An Experimental Study of the Heat Storage and the Discharge Performance and an Economic Performance Analysis of a Flat Plate Phase Change Material (PCM) Storage Tank
Abstract
:1. Introduction
2. Mathematical Model
2.1. Energy Conservation Equation
2.2. Dimensionless
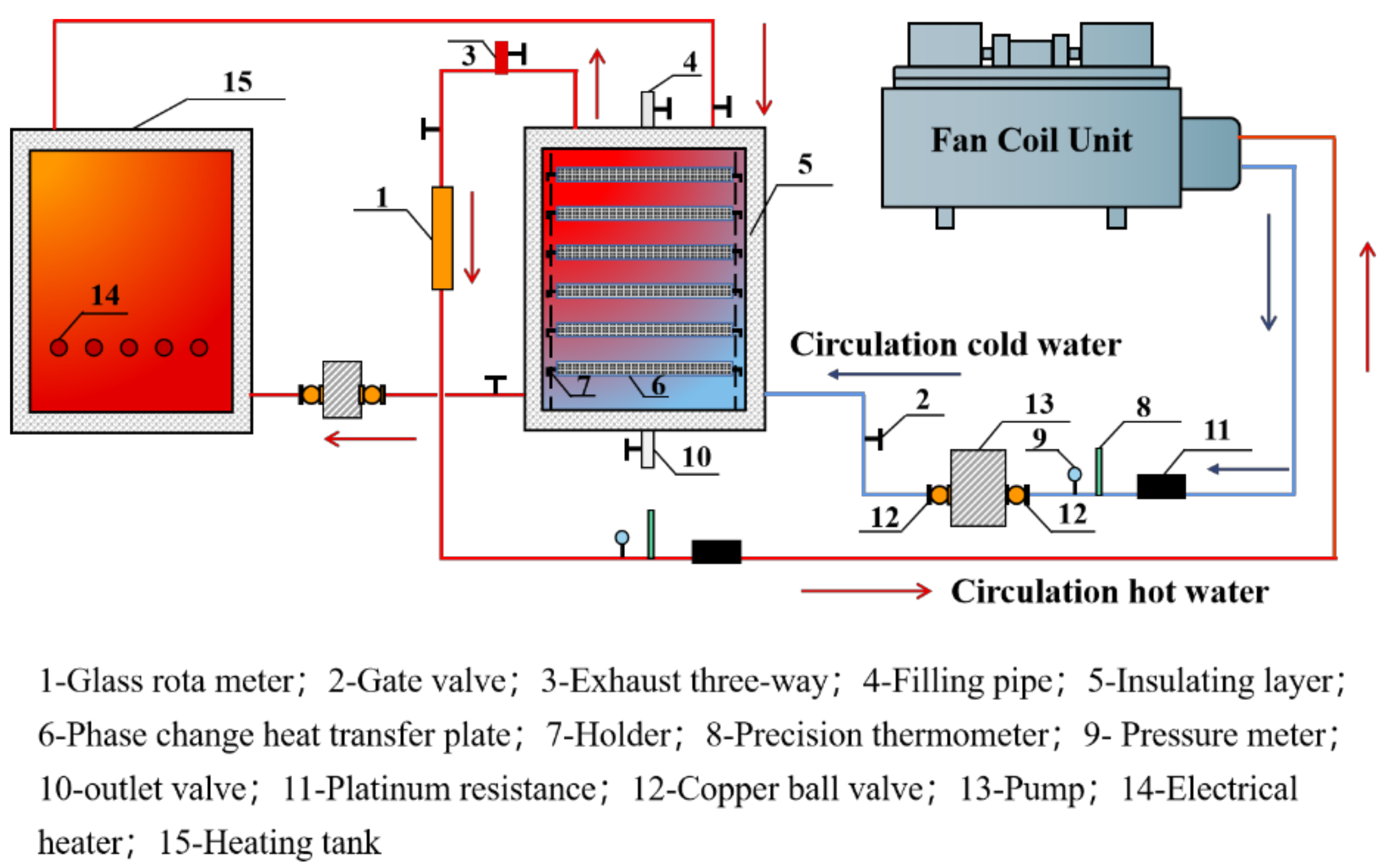
3. Experimental Research
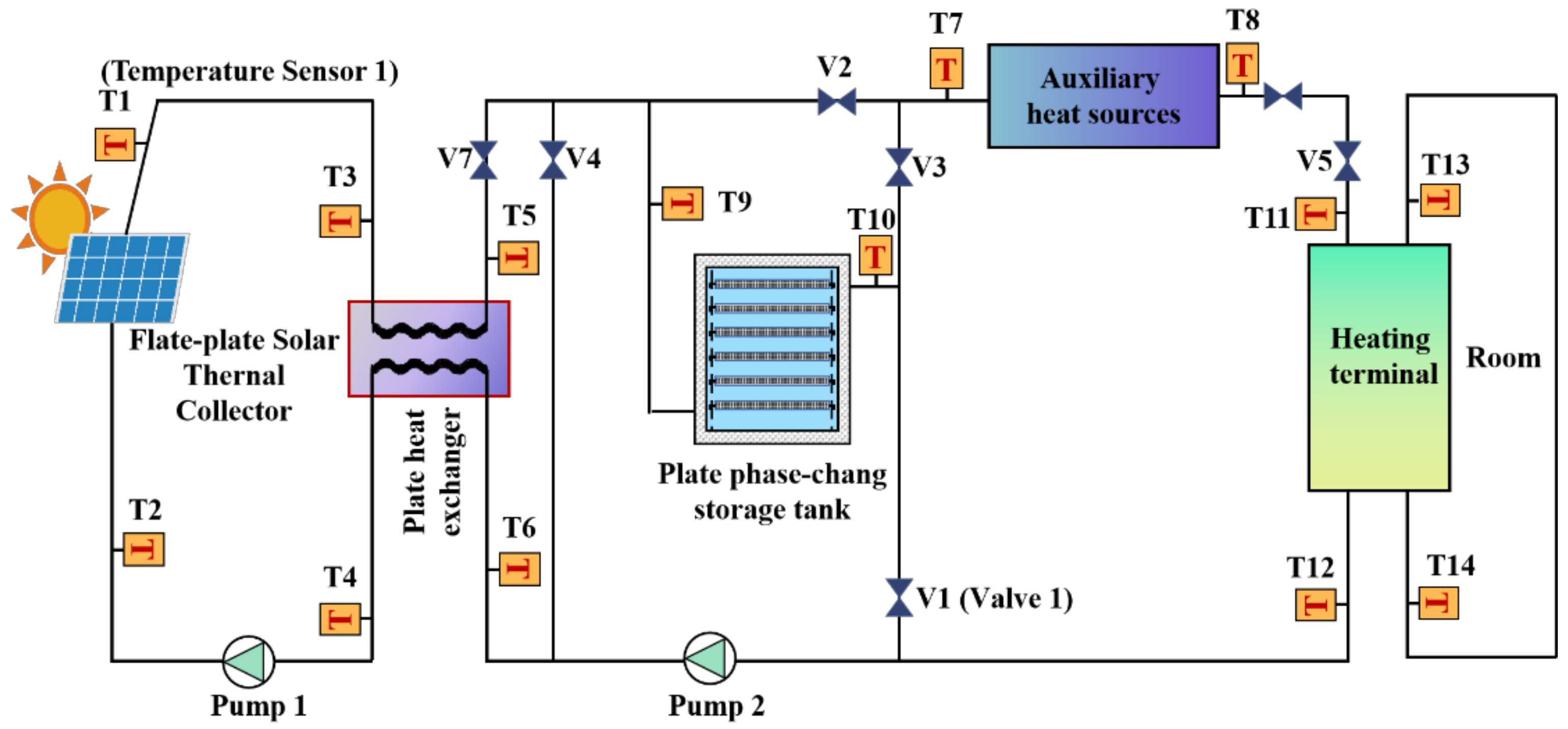
3.1. Experimental Principle
3.2. Test Bench Construction
3.3. Data Processing
3.4. Results and Discussion
- (1)
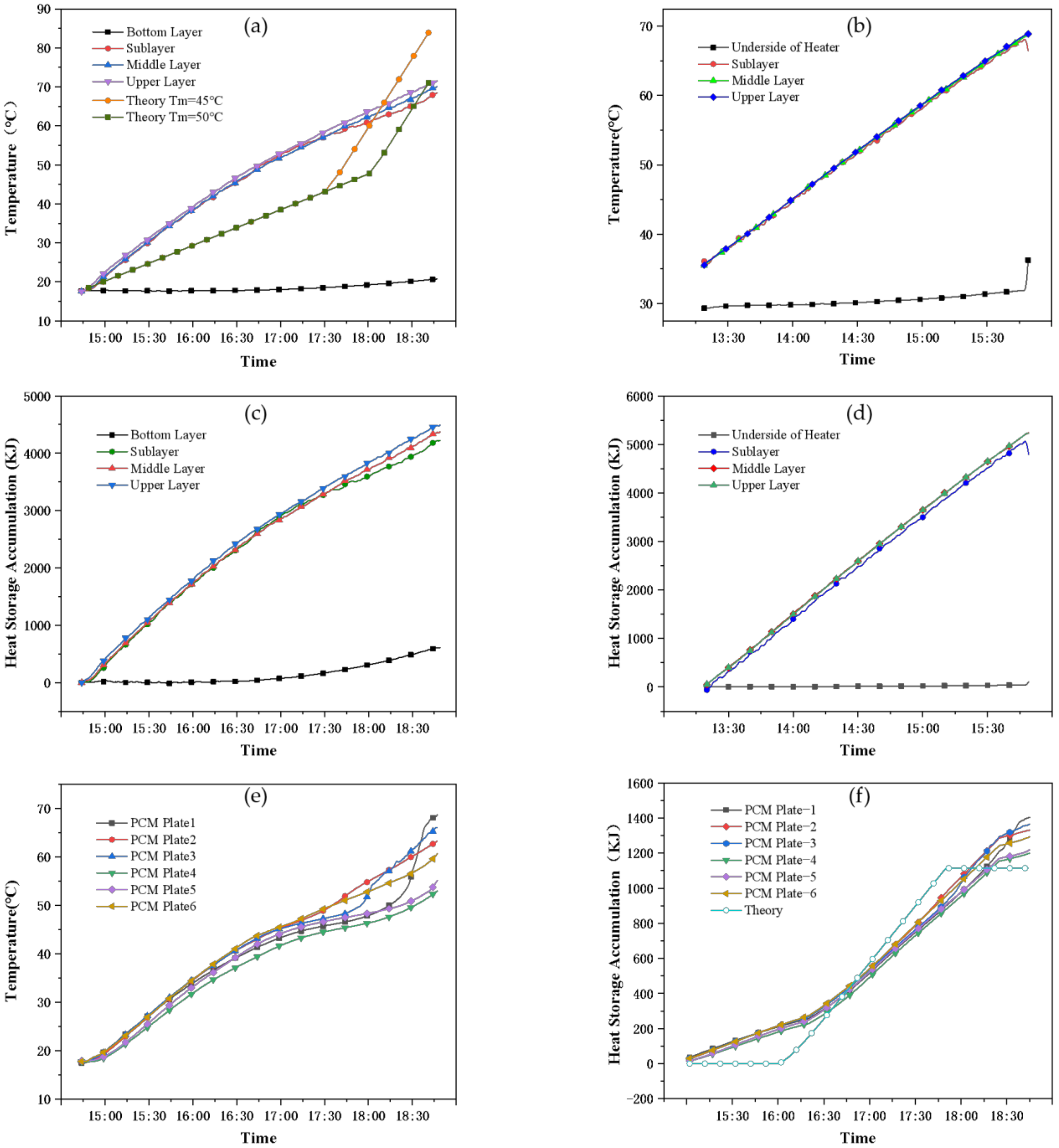
- Working condition 1: only open 1.5 kW heater, static heating process
- (2)
- Working condition 2: only open LOW gear position of FCU, system heating operation process
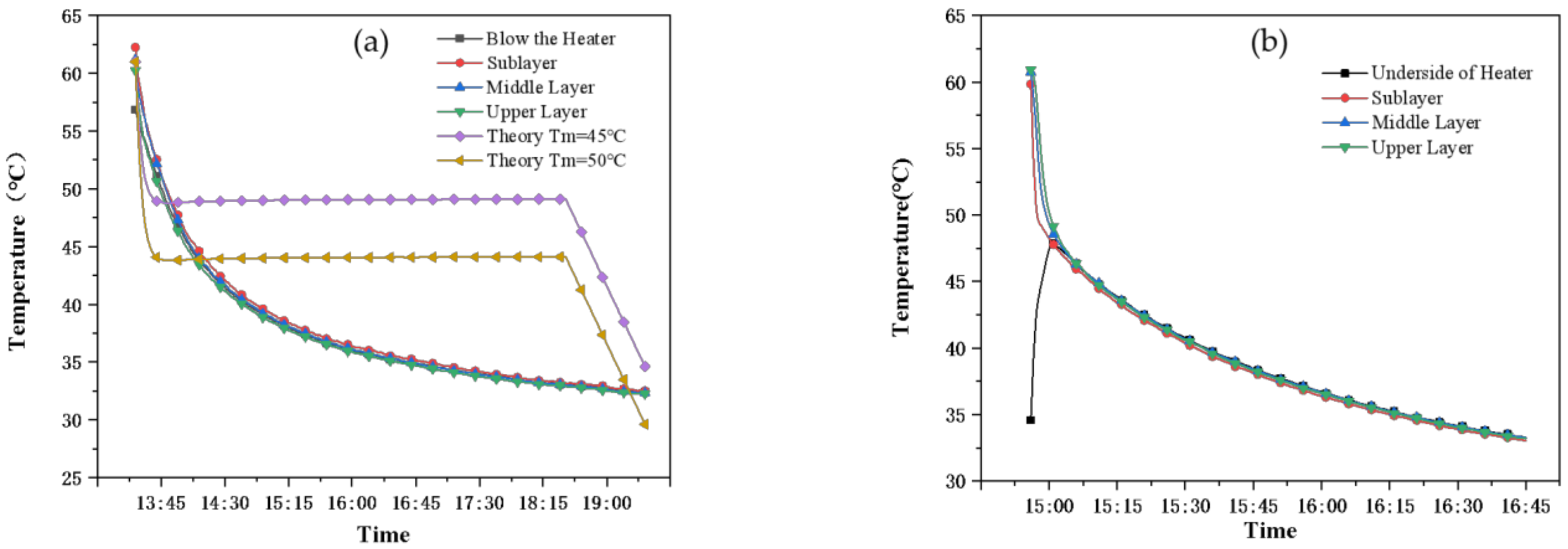
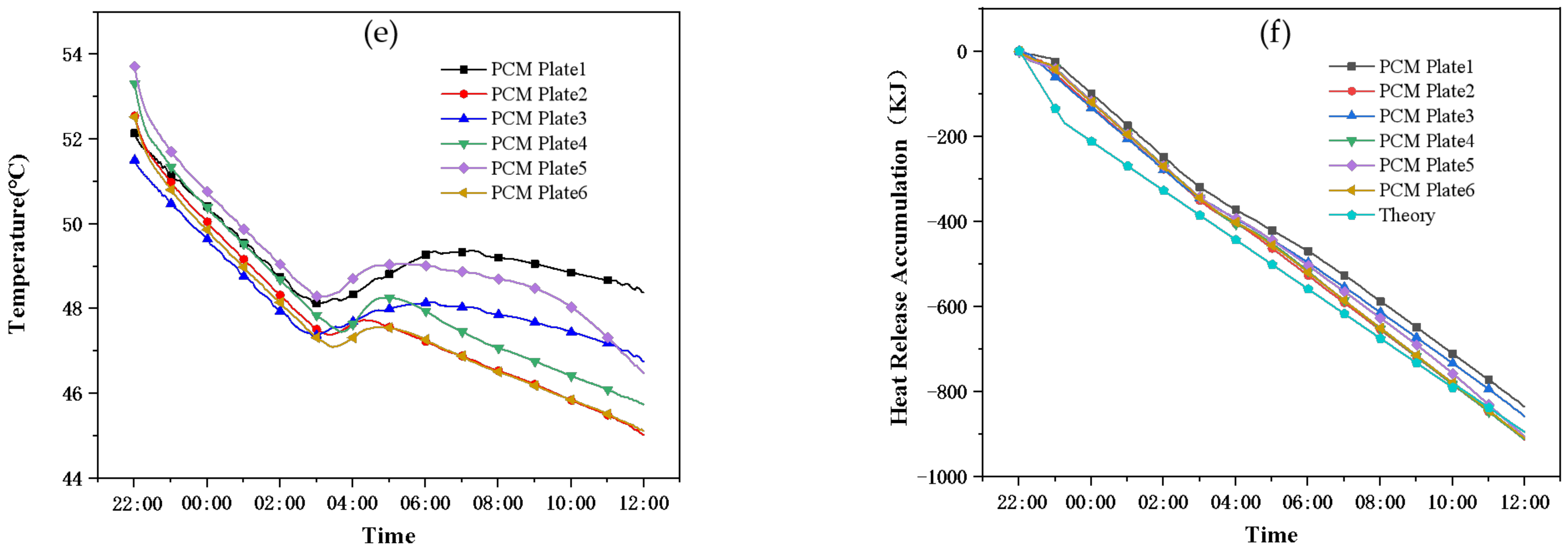
- (3)
- Working condition 3: natural cooling process
4. Energy Saving Comparative Analysis
5. Economic Analysis
5.1. Water Tank Initial Investment Analysis
- ①
- Stainless steel unit quality cost: CNY 17.1/kg.
- ②
- Paraffin wax unit quality cost: CNY 7.6/kg.
5.2. Energy Saving Analysis in Operation of SHS
5.3. Analysis of Payback Period of Energy Saving Reconstruction Investment
6. Conclusions
- (1)
- Experimental condition 1: in the heat storage process, the PCM only occupy less than 20% of the space of the PCM storage tank, but the heat storage can reach 50% of the total heat storage. Under the premise of absorbing equal heat, within two hours, the temperature of the common tank is increased by 35 °C, the temperature of the PCM storage tank is only increased by 25 °C, and the period of the phase change is approximately 1.5 h.
- (2)
- Experimental condition 2: in the heat discharge process, the water temperature of the common tank decreased by 20 °C for 1.5 h, while the PCM storage tank required 5 h for the same temperature drop, and the internal disturbance was strengthened to strengthen the thermal conductivity. The phase change process lasted for approximately 3 h throughout the heat discharge process so that the water temperature was basically maintained at 45–50 °C during this period. The heat release of the PCM was 4800 kJ, accounting for 42.5% of the total heat release of the water tank.
- (3)
- Experimental condition 3: in the natural cooling process, after 12 h of heat discharge at night, the total heat discharge of the PCM storage tank is 8000 kJ, among which the heat discharge of the PCM is 4800 kJ (accounting for 60%), the heat discharge of the common tank is 8186 kJ. The heat discharge of the two water tanks is similar, while the temperature of the common tank dropped by 12 °C and that of the PCM storage tank only dropped by 7 °C, which can effectively reduce the temperature drop and it is conducive to reducing the auxiliary energy opening time of the next day and saving energy.
- (4)
- Through system simulation, it is found that the SHS-PCM saves 34% more energy than the SHS-without PCM, and the volume of the water tank is reduced to 1/5 of common water tank.
- (5)
- Through economic analysis, it is found that compared with the SHS-without PCM, the initial investment cost of the SHS-PCM increases by CNY 9858, the annual operation cost saves CNY 12,100, and the payback period for energy saving renovation projects is 0.81 years, which possesses certain energy saving potential and economic benefits.
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
Nomenclature
| area (m2) | |
| weld size (m) | |
| Biot number | |
| constant pressure specific heat (J·kg−1·K−1) | |
| Fourier number | |
| height (m) | |
| convection heat transfer coefficient (W·m−2·K−1) | |
| heat transfer coefficient (W·m−2·K−1) | |
| phase change plate length (m) | |
| length (m) | |
| mass flow (kg·s−1) | |
| heat transfer unit number | |
| wet perimeter (m) | |
| thermal resistance (m2·K·W−1) | |
| Stanton number | |
| calculation step (s) | |
| phase change plate width (m) | |
| dimensionless length | |
| phase change distance (m) | |
| dimensionless thickness | |
| Greek symbols | |
| Density (kg·m−3) | |
| thermal conductivity (W·m−1·K−1) | |
| dimensionless temperature | |
| dimensionless thermal resistance | |
| specific volume (m3·kg−1) | |
| Subscripts | |
| fluid | |
| input | |
| phase change material | |
| maximum | |
| package plate wall | |
| Abbreviation | |
| AHS | auxiliary heat source |
| COP | coefficient of performance |
| CWT | conventional water tank |
| EAH | electric auxiliary heater |
| FCU | fan coil unit |
| HTF | heat transfer fluid |
| PCM | phase change material |
| SHS | solar heating system |
| SHS-PCM | solar heating system with PCM storage tank |
| SHS-without PCM | solar heating system with common tank |
| SWHS | solar water heating system |
References
- Chen, H.; Ji, J.; Pei, G.; Yang, J.; Zhang, Y. Experimental and numerical comparative investigation on a concentrating photovoltaic system. J. Clean. Prod. 2018, 174, 1288–1298. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Li, J.; Cai, B.; Zhang, F.; Lu, T.; Yang, J.; Jiang, L.; Zhang, Y.; Zhou, J. Experimental research on a solar air-source heat pump system with phase change energy storage. Energy Build. 2020, 228, 110451. [Google Scholar] [CrossRef]
- Teamah, H.; Lightstone, M.; Cotton, J. An alternative approach for assessing the benefit of phase change materials in solar domestic hot water systems. Sol. Energy 2017, 158, 875–888. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Dou, B.; Zhang, G.; Wu, W. Influence of inlet structure on thermal stratification in a heat storage tank with PCMs: CFD and experimental study. Appl. Therm. Eng. 2019, 162, 114151. [Google Scholar] [CrossRef]
- Ghahremannezhad, A.; Xu, H.; Salimpour, M.R.; Wang, P.; Vafai, K. Thermal performance analysis of phase change materials (PCMs) embedded in gradient porous metal foams. Appl. Therm. Eng. 2020, 179, 115731. [Google Scholar] [CrossRef]
- Iasiello, M.; Mameli, M.; Filippeschi, S.; Bianco, N. Simulations of paraffine melting inside metal foams at different gravity levels with preliminary experimental validation. In Proceedings of the 37th UIT Heat Transfer Conference, Journal of Physics: Conference Series, Padova, Italy, 24–26 June 2020; Volume 1599. [Google Scholar] [CrossRef]
- Bianco, N.; Busiello, S.; Iasiello, M.; Mauro, G.M. Finned heat sinks with phase change materials and metal foams: Pareto optimization to address cost and operation time. Appl. Therm. Eng. 2021, 197, 117436. [Google Scholar] [CrossRef]
- Tang, J.; Xie, Y.; Chang, S.; Yan, Z.; Wu, H.; Zhang, H. Performance analysis of acceleration effect on paraffin melting in finned copper foam. Appl. Therm. Eng. 2021, 202, 117826. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Ding, P.; Long, E. Experimental and simulated optimization study on dynamic heat discharge performance of multi-units water tank with PCM. Indoor Built Environ. 2020, 30, 1531–1545. [Google Scholar] [CrossRef]
- Hu, M.-H.; Xu, T.; Chiu, J.N. Experimental analysis of submerged coil and encapsulated slab latent heat storage. Appl. Therm. Eng. 2022, 209, 118259. [Google Scholar] [CrossRef]
- Xie, L.; Lv, Y.; Lu, J.; Li, Y.; Liu, S.; Zou, Q.; Wang, X. Heating storage performance of a water tank–combined phase change material:An experimental case study. Adv. Mech. Eng. 2017, 9, 1687814017724071. [Google Scholar] [CrossRef] [Green Version]
- Koželj, R.; Mlakar, U.; Zavrl, E.; Stritih, U.; Stropnik, R. An experimental and numerical analysis of an improved thermal storage tank with encapsulated PCM for use in retrofitted buildings for heating. Energy Build. 2021, 248, 111196. [Google Scholar] [CrossRef]
- Besagni, G.; Croci, L. Experimental study of a pilot-scale fin-and-tube phase change material storage. Appl. Therm. Eng. 2019, 160, 114089. [Google Scholar] [CrossRef]
- Pop, O.G.; Balan, M.C. A numerical analysis on the performance of DHW storage tanks with immersed PCM cylinders. Appl. Therm. Eng. 2021, 197, 117386. [Google Scholar] [CrossRef]
- Zhu, C.; Li, B.; Yan, S.; Luo, Q.; Li, C. Experimental research on solar phase change heat storage evaporative heat pump system. Energy Convers. Manag. 2021, 229, 113683. [Google Scholar] [CrossRef]
- Ding, Z.; Wu, W.; Chen, Y.; Li, Y. Dynamic simulation and parametric study of solar water heating system with phase change materials in different climate zones. Sol. Energy 2020, 205, 399–408. [Google Scholar] [CrossRef]
- Zhao, J.; Ji, Y.; Yuan, Y.; Zhang, Z.; Lu, J. Seven Operation Modes and Simulation Models of Solar Heating System with PCM Storage Tank. Energies 2017, 10, 2128. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Ji, Y.; Yuan, Y.; Zhang, Z.; Lu, J. Energy-Saving Analysis of Solar Heating System with PCM Storage Tank. Energies 2018, 11, 237. [Google Scholar] [CrossRef] [Green Version]
- GB 50495-2019; Technical Standard for Solar Heating System. Ministry of Housing and Urban-Rural Development of the People’s Republic of China, China Architecture & Building Press: Beijing, China, 2019.
- Network, G.I. Latest Price Inquiry of Paraffin. Available online: https://www.cngold.org/shila/c7889490.html (accessed on 10 December 2021).
- Shaanxi Iron and Steel Co. Ltd. (China). Steel Benchmark Price Index. Available online: https://www.shaangang.com/info/2015061510/201507291415245127/202112210327219393.shtml (accessed on 21 December 2021).
- People’s Government of Tibet Autonomous Region. Notification of the People’s Government of Tibet Autonomous Region on Adjusting the Sale Electricity Price of the Region. Available online: http://www.xizang.gov.cn/zwgk/xxgk_424/zxxxgk/201902/t20190223_63441.html (accessed on 1 July 2014).







| PCM | Latent Heat | Liquid Specific Heat Capacity | Solid Specific Heat Capacity | Density | Thermal Conductivity |
|---|---|---|---|---|---|
| kJ | kJ/(kg·°C) | kJ/(kg·°C) | kg/m3 | W/(m·K) | |
| Paraffin | 200 | 2.9 | 3.2 | 0.87 × 103 | 0.4 |
| Working Condition | Process Description | Heater Power | FCU Gear Position | Pump Switching State |
|---|---|---|---|---|
| 1 | Heat storage process | 1.5 kW | Stop | Off |
| 2 | Heat discharge process | 0 kW | Low | On |
| 3 | Natural cooling process | 0 kW | Stop | Off |
| Unit | Common Tank | PCM Storage Tank | |
|---|---|---|---|
| Heat storage capacity | GJ | 0.63 | 0.63 |
| Volume of tank | m3 | 3 × 2.5 × 4 | 3 × 2.5 × 0.8 |
| Volume of PCM plate | m3 | — | 3 × 2.5 × 0.08 |
| Number of PCM plate | — | 6 | |
| PCM | — | paraffin wax | |
| Phase change temperature | °C | — | 47 |
| Terminal form | FCU | FCU | |
| Supply and return heating temperature | °C | 45/40 | 45/40 |
| PCM Storage Tank | |||
|---|---|---|---|
| Material | Quantity | Unit Price/CNY | Total Price/CNY |
| Insulation Water Tank | 1 | 10,600 | 10,600 |
| Package Plate | 6 | 1076 | 6456 |
| Paraffin Plate | 6 | 3,967 | 23,802 |
| Total | 40,858 | ||
| Common Tank | |||
| Insulation Water Tank | 1 | 31,000 | 31,000 |
| Total | 31,000 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Gao, J.; Liao, J.; Zhou, B.; Bai, Y.; Qiang, T. An Experimental Study of the Heat Storage and the Discharge Performance and an Economic Performance Analysis of a Flat Plate Phase Change Material (PCM) Storage Tank. Energies 2022, 15, 4023. https://doi.org/10.3390/en15114023
Zhao J, Gao J, Liao J, Zhou B, Bai Y, Qiang T. An Experimental Study of the Heat Storage and the Discharge Performance and an Economic Performance Analysis of a Flat Plate Phase Change Material (PCM) Storage Tank. Energies. 2022; 15(11):4023. https://doi.org/10.3390/en15114023
Chicago/Turabian StyleZhao, Juan, Junmei Gao, Junhui Liao, Botao Zhou, Yifei Bai, and Tianwei Qiang. 2022. "An Experimental Study of the Heat Storage and the Discharge Performance and an Economic Performance Analysis of a Flat Plate Phase Change Material (PCM) Storage Tank" Energies 15, no. 11: 4023. https://doi.org/10.3390/en15114023





