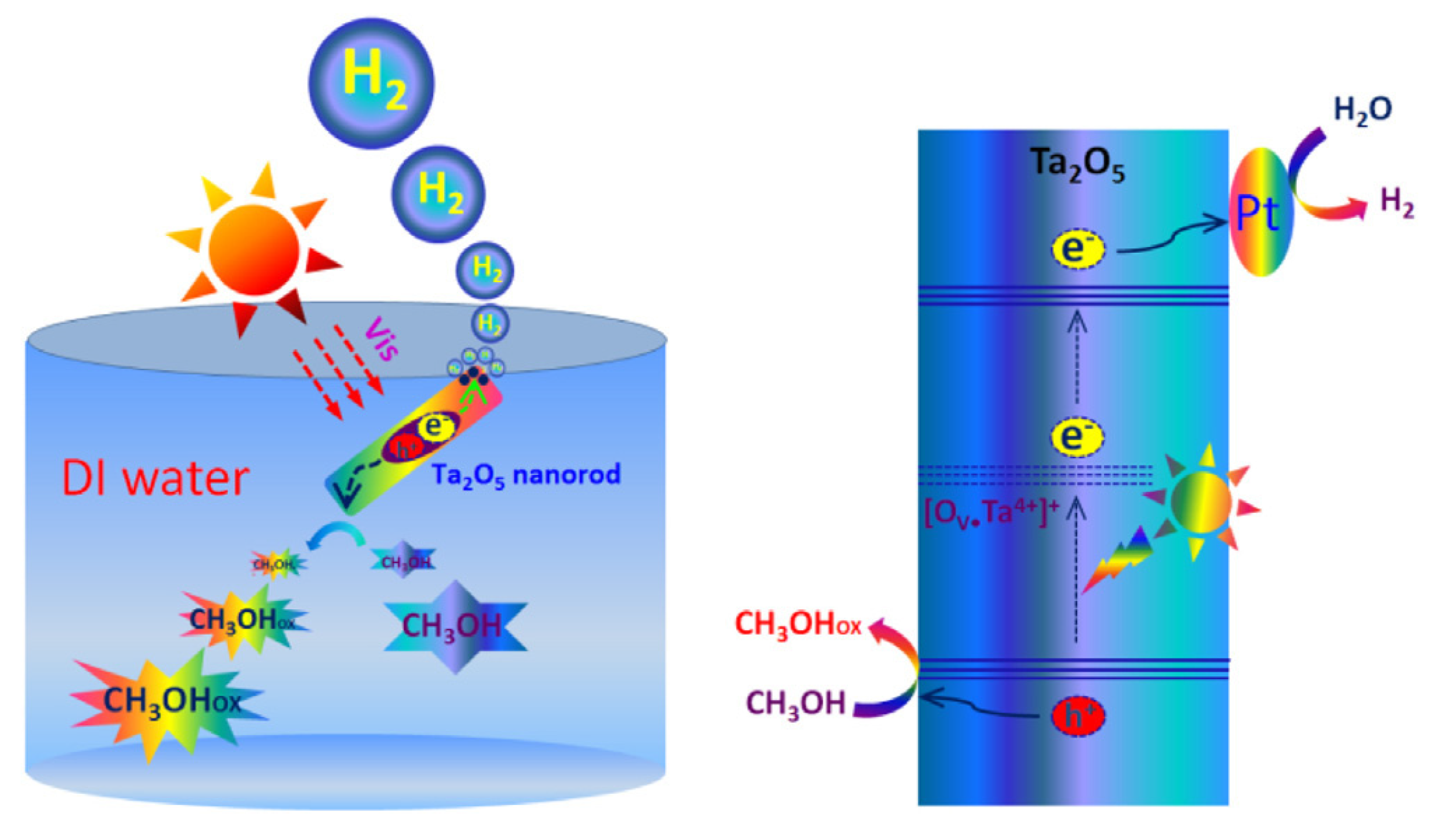
| Ta2O5 | Ta4+ | Xe lamp (>400 nm) | CH3OH | 23.35 mmol g−1 h−1 | [118] |
| TiO2/ZnO | nanowires | Xe lamp (200–2500 nm) | CH3OH | 1.985 μmol·g−1·h−1 | [119] |
| CdS–ZnO | Core-shell nanowires | Xe lamp | ascorbic acid | more than 1200 μmol·g−1·h−1 | [120] |
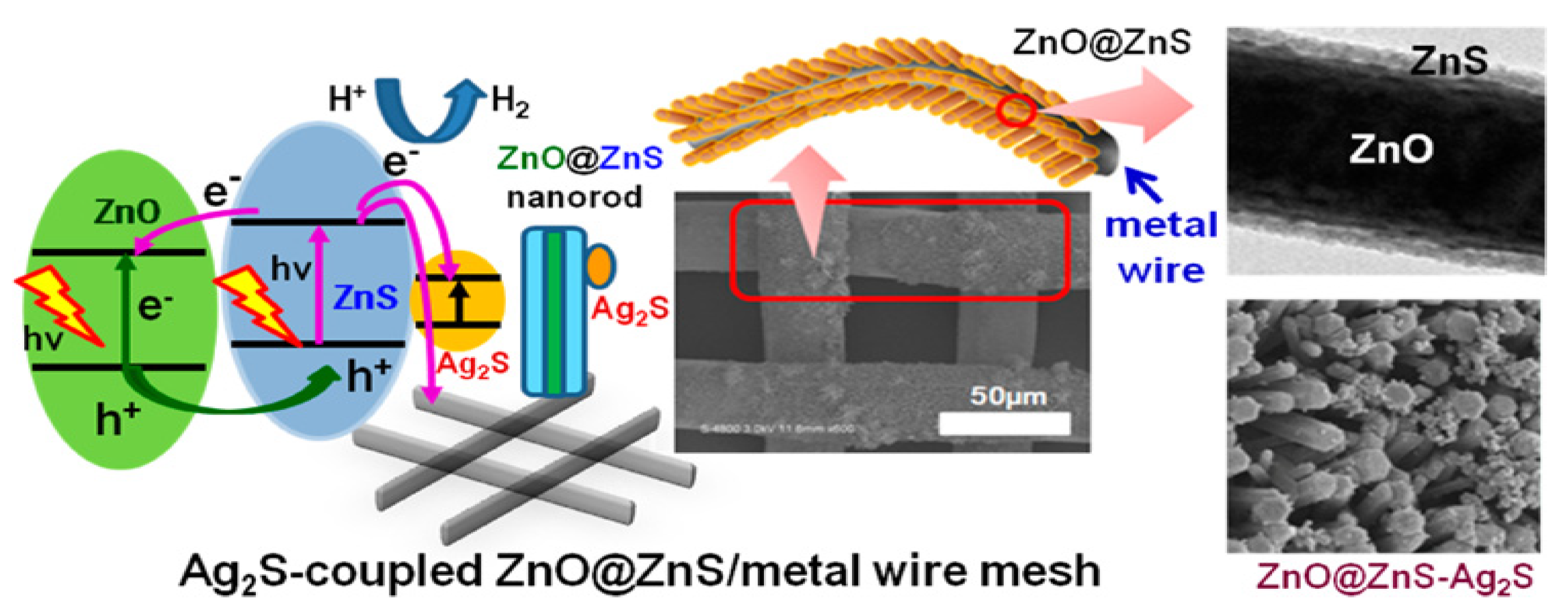
| ZnO | Ag2S–ZnO@ZnS core-shell nanorods | visible light | Na2S/Na2SO3/NaCl | 168 μmol l g−1 h−1 | [121] |
4. Two-Dimension (2D) Photocatalysts
| SCs | Modification on SCs | Irradiation Source | Scavenger | Findings | Ref. |
|---|---|---|---|---|---|
| TiO2 | ultrathin nanosheets | AM1.5 | CH3OH | 540.7 µmol g−1h−1 | [122] |
| TiO2 | Ti3+ | simulated solar light | CH3OH | 28.8 µmol g−1h−1 | [123] |
| TiO2 | N-doping | simulated solar light | TEA | 14.85× enhancement | [124] |
| TiO2 | Pt loading | Xe lamp (solar simulator) | CH3OH | 13,460.7 µmol g−1h−1 | [125] |
| Ta2O5 | Pd-Pt loading | Xe lamp | CH3OH | 21,529.52 µmol g−1h−1 | [126] |
| TiO2 | Co loading | Xe lamp | CH3OH | 12× enhancement | [127] |
| TiO2 | Co3O4 loading | Xe lamp (400 nm) | ENR/CIP/IBU | 3.75 mmol h−1g−1 | [128] |
| CdS | Pt loading | Xe lamp (435 nm) | Na2S /Na2SO3 | improved activity | [129] |
| BiOIO3 | nanoplates | UV-vis | CH3OH | 7× enhancement | [130] |
| g-C3N4 | CuS loading | simulated solar light | TEA | 126.5 µmol g−1h−1 | [131] |
| WO3 | WS2 | Xe lamp | lactic acid | 673 µmol g−1h−1 | [132] |
5. Three-Dimension (3D) Photocatalysts
| PCs | Modification on PCs | Irradiation Source | Scavenger | Findings | Ref. |
|---|---|---|---|---|---|
| TNTs | - | Xe lamp | - | improved activity | [154] |
| TiO2 | Pt loading | Xe lamp | CH3OH | 2× enhancement * | [157] |
| IOT | Au loading | UV lamp (>365 nm) and solar | C2H5OH | high activity | [158] |
| TiO2 nanorods | Au NP loading | Xe lamp (>420 nm) | - | improved activity | [160] |
| Mo:BiVO4 IO | Au NP deposition | solar light stimulator | - | 4× enhancement | [161] |
| WO3 | Au/CdS loading | Xe lamp (>420 nm) | Na2S/Na2SO3 | high activity * | [163] |
| TiO2 | Au/CdS loading | Xe lamp (>420 nm) | Na2S/Na2SO3 | high activity | [165] |
| TNTs | Cu NP loading | Xe lamp (>420 nm) | ethylene glycol | high activity | [166] |
| IOT | F_IOT/Au infilt. | Hg/Xe lamp | C2H5OH | high activity | [167] |
| IOT | CuO/BiVO4 loading | Hg lamp (365 nm) | C2H5OH | enh. Activity ** | [168] |
| IOT | Ti3+/Au−Pt NPs load. | UV lamp (<400 nm) | C2H5OH | high activity | [169] |
| CdS IO | - | λ ≥ 420 nm | Na2S/Na2SO3 | 2× enhancement * | [170] |
| TNTs | Ti3+ | Xe lamp | - | 10× enhancement * | [171] |
| g-C3N4 IO | - | Xe lamp | TEA | 6× enhancement * | [172] |
| IOT | Ag NP loading | Xe lamp | - | high activity | [173] |
6. Composite-Structure (Multi-Dimensional) Photocatalysts
| SCs | Modification on SCs | Irradiation Source | Scavenger | Findings | Ref. |
|---|---|---|---|---|---|
| α-Fe2O3 | Zn-AgIn5S8 QDs | Xe lamp (λ ≥ 420 nm) | L-ascorbic acid | 1.7 mmol g−1 h−1 | [173] |
| CoP | TiO2 | Xe lamp | CH3OH | 0.06 mmol g−1 h−1 | [174] |
| g-C3N4 | TiO2 | Xe lamp | TEA | 7.7× enhancement | [175] |
| CdS | Co9S8 nanoparticles | halogen light (5% UV light) | Na2S/Na2SO3 | 5.15 mmol h−1 g−1 6.8× enhancement | [179] |
| TiO2/CdS | Xe lamp (>400 nm) | Xe lamp (>400 nm) | lactic acid | 35.3 µmol g−1h−1 | [180] |
| CdS | MoS2 | Xe lamp | lactic acid | 11.85 mmol g−1 h−1 11× enhancement | [181] |
| TiO2 | CdSe | UV cut-off filter (λ ≥ 400 nm) | Na2S/Na2SO3 | 3650 µmol g−1 h−1 | [185] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsang, C.H.A.; Huang, H.B.; Xuan, J.; Wang, H.Z.; Leung, D.Y.C. Graphene materials in green energy applications: Recent development and future perspective. Renew. Sustain. Energy Rev. 2020, 120, 109656. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Xie, Z.J.; Peng, Y.P.; Yu, L.; Xing, C.Y.; Qiu, M.; Hu, J.Q.; Zhang, H. Solar-Inspired Water Purification Based on Emerging 2D Materials: Status and Challenges. Sol. RRL 2020, 4, 1900400. [Google Scholar] [CrossRef]
- Saidur, R.; Rahim, N.A.; Islam, M.R.; Solangi, K.H. Environmental impact of wind energy. Renew. Sustain. Energy Rev. 2011, 15, 2423–2430. [Google Scholar] [CrossRef]
- Pickering, M.D.; Horsburgh, K.J.; Blundell, J.R.; Hirschi, J.J.M.; Nicholls, R.J.; Verlaan, M.; Wells, N.C. The impact of future sea-level rise on the global tides. Cont. Shelf Res. 2017, 142, 50–68. [Google Scholar] [CrossRef] [Green Version]
- Kuriqi, A.; Pinheiro, A.N.; Sordo-Ward, A.; Garrote, L. Water-energy-ecosystem nexus: Balancing competing interests at a run-of-river hydropower plant coupling a hydrologic-ecohydraulic approach. Energy Convers. Manag. 2020, 223, 113267. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Cobo, S.; Heidkamp, J.; Jacques, P.A.; Fize, J.; Fourmond, V.; Guetaz, L.; Jousselme, B.; Ivanova, V.; Dau, H.; Palacin, S.; et al. A Janus cobalt-based catalytic material for electro-splitting of water. Nat. Mater. 2012, 11, 802–807. [Google Scholar] [CrossRef]
- Yilmaz, C.; Tan, C.F.; Lim, Y.F.; Ho, C.W. Pseudomorphic Transformation of Interpenetrated Prussian Blue Analogs into Defective Nickel Iron Selenides for Enhanced Electrochemical and Photo-Electrochemical Water Splitting. Adv. Energy Mater. 2019, 9, 1802983. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.U.M.; Al-Shahry, M.; Ingler, W.B., Jr. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Lu, D.L.; Hitoki, G.; Katou, E.; Kondo, J.N.; Hara, M.; Domen, K. Porous single-crystalline TaON and Ta3N5 particles. Chem. Mater. 2004, 16, 1603–1605. [Google Scholar] [CrossRef]
- Park, H.; Ou, H.H.; Kim, M.; Kang, U.; Han, D.S.; Hoffmann, M.R. Photocatalytic H2 production on trititanate nanotubes coupled with CdS and platinum nanoparticles under visible light: Revisiting H2 production and material durability. Faraday Discuss. 2017, 198, 419–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B. Photocatalysis A to Z-What we know and what we do not know in a scientific sense. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 157–178. [Google Scholar] [CrossRef] [Green Version]
- Ohtani, B. Preparing articles on photocatalysis-beyond the illusions, misconceptions, and speculation. Chem. Lett. 2008, 37, 217–229. [Google Scholar] [CrossRef] [Green Version]
- Osterloh, F.E. Photocatalysis versus Photosynthesis: A Sensitivity Analysis of Devices for Solar Energy Conversion and Chemical Transformations. ACS Energy Lett. 2017, 2, 445–453. [Google Scholar] [CrossRef]
- Takanabe, K. Photocatalytic Water Splitting: Quantitative Approaches toward Photocatalyst by Design. ACS Catal. 2017, 7, 8006–8022. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Z.; Zheng, Y.; Hu, Y.H. Recent progress in visible light photocatalytic conversion of carbon dioxide. J. Mat. Chem. A 2019, 7, 865–887. [Google Scholar] [CrossRef]
- Pan, H.; Heagy, M.D. Photons to Formate: A Review on Photocatalytic Reduction of CO2 to Formic Acid. Nanomaterials 2020, 10, 2422. [Google Scholar] [CrossRef]
- White, J.L.; Baruch, M.F.; Pander, J.E.; Hu, Y.; Fortmeyer, I.C.; Park, J.E.; Zhang, T.; Liao, K.; Gu, J.; Yan, Y.; et al. Light-Driven Heterogeneous Reduction of Carbon Dioxide: Photocatalysts and Photoelectrodes. Chem. Rev. 2015, 115, 12888–12935. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Suzuki, N.; Terashima, C.; Fujishima, A. Recent Improvements in the Production of Solar Fuels: From CO2 Reduction to Water Splitting and Artificial Photosynthesis. Bull. Chem. Soc. Jpn. 2019, 92, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Teng, C.; Wang, S.; Min, Q. Recent Advances in TiO2-Based Heterojunctions for Photocatalytic CO2 Reduction With Water Oxidation: A Review. Front. Chem. 2021, 9, 637501. [Google Scholar] [CrossRef]
- Takata, T.; Tanaka, A.; Hara, M.; Kondo, J.N.; Domen, K. Recent progress of photocatalysts for overall water splitting. Catal. Today 1998, 44, 17–26. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Takata, T.; Saito, N.; Inoue, Y.; Domen, K. Photocatalyst releasing hydrogen from water. Nature 2006, 440, 295. [Google Scholar] [CrossRef] [PubMed]
- Yamasita, D.; Takata, T.; Hara, M.; Kondo, J.N.; Domen, K. Recent progress of visible-light-driven heterogeneous photocatalysts for overall water splitting. Solid State Ion. 2004, 172, 591–595. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nemoto, H.; Saito, K.; Kudo, A. Solar Water Splitting Using Powdered Photocatalysts Driven by Z-Schematic Interparticle Electron Transfer without an Electron Mediator. J. Phys. Chem. C 2009, 113, 17536–17542. [Google Scholar] [CrossRef]
- Maeda, K.; Higashi, M.; Lu, D.L.; Abe, R.; Domen, K. Efficient Nonsacrificial Water Splitting through Two-Step Photoexcitation by Visible Light using a Modified Oxynitride as a Hydrogen Evolution Photocatalyst. J. Am. Chem. Soc. 2010, 132, 5858–5868. [Google Scholar] [CrossRef]
- Abe, R.; Higashi, M.; Domen, K. Overall Water Splitting under Visible Light through a Two-Step Photoexcitation between TaON and WO3 in the Presence of an Iodate-Iodide Shuttle Redox Mediator. ChemSusChem 2011, 4, 228–237. [Google Scholar] [CrossRef]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photoch. Photobio. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Kudo, A. Recent progress in the development of visible light-driven powdered photocatalysts for water splitting. Int. J. Hydrogen Energy 2007, 32, 2673–2678. [Google Scholar] [CrossRef]
- Kudo, A.; Kato, H.; Tsuji, I. Strategies for the development of visible-light-driven photocatalysts for water splitting. Chem. Lett. 2004, 33, 1534–1539. [Google Scholar] [CrossRef]
- Liu, G.; Yu, J.C.; Lu, G.Q.; Cheng, H.M. Crystal facet engineering of semiconductor photocatalysts: Motivations, advances and unique properties. Chem. Commun. 2011, 47, 6763–6783. [Google Scholar] [CrossRef] [PubMed]
- Babu, V.J.; Vempati, S.; Uyar, T.; Ramakrishna, S. Review of one-dimensional and two-dimensional nanostructured materials for hydrogen generation. Phys. Chem. Chem. Phys. 2015, 17, 2960–2986. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D Nanomaterials for Photocatalytic Hydrogen Production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef] [Green Version]
- Faraji, M.; Yousefi, M.; Yousefzadeh, S.; Zirak, M.; Naseri, N.; Jeon, T.H.; Choi, W.; Moshfegh, A.Z. Two-dimensional materials in semiconductor photoelectrocatalytic systems for water splitting. Energy Environ. Sci. 2019, 12, 59–95. [Google Scholar] [CrossRef]
- Tong, R.; Ng, K.W.; Wang, X.; Wang, S.; Wang, X.; Pan, H. Two-dimensional materials as novel co-catalysts for efficient solar-driven hydrogen production. J. Mat. Chem. A 2020, 8, 23202–23230. [Google Scholar] [CrossRef]
- Kraeutler, B.; Bard, A.J. Heterogeneous photocatalytic preparation of supported catalysts. Photodeposition of platinum on TiO2 powder and other substrates. J. Am. Chem. Soc. 1978, 100, 4317–4318. [Google Scholar] [CrossRef]
- Herrmann, J.-M.; Disdier, J.; Pichat, P. Photoassisted platinum deposition on TiO2 powder using various platinum complexes. J. Phys. Chem. 1986, 90, 6028–6034. [Google Scholar] [CrossRef]
- Ohtani, B.; Osaki, H.; Nishimoto, S.; Kagiya, T. A novel photocatalytic process of amine N-alkylation by platinized semiconductor particles suspended in alcohols. J. Am. Chem. Soc. 1986, 108, 308–310. [Google Scholar] [CrossRef]
- Kowalska, E.; Remita, H.; Colbeau-Justin, C.; Hupka, J.; Belloni, J. Modification of titanium dioxide with platinum ions and clusters: Application in photocatalysis. J. Phys. Chem. C 2008, 112, 1124–1131. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Endo, M.; Wang, K.L.; Balcytis, A.; Nitta, A.; Mendez-Medrano, M.G.; Colbeau-Justin, C.; Juodkazis, S.; Ohtani, B.; et al. Noble metal-modified faceted anatase titania photocatalysts: Octahedron versus decahedron. Appl. Catal. B Environ. 2018, 237, 574–587. [Google Scholar] [CrossRef]
- Wei, Z.; Endo, M.; Wang, K.; Charbit, E.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. Noble metal-modified octahedral anatase titania particles with enhanced activity for decomposition of chemical and microbiological pollutants. Chem. Eng. J. 2017, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.L.; Wei, Z.S.; Ohtani, B.; Kowalska, E. Interparticle electron transfer in methanol dehydrogenation on platinum-loaded titania particles prepared from P25. Catal. Today 2018, 303, 327–333. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, C.; Dragoe, D.; Beaunier, P.; Colbeau-Justin, C.; Remita, H. Highly Promoted Photocatalytic Hydrogen Generation by Multiple Electron Transfer Pathways. Appl. Catal. B Environ. 2021, 281, 119457. [Google Scholar] [CrossRef]
- Abe, R.; Shinmei, K.; Koumura, N.; Hara, K.; Ohtani, B. Visible-Light-Induced Water Splitting Based on Two-Step Photoexcitation between Dye-Sensitized Layered Niobate and Tungsten Oxide Photocatalysts in the Presence of a Triiodide/Iodide Shuttle Redox Mediator. J. Am. Chem. Soc. 2013, 135, 16872–16884. [Google Scholar] [CrossRef]
- Muniz-Miranda, M.; Gellini, C.; Simonelli, A.; Tiberi, M.; Giammanco, F.; Giorgetti, E. Characterization of Copper nanoparticles obtained by laser ablation in liquids. Appl. Phys. A 2013, 110, 829–833. [Google Scholar] [CrossRef]
- Nilius, N.; Ernst, N.; Freund, H. On energy transfer processes at cluster-oxide interfaces: Silver on titania. Chem. Phys. Lett. 2001, 349, 351–357. [Google Scholar] [CrossRef]
- Endo-Kimura, M.; Karabiyik, B.; Wang, K.; Wei, Z.; Ohtani, B.; Markowska-Szczupak, A.; Kowalska, E. Vis-responsive copper-modified titania for decomposition of organic compounds and microorganisms. Catalysts 2020, 10, 1194. [Google Scholar] [CrossRef]
- Janczarek, M.; Wei, Z.; Endo, M.; Ohtani, B.; Kowalska, E. Silver- and copper-modified decahedral anatase tiania particles as visible light-responsive plasmonic photocatalyst. J. Photon. Energy 2017, 7, 012008. [Google Scholar] [CrossRef] [Green Version]
- Ombaka, L.M.; Curti, M.; McGettrick, J.D.; Davies, M.L.; Bahnemann, D.W. Nitrogen/Carbon-Coated Zero-Valent Copper as Highly Efficient Co-catalysts for TiO2 Applied in Photocatalytic and Photoelectrocatalytic Hydrogen Production. ACS Appl. Mater. Interfaces 2020, 12, 30365–30380. [Google Scholar] [CrossRef]
- DeSario, P.A.; Pietron, J.J.; Brintlinger, T.H.; McEntee, M.; Parker, J.F.; Baturina, O.; Stroud, R.M.; Rolison, D.R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic activity of S-doped TiO2 photocatalyst under visible light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef] [Green Version]
- Reszczynska, J.; Grzyb, T.; Wei, Z.S.; Klein, M.; Kowalska, E.; Ohtani, B.; Zaleska-Medynska, A. Photocatalytic activity and luminescence properties of RE3+-TiO2 nanocrystals prepared by sol-gel and hydrothermal methods. Appl. Catal. B Environ. 2016, 181, 825–837. [Google Scholar] [CrossRef]
- Luna, A.L.; Novoseltceva, E.; Louran, E.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Synergetic effect of Ni and Au nanoparticles synthesized on titania particles for efficient photocatalytic hydrogen production. Appl. Catal. B Environ. 2016, 191, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Bielan, Z.; Endo-Kimura, M.; Janczarek, M.; Zhang, D.; Kowalski, D.; Zielińska-Jurek, A.; Markowska-Szczupak, A.; Ohtani, B.; Kowalska, E. On the mechanism of photocatalytic reactions on CuxO@TiO2 core-shell photocatalysts. J. Mat. Chem. A 2021, 9, 10135–10145. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Kowalska, E.; Abe, R.; Ohtani, B. Visible light-induced photocatalytic reaction of gold-modified titanium(IV) oxide particles: Action spectrum analysis. Chem. Commun. 2009, 241–243. [Google Scholar] [CrossRef]
- Ueno, K.; Misawa, H. Surface plasmon-enhanced photochemical reactions. J. Photoch. Photobio. C 2013, 15, 31–52. [Google Scholar] [CrossRef]
- Verbruggen, S.W. TiO2 photocatalysis for the degradation of pollutants in gas phase: From morphological design to plasmonic enhancement. J. Photoch. Photobio. C Photochem. Rev. 2015, 24, 64–82. [Google Scholar] [CrossRef]
- Wei, Z.; Janczarek, M.; Wang, K.; Zheng, S.; Kowalska, E. Morphology-governed performance of plasmonic photocatalysts. Catalysts 2020, 10, 1070. [Google Scholar] [CrossRef]
- Malankowska, A.; Kobylanski, M.P.; Mikolajczyk, A.; Cavdar, O.; Nowaczyk, G.; Jarek, M.; Lisowski, W.; Michalska, M.; Kowalska, E.; Ohtani, B.; et al. TiO2 and NaTaO3 Decorated by Trimetallic Au/Pd/Pt Core-Shell Nanoparticles as Efficient Photocatalysts: Experimental and Computational Studies. ACS Sustain. Chem. Eng. 2018, 6, 16665–16682. [Google Scholar] [CrossRef]
- Kowalska, E.; Yoshiiri, K.; Wei, Z.; Zheng, S.; Kastl, E.; Remita, H.; Ohtani, B.; Rau, S. Hybrid photocatalysts composed of titania modified with plasmonic nanoparticles and ruthenium complexes for decomposition of organic compounds. Appl. Catal. B Environ. 2015, 178, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Medrano, M.G.; Kowalska, E.; Lehoux, A.; Herissan, A.; Ohtani, B.; Rau, S.; Colbeau-Justin, C.; Rodriguez-Lopez, J.L.; Remita, H. Surface Modification of TiO2 with Au Nanoclusters for Efficient Water Treatment and Hydrogen Generation under Visible Light. J. Phys. Chem. C 2016, 120, 25010–25022. [Google Scholar] [CrossRef]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. Au/TiO2 Superstructure-Based Plasmonic Photocatalysts Exhibiting Efficient Charge Separation and Unprecedented Activity. J. Am. Chem. Soc. 2014, 136, 458–465. [Google Scholar] [CrossRef]
- Bian, Z.F.; Tachikawa, T.; Zhang, P.; Fujitsuka, M.; Majima, T. A nanocomposite superstructure of metal oxides with effective charge transfer interfaces. Nat. Commun. 2014, 5, 3038. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Han, B.; Lou, Y.; Liu, Z.; Qian, G.; Wang, Z. Rational design and fabrication of TiO2 nano heterostructure with multi-junctions for efficient photocatalysis. Int. J. Hydrogen Energy 2020, 45, 28640–28650. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. Defective Dopant-Free TiO2 as an Efficient Visible Light-Active Photocatalyst. Catalysts 2021, 11, 978. [Google Scholar] [CrossRef]
- Luna, A.L.; Dragoe, D.; Wang, K.L.; Beaunier, P.; Kowalska, E.; Ohtani, B.; Uribe, D.B.; Valenzuela, M.A.; Remita, H.; Colbeau-Justin, C. Photocatalytic Hydrogen Evolution Using Ni-Pd/TiO2: Correlation of Light Absorption, Charge-Carrier Dynamics, and Quantum Efficiency. J. Phys. Chem. C 2017, 121, 14302–14311. [Google Scholar] [CrossRef]
- Huang, H.; Lin, J.; Fan, L.; Wang, X.; Fu, X.; Long, J. Heteroatomic Ni, Sn Clusters-Grafted Anatase TiO2 Photocatalysts: Structure, Electron Delocalization, and Synergy for Solar Hydrogen Production. J. Phys. Chem. C 2015, 119, 10478–10492. [Google Scholar] [CrossRef]
- Amano, F.; Yasumoto, T.; Prieto-Mahaney, O.O.; Uchida, S.; Shibayama, T.; Ohtani, B. Photocatalytic activity of octahedral single-crystalline mesoparticles of anatase titanium(IV) oxide. Chem. Commun. 2009, 2311–2313. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Kowalska, E.; Verrett, J.; Colbeau-Justin, C.; Remita, H.; Ohtani, B. Morphology-dependent photocatalytic activity of octahedral anatase particles prepared by ultrasonication-hydrothermal reaction of titanates. Nanoscale 2015, 7, 12392–12404. [Google Scholar] [CrossRef] [Green Version]
- Amano, F.; Prieto-Mahaney, O.O.; Terada, Y.; Yasumoto, T.; Shibayama, T.; Ohtani, B. Decahedral single-crystalline particles of anatase titanium(IV) oxide with high photocatalytic activity. Chem. Mater. 2009, 21, 2601–2603. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E.; Ohtani, B. Decahedral-shaped anatase titania photocatalyst particles: Synthesis in a newly developed coaxial-flow gas-phase reactor. Chem. Eng. J. 2016, 289, 502–512. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Janczarek, M.; Endo, M.; Colbeau-Justin, C.; Ohtani, B.; Kowalska, E. Silver-modified octahedral anatase particles as plasmonic photocatalyst. Catal. Today 2018, 310, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Rosa, L.; Wang, K.; Endo, M.; Juodkazi, S.; Ohtani, B.; Kowalska, E. Size-controlled gold nanoparticles on octahedral anatase particles as efficient plasmonic photocatalyst. Appl. Catal. B Environ. 2017, 206, 393–405. [Google Scholar] [CrossRef]
- Amoli, V.; Sibi, M.G.; Banerjee, B.; Anand, M.; Maurya, A.; Farooqui, S.A.; Bhaumik, A.; Sinha, A.K. Faceted Titania Nanocrystals Doped with Indium Oxide Nanoclusters As a Superior Candidate for Sacrificial Hydrogen Evolution without Any Noble-Metal Cocatalyst under Solar Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 810–822. [Google Scholar] [CrossRef]
- Li, W.; Elzatahry, A.; Aldhayan, D.; Zhao, D. Core–shell structured titanium dioxide nanomaterials for solar energy utilization. Chem. Soc. Rev. 2018, 47, 8203–8237. [Google Scholar] [CrossRef] [PubMed]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Bielan, Z.; Sulowska, A.; Dudziak, S.; Siuzdak, K.; Ryl, J.; Zielinska-Jurek, A. Defective TiO2 core-shell magnetic photocatalyst modified with plasmonic nanoparticles for visible light-induced photocatalytic activity. Catalysts 2020, 10, 672. [Google Scholar] [CrossRef]
- Zielinska-Jurek, A.; Bielan, Z.; Wysocka, I.; Strychalska, J.; Janczarek, M.; Klimczuk, T. Magnetic semiconductor photocatalysts for the degradation of recalcitrant chemicals from flow back water. J. Environ. Manag. 2017, 195, 157–165. [Google Scholar] [CrossRef]
- Bielan, Z.; Kowalska, E.; Dudziak, S.; Wang, K.; Ohtani, B.; Zielinska-Jurek, A. Mono- and bimetallic (Pt/Cu) titanium(IV) oxide core-shell photocatalysts with UV/Vis light activity and magnetic separability. Catal. Today 2021, 361, 198–209. [Google Scholar] [CrossRef]
- Janczarek, M.; Kowalska, E. On the Origin of Enhanced Photocatalytic Activity of Copper-Modified Titania in the Oxidative Reaction Systems. Catalysts 2017, 7, 317. [Google Scholar] [CrossRef] [Green Version]
- Endo-Kimura, M.; Janczarek, M.; Bielan, Z.; Zhang, D.; Wang, K.; Markowska-Szczupak, A.; Kowalska, E. Photocatalytic and antimicrobial properties of Ag2O/TiO2 heterojunction. ChemEngineering 2019, 3, 3. [Google Scholar] [CrossRef] [Green Version]
- Janczarek, M.; Endo, M.; Zhang, D.; Wang, K.; Kowalska, E. Enhanced photocatalytic and antimicrobial prformance of cuprous oxide/titania: The effect of titania matrix. Materials. 2018, 11, 2069. [Google Scholar] [CrossRef] [Green Version]
- Mendez-Medrano, M.G.; Kowalska, E.; Ohtani, B.; Uribe, D.B.; Colbeau-Justin, C.; Rau, S.; Rodriguez-Lopez, J.L.; Remita, H. Heterojunction of CuO nanoclusters with TiO2 for photo-oxidation of organic compounds and for hydrogen production. J. Chem. Phys. 2020, 153, 034705. [Google Scholar] [CrossRef]
- Zubair, M.; Svenum, I.-H.; Rønning, M.; Yang, J. Facile synthesis approach for core-shell TiO2–CdS nanoparticles for enhanced photocatalytic H2 generation from water. Catal. Today 2019, 328, 15–20. [Google Scholar] [CrossRef]
- Paszkiewicz-Gawron, M.; Kowalska, E.; Endo-Kimura, M.; Zwara, J.; Pancielejko, A.; Wang, K.; Lisowski, W.; Łuczak, J.; Zaleska-Medynska, A.; Grabowska-Musiał, E. Stannates, titanates and tantalates modified with carbon and graphene quantum dots for enhancement of visible-light photocatalytic activity. Appl. Surf. Sci. 2021, 541, 148425. [Google Scholar] [CrossRef]
- Dong, Y.; Han, Q.; Hu, Q.; Xu, C.; Dong, C.; Peng, Y.; Ding, Y.; Lan, Y. Carbon quantum dots enriching molecular nickel polyoxometalate over CdS semiconductor for photocatalytic water splitting. Appl. Catal. B Environ. 2021, 293, 120214. [Google Scholar] [CrossRef]
- Zhang, P.; Zeng, G.; Song, T.; Huang, S.; Wang, T.; Zeng, H. Synthesis of a plasmonic CuNi bimetal modified with carbon quantum dots as a non-semiconductor-driven photocatalyst for effective water splitting. J. Catal. 2019, 369, 267–275. [Google Scholar] [CrossRef]
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634. [Google Scholar] [CrossRef] [Green Version]
- Giovannetti, R.; Rommozzi, E.; Zannotti, M.; D’Amato, C.A. Recent Advances in Graphene Based TiO2 Nanocomposites (GTiO2Ns) for Photocatalytic Degradation of Synthetic Dyes. Catalysts 2017, 7, 305. [Google Scholar] [CrossRef]
- Wang, K.L.; Endo-Kimura, M.; Belchi, R.; Zhang, D.; Habert, A.; Boucle, J.; Ohtani, B.; Kowalska, E.; Herlin-Boime, N. Carbon/graphene-modified titania with enhanced photocatalytic activity under UV and vis irradiation. Materials. 2019, 12, 4158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, J.-P.; Wang, L.-C.; Luo, J.; Nie, Y.-C.; Xing, Q.-J.; Luo, X.-B.; Du, H.-M.; Luo, S.-L.; Suib, S.L. Synthesis and efficient visible light photocatalytic H2 evolution of a metal-free g-C3N4/graphene quantum dots hybrid photocatalyst. Appl. Catal. B Environ. 2016, 193, 103–109. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Hua, Y.; Zhu, F.; Sun, L.; Gu, W.; Shi, W. Constructing nitrogen doped graphene quantum dots-ZnNb2O6/g-C3N4 catalysts for hydrogen production under visible light. Appl. Catal. B Environ. 2017, 206, 531–537. [Google Scholar] [CrossRef]
- Qian, J.; Chen, Z.; Chen, F.; Wang, Y.; Wu, Z.; Zhang, W.; Wu, Z.; Li, P. Exploration of CeO2–CuO Quantum Dots in Situ Grown on Graphene under Hypha Assistance for Highly Efficient Solar-Driven Hydrogen Production. Inorg. Chem. 2018, 57, 14532–14541. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, K.; Kim, W.D.; Lee, S.; Shin, D.J.; Lee, D.C. Thin Amorphous TiO2 Shell on CdSe Nanocrystal Quantum Dots Enhances Photocatalysis of Hydrogen Evolution from Water. J. Phys. Chem. C 2014, 118, 23627–23634. [Google Scholar] [CrossRef]
- Ohtani, B.; Ogawa, Y.; Nishimoto, S.I. Photocatalytic Activity of Amorphous-Anatase Mixture of Titanium(IV) Oxide Particles Suspended in Aqueous Solutions. J. Phys. Chem. B 1997, 101, 3746–3752. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef] [Green Version]
- Beranek, R.; Hildebrand, H.; Schmuki, P. Self-Organized Porous Titanium Oxide Prepared in H2SO4/HF Electrolytes. Electrochem. Solid State Lett. 2003, 6, B12–B14. [Google Scholar] [CrossRef]
- Kowalski, D.; Kim, D.; Schmuki, P. TiO2 nanotubes, nanochannels and mesosponge: Self-organized formation and applications. Nano Today 2013, 8, 235–264. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of Highly-Ordered TiO2 Nanotube Arrays in Dye-Sensitized Solar Cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, D.; Schmuki, P. Polypyrrole self-organized nanopore arrays formed by controlled electropolymerization in TiO2 nanotube template. Chem. Commun. 2010, 46, 8585–8587. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, D.; Mallet, J.; Thomas, S.; Nemaga, A.W.; Michel, J.; Guery, C.; Molinari, M.; Morcrette, M. Electrochemical synthesis of 1D core-shell Si/TiO2 nanotubes for lithium ion batteries. J. Power Sources 2017, 361, 243–248. [Google Scholar] [CrossRef]
- Kowalski, D.; Mallet, J.; Thomas, S.; Rysz, J.; Bercu, B.; Michel, J.; Molinari, M. Self-organization of TiO2 nanotubes in mono-, di- and tri-ethylene glycol electrolytes. Electrochim. Acta 2016, 204, 287–293. [Google Scholar] [CrossRef]
- Nischk, M.; Mazierski, P.; Wei, Z.S.; Siuzdak, K.; Kouame, N.A.; Kowalska, E.; Remita, H.; Zaleska-Medynska, A. Enhanced photocatalytic, electrochemical and photoelectrochemical properties of TiO2 nanotubes arrays modified with Cu, AgCu and Bi nanoparticles obtained via radiolytic reduction. Appl. Surf. Sci. 2016, 387, 89–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazierski, P.; Malankowska, A.; Kobylanski, M.; Diak, M.; Kozak, M.; Winiarski, M.J.; Klimczuk, T.; Lisowski, W.; Nowaczyk, G.; Zaleska-Medynska, A. Photocatalytically Active TiO2/Ag2O Nanotube Arrays Interlaced with Silver Nanoparticles Obtained from the One-Step Anodic Oxidation of Ti-Ag Alloys. ACS Catal. 2017, 7, 2753–2764. [Google Scholar] [CrossRef]
- Wang, G.M.; Feng, H.Q.; Hu, L.S.; Jin, W.H.; Hao, Q.; Gao, A.; Peng, X.; Li, W.; Wong, K.Y.; Wang, H.Y.; et al. An antibacterial platform based on capacitive carbon-doped TiO2 nanotubes after direct or alternating current charging. Nat. Commun. 2018, 9, 2055. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hoivik, N.; Wang, K.Y. Recent advance on engineering titanium dioxide nanotubes for photochemical and photoelectrochemical water splitting. Nano Energy 2016, 30, 728–744. [Google Scholar] [CrossRef]
- Chu, J.; Sun, Y.; Han, X.; Zhang, B.; Du, Y.; Song, B.; Xu, P. Mixed Titanium Oxide Strategy for Enhanced Photocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2019, 11, 18475–18482. [Google Scholar] [CrossRef]
- Yang, W.; Li, M.; Pan, K.; Guo, L.; Wu, J.; Li, Z.; Yang, F.; Lin, K.; Zhou, W. Surface engineering of mesoporous anatase titanium dioxide nanotubes for rapid spatial charge separation on horizontal-vertical dimensions and efficient solar-driven photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 586, 75–83. [Google Scholar] [CrossRef]
- Liu, N.; Schneider, C.; Freitag, D.; Hartmann, M.; Venkatesan, U.; Müller, J.; Spiecker, E.; Schmuki, P. Black TiO2 Nanotubes: Cocatalyst-Free Open-Circuit Hydrogen Generation. Nano Lett. 2014, 14, 3309–3313. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhu, L.; Ba, N.; Gao, F.; Xie, H. Effects of NH4F quantity on N-doping level, photodegradation and photocatalytic H2 production activities of N-doped TiO2 nanotube array films. Mater. Res. Bull. 2017, 86, 268–276. [Google Scholar] [CrossRef]
- Chen, W.-T.; Dosado, A.G.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I.N. Highly reactive anatase nanorod photocatalysts synthesized by calcination of hydrogen titanate nanotubes: Effect of calcination conditions on photocatalytic performance for aqueous dye degradation and H2 production in alcohol-water mixtures. Appl. Catal. A Gen. 2018, 565, 98–118. [Google Scholar] [CrossRef]
- Galdámez-Martínez, A.; Bai, Y.; Santana, G.; Sprick, R.S.; Dutt, A. Photocatalytic hydrogen production performance of 1-D ZnO nanostructures: Role of structural properties. Int. J. Hydrogen Energy 2020, 45, 31942–31951. [Google Scholar] [CrossRef]
- Yu, X.; Li, W.; Li, Z.; Liu, J.; Hu, P. Defect engineered Ta2O5 nanorod: One-pot synthesis, visible-light driven hydrogen generation and mechanism. Appl. Catal. B Environ. 2017, 217, 48–56. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, T.; He, H.; Fu, Y.; Zhang, X.; Sun, J.; Xing, L.; Liu, B.; Zhang, Y.; Xue, X. Enhanced H2 Production of TiO2/ZnO Nanowires Co-Using Solar and Mechanical Energy through Piezo-Photocatalytic Effect. ACS Sustain. Chem. Eng. 2018, 6, 10162–10172. [Google Scholar] [CrossRef]
- Tso, S.; Li, W.-S.; Wu, B.-H.; Chen, L.-J. Enhanced H2 production in water splitting with CdS-ZnO core-shell nanowires. Nano Energy 2018, 43, 270–277. [Google Scholar] [CrossRef]
- Hsu, M.-H.; Chang, C.-J.; Weng, H.-T. Efficient H2 Production Using Ag2S-Coupled ZnO@ZnS Core–Shell Nanorods Decorated Metal Wire Mesh as an Immobilized Hierarchical Photocatalyst. ACS Sustain. Chem. Eng. 2016, 4, 1381–1391. [Google Scholar] [CrossRef]
- Hu, S.; Qiao, P.; Zhang, L.; Jiang, B.; Gao, Y.; Hou, F.; Wu, B.; Li, Q.; Jiang, Y.; Tian, C.; et al. Assembly of TiO2 ultrathin nanosheets with surface lattice distortion for solar-light-driven photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 239, 317–323. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Li, D.; Fang, F.; Huang, W. Engineering self-doped surface defects of anatase TiO2 nanosheets for enhanced photocatalytic efficiency. Appl. Surf. Sci. 2021, 540, 148330. [Google Scholar] [CrossRef]
- Liu, X.; Hua, R.; Niu, J.; Zhang, Z.; Zhang, J. N2 plasma treatment TiO2 nanosheets for enhanced visible light-driven photocatalysis. J. Alloys Compd. 2021, 881, 160509. [Google Scholar] [CrossRef]
- Hu, X.; Song, J.; Luo, J.; Zhang, H.; Sun, Z.; Li, C.; Zheng, S.; Liu, Q. Single-atomic Pt sites anchored on defective TiO2 nanosheets as a superior photocatalyst for hydrogen evolution. J. Energy Chem. 2021, 62, 1–10. [Google Scholar] [CrossRef]
- Yu, X.; Liu, G.; Li, W.; An, L.; Li, Z.; Liu, J.; Hu, P. Mesocrystalline Ta2O5 nanosheets supported PdPt nanoparticles for efficient photocatalytic hydrogen production. Int. J. Hydrogen Energy 2018, 43, 8232–8242. [Google Scholar] [CrossRef]
- Wu, X.; Zuo, S.; Qiu, M.; Li, Y.; Zhang, Y.; An, P.; Zhang, J.; Zhang, H.; Zhang, J. Atomically defined Co on two-dimensional TiO2 nanosheet for photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 420, 127681. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Hu, H.; Zeng, G.; Li, C. Recovering Hydrogen Energy from Photocatalytic Treatment of Pharmaceutical-Contaminated Water Using Co3O4 Modified {001}/{101}-TiO2 Nanosheets. ACS EST Eng. 2021, 1, 603–611. [Google Scholar] [CrossRef]
- Feng, J.; An, C.; Dai, L.; Liu, J.; Wei, G.; Bai, S.; Zhang, J.; Xiong, Y. Long-term production of H2 over Pt/CdS nanoplates under sunlight illumination. Chem. Eng. J. 2016, 283, 351–357. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, L.; Wang, W. Internal polar field enhanced H2 evolution of BiOIO3 nanoplates. Int. J. Hydrogen Energy 2016, 41, 10170–10177. [Google Scholar] [CrossRef]
- Yu, S.; Webster, R.D.; Zhou, Y.; Yan, X. Ultrathin g-C3N4 nanosheets with hexagonal CuS nanoplates as a novel composite photocatalyst under solar light irradiation for H2 production. Catal. Sci. Technol. 2017, 7, 2050–2056. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, S.; Liu, D.; Zhang, J.; Peng, T. Layered WS2/WO3 Z-scheme photocatalyst constructed via an in situ sulfurization of hydrous WO3 nanoplates for efficient H2 generation. Appl. Surf. Sci. 2020, 529, 147013. [Google Scholar] [CrossRef]
- Chandra, M.; Pradhan, D. Engineering the Morphology and Crystal Phase of 3 D Hierarchical TiO2 with Excellent Photochemical and Photoelectrochemical Solar Water Splitting. ChemSusChem 2020, 13, 3005–3016. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, H.; Xu, J.; Zhuang, H.; Long, J. 3D flower-like heterostructured TiO2@Ni(OH)2 microspheres for solar photocatalytic hydrogen production. Chin. J. Catal. 2019, 40, 320–325. [Google Scholar] [CrossRef]
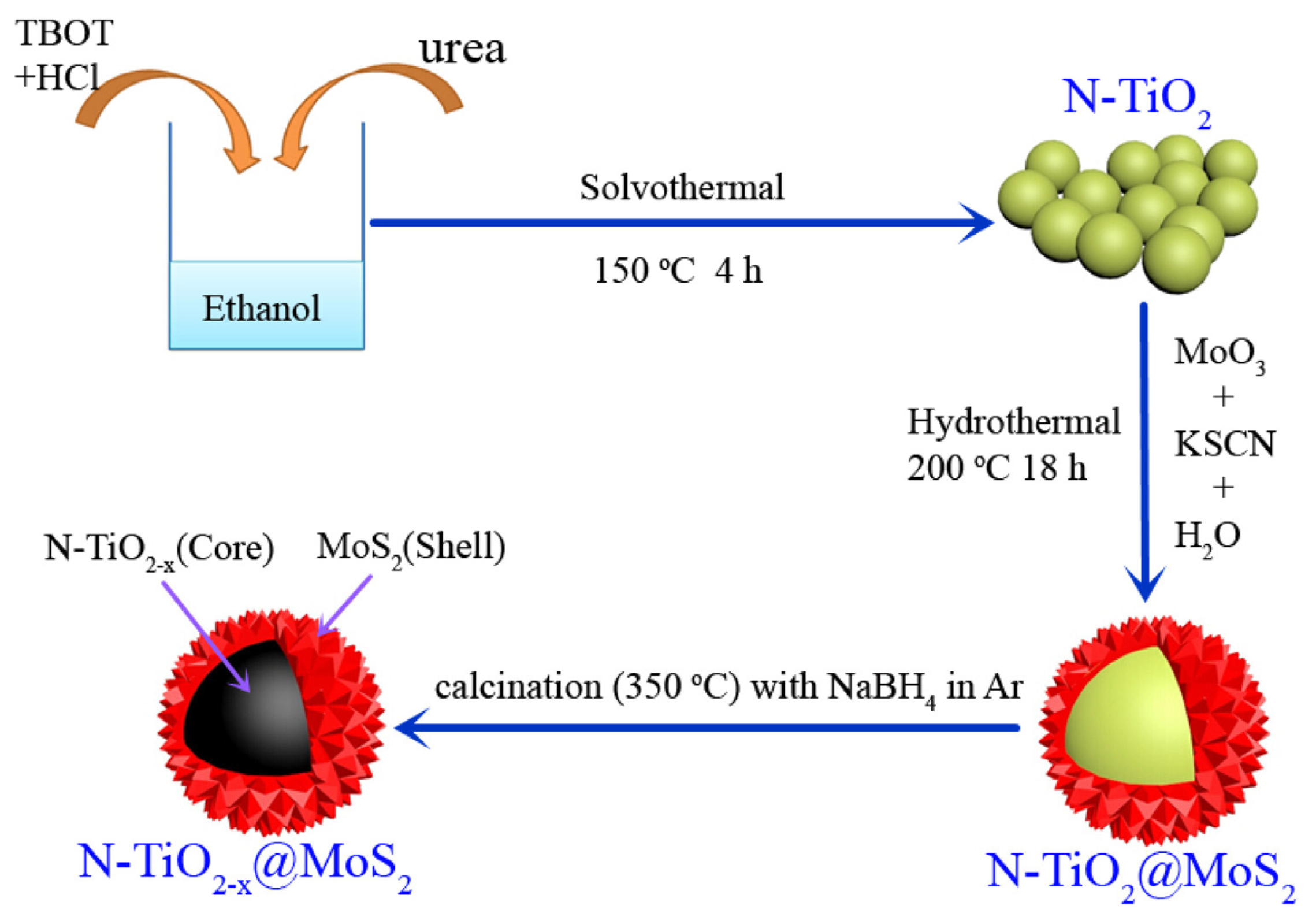
- Liu, X.; Xing, Z.; Zhang, Y.; Li, Z.; Wu, X.; Tan, S.; Yu, X.; Zhu, Q.; Zhou, W. Fabrication of 3D flower-like black N-TiO2-x@MoS2 for unprecedented-high visible-light-driven photocatalytic performance. Appl. Catal. B Environ. 2017, 201, 119–127. [Google Scholar] [CrossRef]
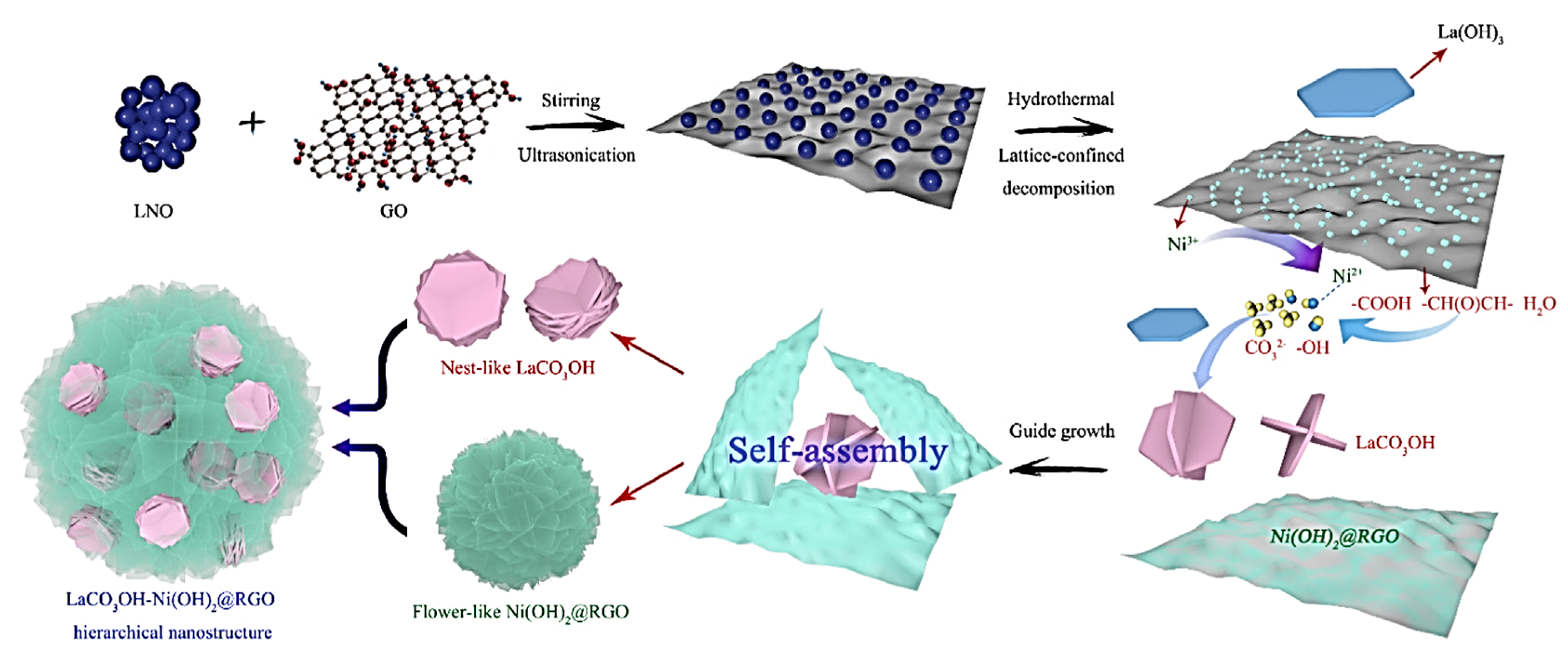
- Lv, T.; Xu, Z.; Hong, W.; Li, G.; Li, Y.; Jia, L. Graphene oxide mediated self-sacrificial synthesis of LaCO3OH-Ni(OH)2@graphene hierarchical composite for photocatalytic H2 evolution and supercapacitor. Chem. Eng. J. 2020, 382, 123021–123032. [Google Scholar] [CrossRef]
- Chao, P.-Y.; Chang, C.-J.; Lin, K.-S.; Wang, C.F. Synergistic effects of morphology control and calcination on the activity of flower-like Bi2WO6-Bi2O3 photocatalysts prepared by an ionic liquid-assisted solvothermal method. J. Alloys Compd. 2021, 883, 160920–160930. [Google Scholar] [CrossRef]
- Sun, L.; Xiao, H.; Cong, S.; Hao, Y.; Xue, M.; Kang, S.-Z. Fabrication of flower-like Sn3O4 hierarchical nanostructure and its photocatalytic activity for H2 evolution from water. Inorg. Chem. Commun. 2019, 106, 116–119. [Google Scholar] [CrossRef]
- Di, T.; Cheng, B.; Ho, W.; Yu, J.; Tang, H. Hierarchically CdS–Ag2S nanocomposites for efficient photocatalytic H2 production. Appl. Surf. Sci. 2019, 470, 196–204. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.; Tian, Y.; Li, H.; Lan, K.; Zu, L.; Xia, Y.; Duan, L.; Li, W.; Zhao, D. Defect-engineering of mesoporous TiO2 microspheres with phase junctions for efficient visible-light driven fuel production. Nano Energy 2019, 66, 104113. [Google Scholar] [CrossRef]
- Zhang, W.; He, H.L.; Tian, Y.; Lan, K.; Liu, Q.; Wang, C.Y.; Liu, Y.; Elzatahry, A.; Che, R.C.; Li, W.; et al. Synthesis of uniform ordered mesoporous TiO2 microspheres with controllable phase junctions for efficient solar water splitting. Chem. Sci. 2019, 10, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Lv, S.; Wang, Y.; Zhou, Y.; Liu, Q.; Song, C.; Wang, D. Oxygen vacancy stimulated direct Z-scheme of mesoporous Cu2O/TiO2 for enhanced photocatalytic hydrogen production from water and seawater. J. Alloys Compd. 2021, 868, 159144. [Google Scholar] [CrossRef]
- Zou, X.; Yang, Y.; Chen, H.; Shi, X.-L.; Song, S.; Chen, Z.-G. Hierarchical meso/macro-porous TiO2/graphitic carbon nitride nanofibers with enhanced hydrogen evolution. Mater. Des. 2021, 202, 109542. [Google Scholar] [CrossRef]
- Meng, A.; Zhu, B.; Zhong, B.; Zhang, L.; Cheng, B. Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity. Appl. Surf. Sci. 2017, 422, 518–527. [Google Scholar] [CrossRef]
- Lv, B.; Lu, L.; Feng, X.; Wu, X.; Wang, X.; Zou, X.; Zhang, F. Efficient photocatalytic hydrogen production using an NH4TiOF3/TiO2/g-C3N4 composite with a 3D camellia-like Z-scheme heterojunction structure. Ceram. Int. 2020, 46, 26689–26697. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Nie, T.; Wang, R.; He, B.; Han, B.; Wang, H.; Tian, Y.; Gong, Y. Enhanced visible-light photocatalytic H2 production of hierarchical g-C3N4 hexagon by one-step self-assembly strategy. Appl. Surf. Sci. 2020, 499, 143942. [Google Scholar] [CrossRef]
- Gultom, N.S.; Abdullah, H.; Kuo, D.-H.; Ke, W.-C. Oriented p–n Heterojunction Ag2O/Zn(O,S) Nanodiodes on Mesoporous SiO2 for Photocatalytic Hydrogen Production. ACS Appl. Energy Mat. 2019, 2, 3228–3236. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Bao, J.; Sheng, X.; Fang, J.; Zhao, S.; Zhang, Y.; Chen, W. Hierarchical Honeycomb Br-, N-Codoped TiO2 with Enhanced Visible-Light Photocatalytic H2 Production. ACS Appl. Mater. Interfaces 2018, 10, 18796–18804. [Google Scholar] [CrossRef]
- Yablonovitch, E. Photonic Band-Gap Structures. J. Opt. Soc. Am. B 1993, 10, 283–295. [Google Scholar] [CrossRef]
- Lopez, C. Materials aspects of photonic crystals. Adv. Mater. 2003, 15, 1679–1704. [Google Scholar] [CrossRef]
- Jovic, V.; Idriss, H.; Waterhouse, G.I.N. Slow photon amplification of gas-phase ethanol photo-oxidation in titania inverse opal photonic crystals. Chem. Phys. 2016, 479, 109–121. [Google Scholar] [CrossRef]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhang, Q.; Zhang, M. Preparation of inverse opal titanium dioxide for photocatalytic performance research. Optic. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Zuliani, A.; Ceresoli, D.; Martinazzo, R.; Selli, E. Exploiting the Photonic Crystal Properties of TiO2 Nanotube Arrays To Enhance Photocatalytic Hydrogen Production. ACS Catal. 2016, 6, 1345–1353. [Google Scholar] [CrossRef]
- Kim, W.T.; Choi, W.Y. Fabrication of TiO2 photonic crystal by anodic oxidation and their optical sensing properties. Sens. Actuators A Phys. 2017, 260, 178–184. [Google Scholar] [CrossRef]
- Cheng, C.W.; Karuturi, S.K.; Liu, L.J.; Liu, J.P.; Li, H.X.; Su, L.T.; Tok, A.I.Y.; Fan, H.J. Quantum-dot-sensitized TiO2 inverse opals for photoelectrochemical hydrogen generation. Small 2012, 8, 37–42. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Li, M.; Shen, W.; Liu, Z.; Wang, J.; Zhao, J.; Jiang, L.; Song, Y. Enhancement of photochemical hydrogen evolution over Pt-loaded hierarchical titania photonic crystal. Energy Environ. Sci. 2010, 3, 1503–1506. [Google Scholar] [CrossRef]
- Waterhouse, G.I.N.; Wahab, A.K.; Al-Oufi, M.; Jovic, V.; Anjum, D.H.; Sun-Waterhouse, D.; Llorca, J.; Idriss, H. Hydrogen production by tuning the photonic band gap with the electronic band gap of TiO2. Sci. Rep. 2013, 3, 2849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raja-Mogan, T.; Ohtani, B.; Kowalska, E. Photonic crystals for plasmonic photocatalysis. Catalysts 2020, 10, 827. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Y.; Lee, S.T.; Yang, S.H.; Kang, Z.H. Coupling surface plasmon resonance of gold nanoparticles with slow-photon-effect of TiO2 photonic crystals for synergistically enhanced photoelectrochemical water splitting. Energ. Environ. Sci. 2014, 7, 1409–1419. [Google Scholar] [CrossRef]
- Zhang, L.W.; Lin, C.Y.; Valev, V.K.; Reisner, E.; Steiner, U.; Baumberg, J.J. Plasmonic enhancement in BiVO4 photonic crystals for efficient water splitting. Small 2014, 10, 3970–3978. [Google Scholar] [CrossRef] [Green Version]
- Raja-Mogan, T.; Lehoux, A.; Takashima, M.; Kowalska, E.; Ohtani, B. Slow photon-induced enhancement of photocatalytic activity of gold nanoparticle-incorporated titania in-verse opal. Chem. Lett. 2021, 50, 711–713. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Jiang, G.; Zhao, Z.; Xu, C.; Wei, Y.; Duan, A.; Liu, J.; Gao, J. A photonic crystal-based CdS–Au–WO3 heterostructure for efficient visible-light photocatalytic hydrogen and oxygen evolution. RSC Adv. 2014, 4, 15689–15694. [Google Scholar] [CrossRef]
- Sordello, F.; Duca, C.; Maurino, V.; Minero, C. Photocatalytic metamaterials: TiO2 inverse opals. Chem. Commun. 2011, 47, 6147–6149. [Google Scholar] [CrossRef]
- Zhao, H.; Hu, Z.Y.; Liu, J.; Li, Y.; Wu, M.; Van Tendeloo, G.; Su, B.L. Blue-edge slow photons promoting visible-light hydrogen production on gradient ternary 3DOM TiO2-Au-CdS photonic crystals. Nano Energy 2018, 47, 266–274. [Google Scholar] [CrossRef]
- Zhang, S.S.; Peng, B.Y.; Yang, S.Y.; Wang, H.G.; Yu, H.; Fang, Y.P.; Peng, F. Non-noble metal copper nanoparticles-decorated TiO2 nanotube arrays with plasmon-enhanced photocatalytic hydrogen evolution under visible light. Int. J. Hydrogen Energy 2015, 40, 303–310. [Google Scholar] [CrossRef]
- Rahul, T.K.; Sandhyarani, N. In situ gold-loaded fluorinated titania inverse opal photocatalysts for enhanced solar-light-driven hydrogen production. ChemNanoMat 2017, 3, 503–510. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Photocatalytic H2 production over inverse opal TiO2 catalysts. Catal. Today 2019, 321–322, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Rahul, T.K.; Mohan, M.; Sandhyarani, N. Enhanced solar hydrogen evolution over in situ gold-platinum bimetallic nanoparticle-loaded Ti3+ self-doped titania photocatalysts. ACS Sustain. Chem. Eng. 2018, 6, 3049–3059. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, C.; Chen, H.; Zhao, H.; Liu, J.; Li, Y.; Su, B. Cadmium Sulfide Inverse Opal for Photocatalytic Hydrogen Production. Acta Phys. -Chim. Sin. 2020, 36, 1803014. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Yang, X.L.; Hedhili, M.N.; Ahmed, E.; Shi, L.; Wang, P. Microwave-Assisted Self-Doping of TiO2 Photonic Crystals for Efficient Photoelectrochemical Water Splitting. ACS Appl. Mater. Inter. 2014, 6, 691–696. [Google Scholar] [CrossRef]
- <monospace>Chen, Y.; Li, L.; Xu, Q.; Düren, T.; Fan, J.; Ma, D. Controllable Synthesis of g-C3N4 Inverse Opal Photocatalysts for Superior Hydrogen Evolution. Acta Phys.-Chim. Sin. 2021, 37, 2009080. [Google Scholar]
- Temerov, F.; Pham, K.; Juuti, P.; Mäkelä, J.M.; Grachova, E.V.; Kumar, S.; Eslava, S.; Saarinen, J.J. Silver-Decorated TiO2 Inverse Opal Structure for Visible Light-Induced Photocatalytic Degradation of Organic Pollutants and Hydrogen Evolution. ACS Appl. Mater. Interfaces 2020, 12, 41200–41210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Mao, B.; Li, D.; Liu, Y.; Li, F.; Dong, W.; Jiang, T.; Shi, W. 0D/2D Z-scheme heterojunctions of Zn-AgIn5S8 QDs/α-Fe2O3 nanosheets for efficient visible-light-driven hydrogen production. Chem. Eng. J. 2021, 417, 128275–128284. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, J.; Tang, H.; Yu, X.; Shen, J. Construction 0D TiO2 nanoparticles/2D CoP nanosheets heterojunctions for enhanced photocatalytic H2 evolution activity. J. Mater. Sci. Technol. 2020, 56, 196–205. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Li, X.; Cen, C. CdS nanorod arrays with TiO2 nano-coating for improved photostability and photocatalytic activity. Phys. Chem. Chem. Phys. 2014, 16, 15339–15345. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Wu, L.; Lin, J.; Li, P.; Li, Q. Microemulsion-mediated solvothermal synthesis of nanosized CdS-sensitized TiO2 crystalline photocatalyst. Chem. Commun. 2003, 1552–1553. [Google Scholar] [CrossRef]
- Peter, L.M.; Riley, D.J.; Tull, E.J.; Wijayantha, K.G.U. Photosensitization of nanocrystalline TiO2 by self-assembled layers of CdS quantum dots. Chem. Commun. 2002, 1030–1031. [Google Scholar] [CrossRef]
- Tan, P.; Liu, Y.; Zhu, A.; Zeng, W.; Cui, H.; Pan, J. Rational Design of Z-Scheme System Based on 3D Hierarchical CdS Supported 0D Co9S8 Nanoparticles for Superior Photocatalytic H2 Generation. ACS Sustain. Chem. Eng. 2018, 6, 10385–10394. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Qu, P.; Xu, Q.; Zheng, J.; Jia, S.; Chen, J.; Zhu, Z. A 2D/1D TiO2 nanosheet/CdS nanorods heterostructure with enhanced photocatalytic water splitting performance for H2 evolution. Int. J. Hydrogen Energy 2018, 43, 7388–7396. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Hydrothermal growth of two dimensional hierarchical MoS2 nanospheres on one dimensional CdS nanorods for high performance and stable visible photocatalytic H2 evolution. Appl. Surf. Sci. 2018, 433, 240–248. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Hu, R.; Lei, Y.-J.; Jia, Z.-Y.; Hu, G.-L.; Li, C.-B.; Gu, Q. Highly efficient and selective photocatalytic CO2 reduction based on water-soluble CdS QDs modified by the mixed ligands in one pot. Catal. Sci. Technol. 2020, 10, 2821–2829. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, M.-L.; Yuan, B.-L.; Cui, H.-J.; Shi, J.-W. Assembly, characterization, and photocatalytic activities of TiO2 nanotubes/CdS quantum dots nanocomposites. J. Nanoparticle Res. 2011, 13, 6661–6672. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Kowalska, E.; Nadolna, J.; Wei, Z.S.; Endo, M.; Ohtani, B.; Zaleska-Medynska, A. Preparation of CdS and Bi2S3 quantum dots co-decorated perovskite-type KNbO3 ternary heterostructure with improved visible light photocatalytic activity and stability for phenol degradation. Dalton Trans. 2018, 47, 15232–15245. [Google Scholar] [CrossRef]
- Yang, M.; Qian, Y.; Du, J.; Yuan, S.; Wang, S.; Zhu, X.; Lin, X.; Li, K.; Li, S.; Kang, D.J. Controlled synthesis of nanoplate, nanoprism and nanopyramid-shaped CdSe decorated on porous TiO2 photocatalysts for visible-light-driven hydrogen evolution. Ceram. Int. 2018, 44, 12555–12563. [Google Scholar] [CrossRef]
- Li, X.C.; Zheng, W.J.; He, G.H.; Zhao, R.; Liu, D. Morphology Control of TiO2 Nanoparticle in Microemulsion and Its Photocatalytic Property. ACS Sustain. Chem. Eng. 2014, 2, 288–295. [Google Scholar] [CrossRef]
- Li, S.Y.; Yang, Y.L.; Su, Q.; Liu, X.Y.; Zhao, H.P.; Zhao, Z.X.; Li, J.; Jin, C. Synthesis and photocatalytic activity of transition metal and rare earth element co-doped TiO2 nano particles. Mater Lett. 2019, 252, 123–125. [Google Scholar] [CrossRef]
- El-Shamy, A.G. New carbon quantum dots nano-particles decorated zinc peroxide (C-dots/ZnO2) nano-composite with superior photocatalytic efficiency for removal of different dyes under UV-A light. Synthetic Met. 2020, 267, 116472. [Google Scholar] [CrossRef]
- Zhou, W.J.; Liu, H.; Boughton, R.I.; Du, G.J.; Lin, J.J.; Wang, J.Y.; Liu, D. One-dimensional single-crystalline Ti-O based nanostructures: Properties, synthesis, modifications and applications. J. Mater. Chem. 2010, 20, 5993–6008. [Google Scholar] [CrossRef]
- Kulathunga, K.M.S.D.B.; Yan, C.F.; Bandara, J. Photocatalytic removal of airborne indoor pollutants by IR illuminated silver coated TiO2 catalyst: Advantage of one-dimensional TiO2 nanostructures in IR active photocatalysis. Colloid. Surface A 2020, 590, 124509. [Google Scholar] [CrossRef]
- Butler, S.Z.; Hollen, S.M.; Cao, L.Y.; Cui, Y.; Gupta, J.A.; Gutierrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.X.; Ismach, A.F.; et al. Progress, Challenges, and Opportunities in Two-Dimensional Materials Beyond Graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef]
- Xu, M.S.; Liang, T.; Shi, M.M.; Chen, H.Z. Graphene-Like Two-Dimensional Materials. Chem. Rev. 2013, 113, 3766–3798. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.S.; Cai, T.M.; Liu, S.; Liu, Y.Y.; Chen, H.J.; Li, Z.T.; Du, J.; Lei, Z.Q.; Peng, H.L. N-doped magnetic three-dimensional carbon microspheres@TiO2 with a porous architecture for enhanced degradation of tetracycline and methyl orange via adsorption/photocatalysis synergy. Chem. Eng. J. 2021, 411, 128615. [Google Scholar] [CrossRef]
- Zhou, Q.L.; Li, L.; Zhang, X.Y.; Yang, H.P.; Cheng, Y.J.; Che, H.T.; Wang, L.; Cao, Y.Z. Construction of heterojunction and homojunction to improve the photocatalytic performance of ZnO quantum dots sensitization three-dimensional ordered hollow sphere ZrO2-TiO2 arrays. Int. J. Hydrogen Energy 2020, 45, 31812–31824. [Google Scholar] [CrossRef]
- Ko, Y.H.; Leem, J.W.; Yu, J.S. Controllable synthesis of periodic flower-like ZnO nanostructures on Si subwavelength grating structures. Nanotechnology 2011, 22, 205604. [Google Scholar] [CrossRef]









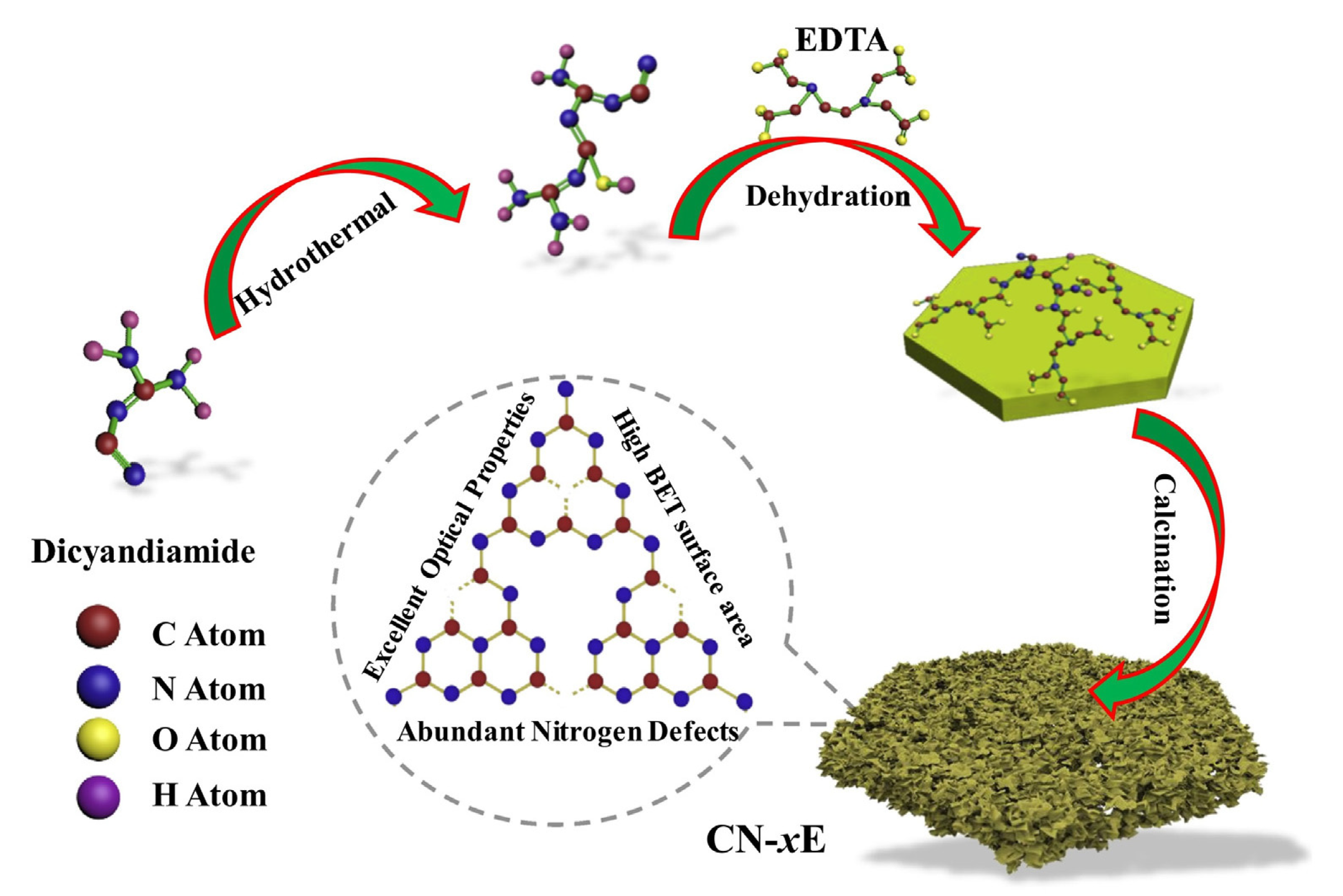
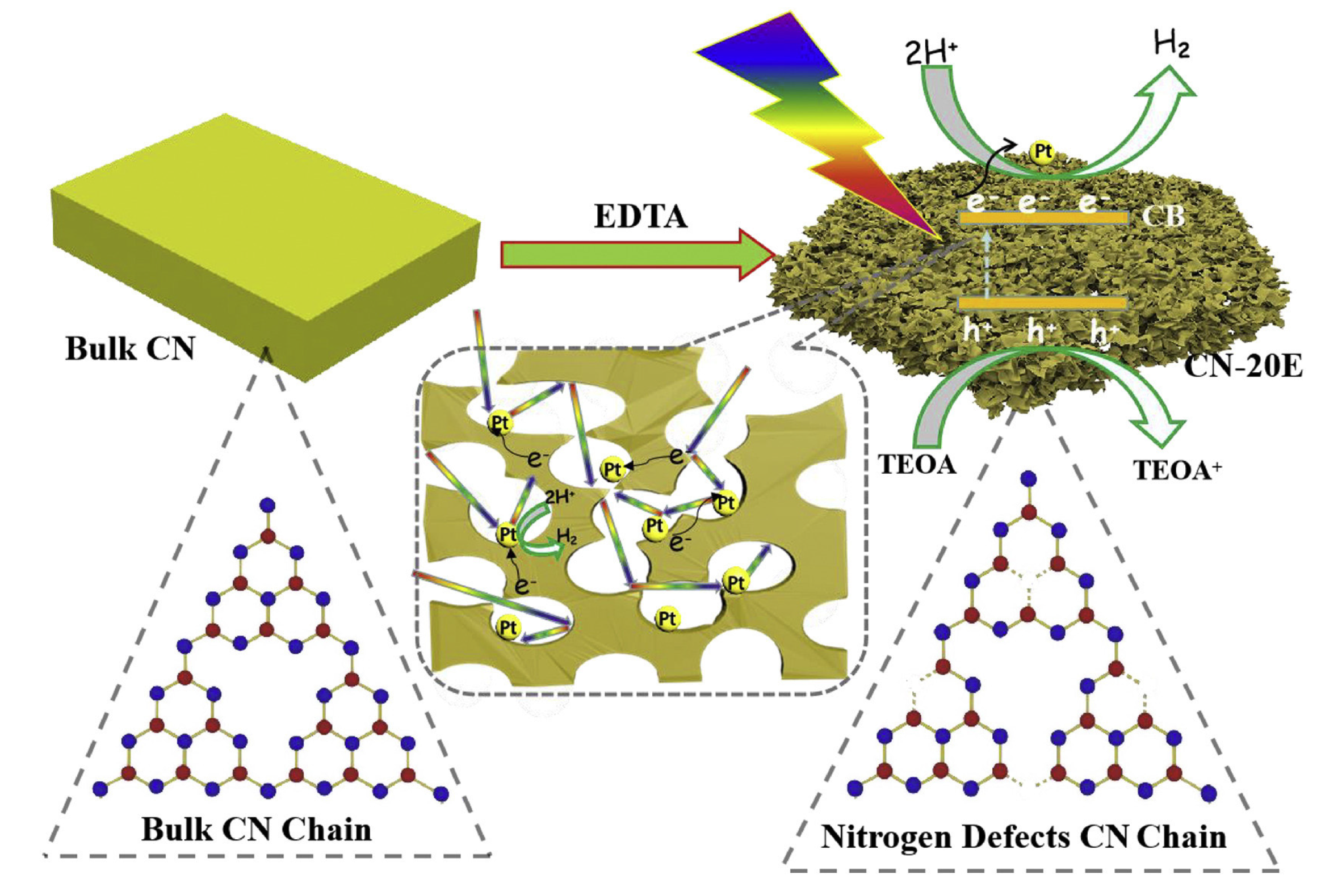


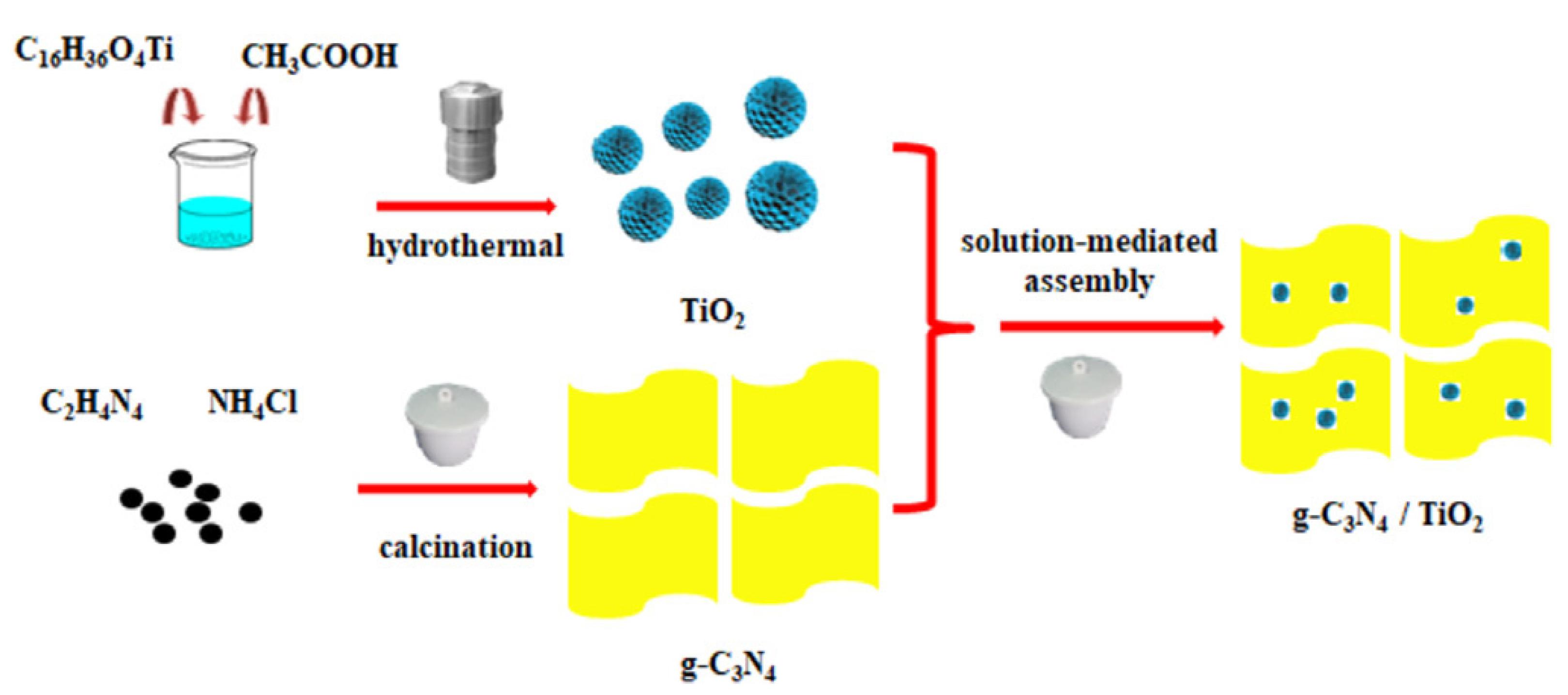


| D | Advantages | Disadvantages | Ref. |
|---|---|---|---|
| 0D | large SSA, many AS, fast CCM from bulk to surface | aggl.; AS inactivation (easily and uncontrollable), difficult recyc., secondary pollutants | [187,188,189] |
| 1D | directivity and conductivity, large SSA, high aspect ratio, fast CCM along the axial direction−low E-HR and high PA | E-T-B uneven in size, poor dispersion | [190,191] |
| 2D | large SSA, high stability, used to stabilize 0D (poor stability/aggl.) by loading 0D on 2D | E-T-B re-stacked due to the hydrophobicity and van der Waals force - reduces SSA | [192,193] |
| 3D | easily recy., high stability, overcome shortcomings of 0D, 1D and 2D aggl., AS of each component, large SSA, high porosity and AS, improved CCM and absorbability | req. surfactants or template (secondary pollutant), a problem of subsequent removal (affecting integrity of the structure) | [194,195,196] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Mogan, T.R.; Wang, K.; Janczarek, M.; Kowalska, E. Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation. Energies 2021, 14, 7223. https://doi.org/10.3390/en14217223
Wei Z, Mogan TR, Wang K, Janczarek M, Kowalska E. Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation. Energies. 2021; 14(21):7223. https://doi.org/10.3390/en14217223
Chicago/Turabian StyleWei, Zhishun, Tharishinny Raja Mogan, Kunlei Wang, Marcin Janczarek, and Ewa Kowalska. 2021. "Morphology-Governed Performance of Multi-Dimensional Photocatalysts for Hydrogen Generation" Energies 14, no. 21: 7223. https://doi.org/10.3390/en14217223