Extraction and Performance Analysis of Hydrocarbons from Waste Plastic Using the Pyrolysis Process
Abstract
:1. Introduction
2. Literature
3. Materials, Process, and Characterisation
3.1. Waste Plastic
3.2. Pyrolysis Process
3.3. Characterisation of Plastic Oil
4. Results and Discussion
4.1. Flash and Fire Point
4.2. pH Value
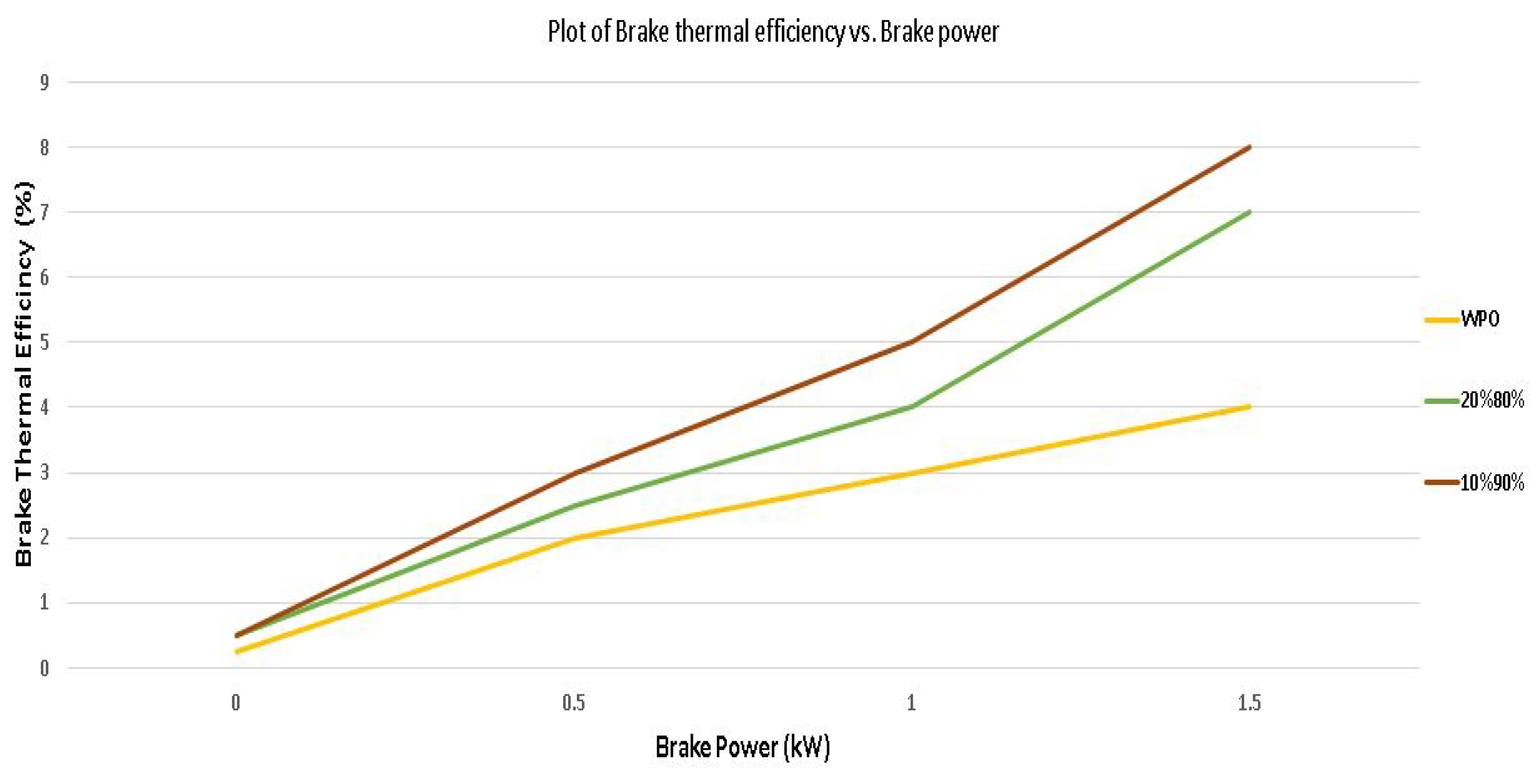
4.3. Engine Performance
4.4. Emission Analysis
4.5. Unburnt Hydro Carbons
5. Conclusions
- Pyrolysis, which has been discovered to be the simplest, effective (in terms of cost), and efficient process for turning waste plastic into fuels solves environmental and energy problems. The majority of plastic garbage’s energy may be transformed into liquid, gas, or charcoal.
- The technical and economic impacts of utilising this oil in a diesel engine are compared, and it is discovered that this oil can replace diesel oil. This procedure yields a liquid with a substantially greater volume and a narrow boiling range. This method yields cleaner fuel than conventional fuels.
- In terms of fuel lubricity and viscosity, a biodiesel concentration of 10% (v/v) was found to be the most effective for improving oil from recycled plastic.
- Adding biodiesel to used plastic oil slightly increased the engine’s thermal braking efficiency.
- Adding biodiesel decreased the amount of hydrocarbon- and oxide-containing nitrogen emissions, whereas carbon monoxide and smoke emissions increased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pérez-Lombard, L.; Ortiz, J.; Pout, C. A review on buildings energy consumption information. Energy Build. 2008, 40, 394–398. [Google Scholar] [CrossRef]
- Malla, S. An outlook of end-use energy demand based on a clean energy and technology transformation of the household sector in Nepal. Energy 2022, 238, 121810. [Google Scholar] [CrossRef]
- Zakeri, B.; Paulavets, K.; Barreto-Gomez, L.; Echeverri, L.G.; Pachauri, S.; Boza-Kiss, B.; Zimm, C.; Rogelj, J.; Creutzig, F.; Ürge-Vorsatz, D.; et al. Pandemic, War, and Global Energy Transitions. Energies 2022, 15, 6114. [Google Scholar] [CrossRef]
- Hafeez, M.; Rehman, S.U.; Faisal, C.N.; Yang, J.; Ullah, S.; Kaium, M.A.; Malik, M.Y. Financial efficiency and its impact on renewable energy demand and CO2 emissions: Do eco-innovations matter for highly polluted Asian economies? Sustainability 2022, 14, 10950. [Google Scholar] [CrossRef]
- Hossain, M.; Fang, Y.R.; Ma, T.; Huang, C.; Peng, W.; Urpelainen, J.; Hebbale, C.; Dai, H. Narrowing fossil fuel consumption in the Indian road transport sector towards reaching carbon neutrality. Energy Policy 2023, 172, 113330. [Google Scholar] [CrossRef]
- Borg, K.; Lennox, A.; Kaufman, S.; Tull, F.; Prime, R.; Rogers, L.; Dunstan, E. Curbing plastic consumption: A review of single-use plastic behaviour change interventions. J. Clean. Prod. 2022, 344, 131077. [Google Scholar] [CrossRef]
- Kuan, Z.J.; Chan, B.K.N.; Gan, S.K.E. Worming the circular economy for biowaste and plastics: Hermetia illucens, Tenebrio molitor, and Zophobas morio. Sustainability 2022, 14, 1594. [Google Scholar] [CrossRef]
- Mashaan, N. Engineering Characterisation of Wearing Course Materials Modified with Waste Plastic. Recycling 2022, 7, 61. [Google Scholar] [CrossRef]
- Ahmad, J.; Majdi, A.; Babeker Elhag, A.; Deifalla, A.F.; Soomro, M.; Isleem, H.F.; Qaidi, S. A step towards sustainable concrete with substitution of plastic waste in concrete: Overview on mechanical, durability and microstructure analysis. Crystals 2022, 12, 944. [Google Scholar] [CrossRef]
- Johnston, B.; Adamus, G.; Ekere, A.I.; Kowalczuk, M.; Tchuenbou-Magaia, F.; Radecka, I. Bioconversion of plastic waste based on mass full carbon backbone polymeric materials to value-added polyhydroxyalkanoates (PHAs). Bioengineering 2022, 9, 432. [Google Scholar] [CrossRef]
- Hossain, R.; Islam, M.T.; Shanker, R.; Khan, D.; Locock, K.E.S.; Ghose, A.; Schandl, H.; Dhodapkar, R.; Sahajwalla, V. Plastic waste management in India: Challenges, opportunities, and roadmap for circular economy. Sustainability 2022, 14, 4425. [Google Scholar] [CrossRef]
- Lai, W.L.; Sharma, S.; Roy, S.; Maji, P.K.; Sharma, B.; Ramakrishna, S.; Goh, K.L. Roadmap to sustainable plastic waste management: A focused study on recycling PET for triboelectric nanogenerator production in Singapore and India. Environ. Sci. Pollut. Res. 2022, 29, 51234–51268. [Google Scholar] [CrossRef] [PubMed]
- Sieber, R.; Kawecki, D.; Nowack, B. Dynamic probabilistic material flow analysis of rubber release from tires into the environment. Environ. Pollut. 2020, 258, 113573. [Google Scholar] [CrossRef] [PubMed]
- Ferronato, N.; Torretta, V. Waste mismanagement in developing countries: A review of global issues. Int. J. Environ. Res. Public Health 2019, 16, 1060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arabiourrutia, M.; Lopez, G.; Artetxe, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Waste tyre valorization by catalytic pyrolysis—A review. Renew. Sustain. Energy Rev. 2020, 129, 109932. [Google Scholar] [CrossRef]
- Zhong, Y.; Xu, J.; Pan, Y.; Yin, Z.; Wang, X.; Zhou, Y.; Huang, Q. Combustion characteristics of aromatic-enriched oil droplets produced by pyrolyzing unrecyclable waste tire rubber. Fuel Process. Technol. 2022, 226, 107093. [Google Scholar] [CrossRef]
- Cheng, K.; Li, J.Y.; Wang, Y.; Ji, W.W.; Cao, Y. Characterization and Risk Assessment of Airborne Polycyclic Aromatic Hydrocarbons From Open Burning of Municipal Solid Waste. Front. Environ. Sci. 2022, 10, 382. [Google Scholar] [CrossRef]
- Mohite, A.S.; Rajpurkar, Y.D.; More, A.P. Bridging the gap between rubbers and plastics: A review on thermoplastic polyolefin elastomers. Polym. Bull. 2022, 79, 1309–1343. [Google Scholar] [CrossRef]
- Banerjee, R.; Ray, S.S. Sustainability and Life Cycle Assessment of Thermoplastic Polymers for Packaging: A Review on Fundamental Principles and Applications. Macromol. Mater. Eng. 2022, 307, 2100794. [Google Scholar] [CrossRef]
- Venkatesan, R.; Santhamoorthy, M.; Alagumalai, K.; Haldhar, R.; Raorane, C.J.; Raj, V.; Kim, S.C. Novel Approach in Biodegradation of Synthetic Thermoplastic Polymers: An Overview. Polymers 2022, 14, 4271. [Google Scholar] [CrossRef]
- Ghai, H.; Sakhuja, D.; Yadav, S.; Solanki, P.; Putatunda, C.; Bhatia, R.K.; Bhatt, A.K.; Varjani, S.; Yang, Y.H.; Bhatia, S.K.; et al. An Overview on Co-Pyrolysis of Biodegradable and Non-Biodegradable Wastes. Energies 2022, 15, 4168. [Google Scholar] [CrossRef]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Andreeßen, C.; Steinbüchel, A. Recent developments in non-biodegradable biopolymers: Precursors, production processes, and future perspectives. Appl. Microbiol. Biotechnol. 2019, 103, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current technologies for plastic waste treatment: A review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Vollmer, I.; Jenks, M.J.; Roelands, M.C.; White, R.J.; van Harmelen, T.; de Wild, P.; van Der Laan, G.P.; Meirer, F.; Keurentjes, J.T.; Weckhuysen, B.M. Beyond mechanical recycling: Giving new life to plastic waste. Angew. Chem. Int. Ed. 2020, 59, 15402–15423. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; Hossain, A.K.; Griffiths, G.; Duraisamy, G.; Krishnasamy, A.; Ravikrishnan, V.; Sodré, J.R. Plastic waste to liquid fuel: A review of technologies, applications, and challenges. Sustain. Energy Technol. Assess. 2022, 53, 102651. [Google Scholar] [CrossRef]
- Kohli, K.; Chandrasekaran, S.R.; Prajapati, R.; Kunwar, B.; Al-Salem, S.; Moser, B.R.; Sharma, B.K. Pyrolytic Depolymerization Mechanisms for Post-Consumer Plastic Wastes. Energies 2022, 15, 8821. [Google Scholar] [CrossRef]
- Majid, M. Renewable energy for sustainable development in India: Current status, future prospects, challenges, employment, and investment opportunities. Energy Sustain. Soc. 2020, 10, 2. [Google Scholar]
- Policy, Energy; Planning Office, Ministry of Energy Thailand. Energy Statistics of Thailand. 2022. Available online: http://www.eppo.go.th/index.php/th/ (accessed on 2 December 2022).
- Raga, S.; Ayele, Y.; te Velde, D.W. Public Debt Profile of Selected African Countries. 2022. Available online: https://cdn.odi.org/media/documents/Public_debt_profile_of_selected_African_countries_PDF.pdf (accessed on 20 October 2022).
- Gabbar, H.A.; Aboughaly, M.; Stoute, C.B. DC thermal plasma design and utilization for the low density polyethylene to diesel oil pyrolysis reaction. Energies 2017, 10, 784. [Google Scholar] [CrossRef]
- Takkalkar, P.; Jatoi, A.S.; Jadhav, A.; Jadhav, H.; Nizamuddin, S. Thermo-mechanical, rheological, and chemical properties of recycled plastics. Plast. Waste Sustain. Asph. Roads 2022, 29–42. [Google Scholar] [CrossRef]
- Sipra, A.T.; Gao, N.; Sarwar, H. Municipal solid waste (MSW) pyrolysis for bio-fuel production: A review of effects of MSW components and catalysts. Fuel Process. Technol. 2018, 175, 131–147. [Google Scholar] [CrossRef]
- Damodharan, D.; Sathiyagnanam, A.; Rana, D.; Kumar, B.R.; Saravanan, S. Extraction and characterization of waste plastic oil (WPO) with the effect of n-butanol addition on the performance and emissions of a DI diesel engine fueled with WPO/diesel blends. Energy Convers. Manag. 2017, 131, 117–126. [Google Scholar] [CrossRef]
- Areeprasert, C.; Asingsamanunt, J.; Srisawat, S.; Kaharn, J.; Inseemeesak, B.; Phasee, P.; Khaobang, C.; Siwakosit, W.; Chiemchaisri, C. Municipal plastic waste composition study at transfer station of Bangkok and possibility of its energy recovery by pyrolysis. Energy Procedia 2017, 107, 222–226. [Google Scholar] [CrossRef]
- Baskaran, R.; Kumar, P.S. Evaluation on performance of CI engine with waste plastic oil-diesel blends as alternative fuel. Int. J. Res. Appl. Sci. Eng. Technol. 2015, 3, 642–646. [Google Scholar]
- Syamsiro, M.; Saptoadi, H.; Kismurtono, M.; Mufrodi, Z.; Yoshikawa, K. Utilization of waste polyethylene pyrolysis oil as partial substitute for diesel fuel in a DI diesel engine. Int. J. Smart Grid Clean Energy 2019, 8, 38–47. [Google Scholar] [CrossRef]
- Sachuthananthan, B.; Reddy, D.; Mahesh, C.; Dineshwar, B. Production of diesel like fuel from municipal solid waste plastics for using in CI engine to study the combustion, performance and emission characteristics. Int. J. Pure Appl. Math. 2018, 119, 85–96. [Google Scholar]
- Kalargaris, I.; Tian, G.; Gu, S. Combustion, performance and emission analysis of a DI diesel engine using plastic pyrolysis oil. Fuel Process. Technol. 2017, 157, 108–115. [Google Scholar] [CrossRef] [Green Version]
- Mani, M.; Nagarajan, G.; Sampath, S. Characterisation and effect of using waste plastic oil and diesel fuel blends in compression ignition engine. Energy 2011, 36, 212–219. [Google Scholar] [CrossRef]
- Ramesha, D.; Kumara, G.P.; Mohammed, A.V.; Mohammad, H.A.; Kasma, M.A. An experimental study on usage of plastic oil and B20 algae biodiesel blend as substitute fuel to diesel engine. Environ. Sci. Pollut. Res. 2016, 23, 9432–9439. [Google Scholar] [CrossRef]
- Senthilkumar, P.; Sankaranarayanan, G. Effect of Jatropha methyl ester on waste plastic oil fueled DI diesel engine. J. Energy Inst. 2016, 89, 504–512. [Google Scholar] [CrossRef]
- Ahamed, M.; Dash, S.; Kumar, A.; Lingfa, P. A critical review on the production of biodiesel from Jatropha, Karanja and Castor feedstocks. Bioresour. Util. Bioprocess 2020, 107–115. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Ashraf, M.U.; Amjad, S.; Jamil, M.A.; Jamil, M.M.; Mujtaba, M. Jatropha curcas biodiesel: A lucrative recipe for Pakistan’s energy sector. Processes 2021, 9, 1129. [Google Scholar] [CrossRef]
- Zahan, K.A.; Kano, M. Biodiesel production from palm oil, its by-products, and mill effluent: A review. Energies 2018, 11, 2132. [Google Scholar] [CrossRef]
- Kaniapan, S.; Hassan, S.; Ya, H.; Patma Nesan, K.; Azeem, M. The utilisation of palm oil and oil palm residues and the related challenges as a sustainable alternative in biofuel, bioenergy, and transportation sector: A review. Sustainability 2021, 13, 3110. [Google Scholar] [CrossRef]
- Keera, S.; El Sabagh, S.; Taman, A. Castor oil biodiesel production and optimization. Egypt. J. Pet. 2018, 27, 979–984. [Google Scholar] [CrossRef]
- Chidambaranathan, B.; Gopinath, S.; Aravindraj, R.; Devaraj, A.; Krishnan, S.G.; Jeevaananthan, J. The production of biodiesel from castor oil as a potential feedstock and its usage in compression ignition Engine: A comprehensive review. Mater. Today Proc. 2020, 33, 84–92. [Google Scholar] [CrossRef]
- Kaewbuddee, C.; Sukjit, E.; Srisertpol, J.; Maithomklang, S.; Wathakit, K.; Klinkaew, N.; Liplap, P.; Arjharn, W. Evaluation of waste plastic oil–biodiesel blends as alternative fuels for diesel engines. Energies 2020, 13, 2823. [Google Scholar] [CrossRef]
- Machiraju, A.; Harinath, V.; Charan, A.K. Extraction of liquid hydrocarbon fuel from waste plastic. Int. J. Creat. Res. Thoughts 2018, 3, 202–207. [Google Scholar]
- Brindhadevi, K.; Hiep, B.T.; Khouj, M.; Garalleh, H.A. A study on biofuel produced from cracking of low density poly ethylenes using TiO2/AlSBA-15 nanocatalysts. Fuel 2022, 323, 124299. [Google Scholar] [CrossRef]
- Kumar, S.; Panda, A.K.; Singh, R.K. A review on tertiary recycling of high-density polyethylene to fuel. Resour. Conserv. Recycl. 2011, 55, 893–910. [Google Scholar] [CrossRef]
- Joseph, J.J.; Josh, F. Production of Bio-Fuel From Plastic Waste. In Proceedings of the Journal of Physics: Conference Series, Eluru, India, 20–22 June 2019; IOP Publishing: Bristol, UK, 2019; Volume 1362, p. 012103. [Google Scholar]
- Yusop, A.F.; Mamat, R.; Yusaf, T.; Najafi, G.; Yasin, M.H.M.; Khathri, A.M. Analysis of particulate matter (pm) emissions in diesel engines using palm oil biodiesel blended with diesel fuel. Energies 2018, 11, 1039. [Google Scholar] [CrossRef]
- Sukjit, E.; Herreros, J.M.; Dearn, K.; Tsolakis, A. Improving ethanol-diesel blend through the use of hydroxylated biodiesel. In Proceedings of the SAE 2014 International Powertrain, Fuels & Lubricants Meeting, Birmingham, UK, 20–24 October 2014. [Google Scholar]
- Pumpuang, A.; Maithomklang, S.; Sukjit, E.; Dejvajara, D.; Samaiklang, P.; Sanluecha, S.; Tongroon, M. Utilization of Castor Oil-Based Ethyl Ester Biodiesel in a Diesel Engine. In Proceedings of the Small Engine Technology Conference & Exposition, Hiroshima, Japan, 19–21 November 2019. [Google Scholar]





| Sr. No. | Parameter | WPO | Diesel |
|---|---|---|---|
| 1 | Flash point (C) | 55 | 45 |
| 2 | Fire point (C) | 61 | 56 |
| 3 | Kinematic viscosity at 40 C (mm/s) | 1.98 | 4.139 |
| 4 | Density at 40 C (kg/m) | 812 | 875 |
| 5 | Calorific value (MJ/kg) | 40.81 | 45.39 |
| 6 | Cetane Number | 48 | 54 |
| 7 | Carbon (wt%) | 81.7 | 84.7 |
| 8 | Hydrogen (wt%) | 10.7 | 13.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramesh, B.T.; Sayyad, J.; Bongale, A.; Bongale, A. Extraction and Performance Analysis of Hydrocarbons from Waste Plastic Using the Pyrolysis Process. Energies 2022, 15, 9381. https://doi.org/10.3390/en15249381
Ramesh BT, Sayyad J, Bongale A, Bongale A. Extraction and Performance Analysis of Hydrocarbons from Waste Plastic Using the Pyrolysis Process. Energies. 2022; 15(24):9381. https://doi.org/10.3390/en15249381
Chicago/Turabian StyleRamesh, B. T., Javed Sayyad, Arunkumar Bongale, and Anupkumar Bongale. 2022. "Extraction and Performance Analysis of Hydrocarbons from Waste Plastic Using the Pyrolysis Process" Energies 15, no. 24: 9381. https://doi.org/10.3390/en15249381







