Effect of Cooking Conditions on Selected Properties of Biodiesel Produced from Palm-Based Waste Cooking Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. WCO Conditioning
2.3. WCO Characterization
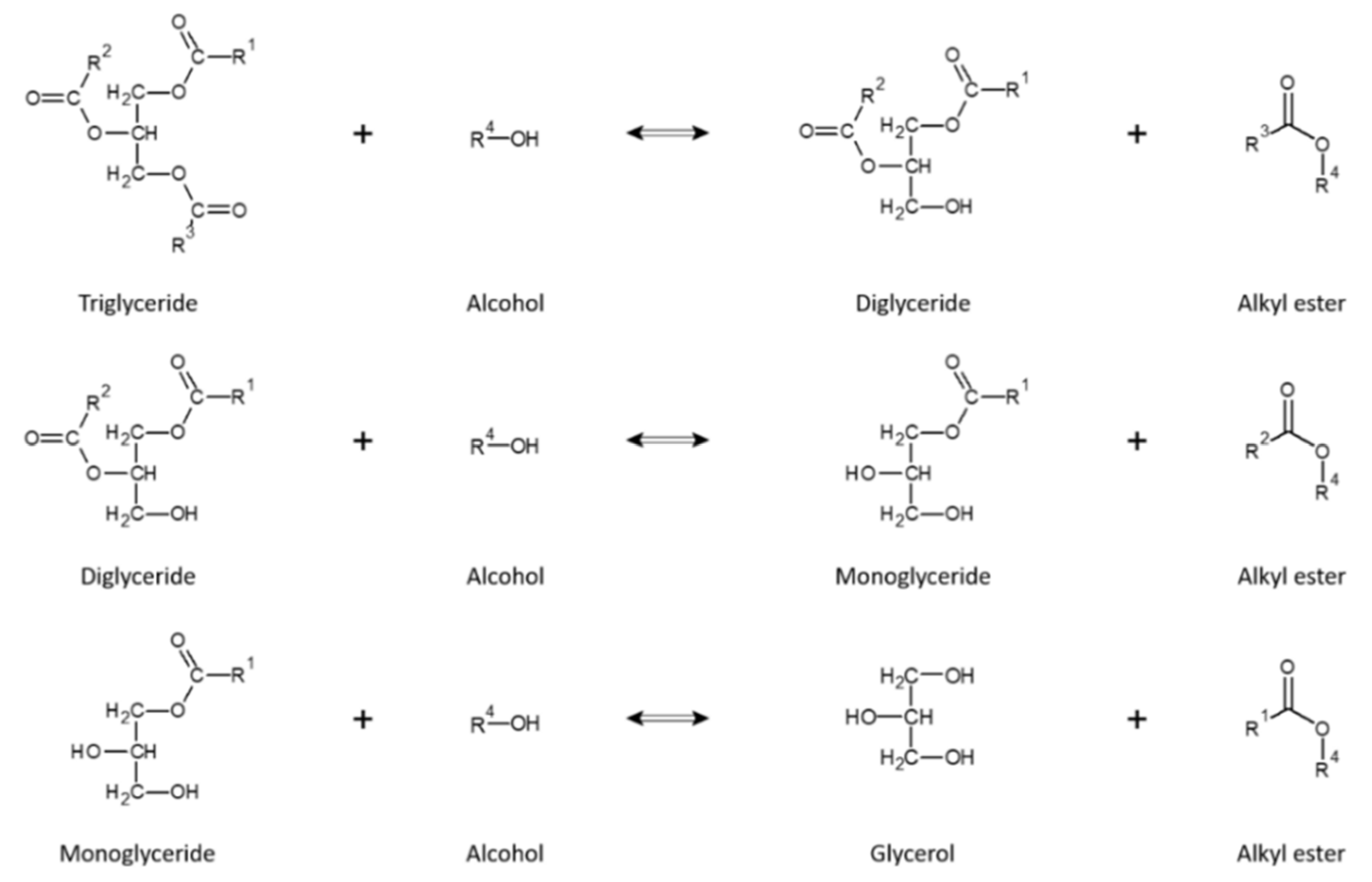
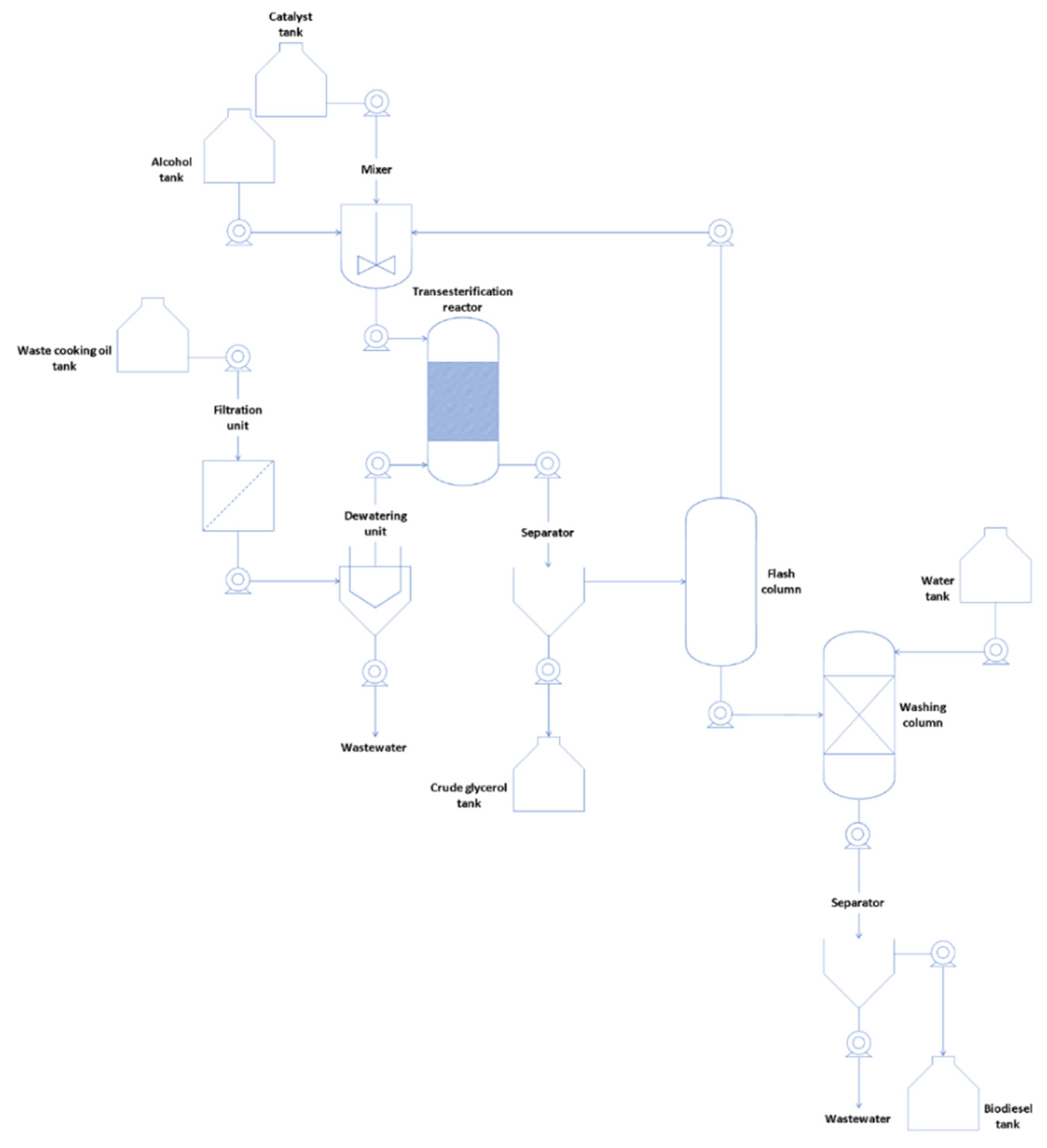
2.4. Biodiesel Production and Characterization
2.5. Statistical Analysis
3. Results and Discussion
3.1. Fatty Acid Composition
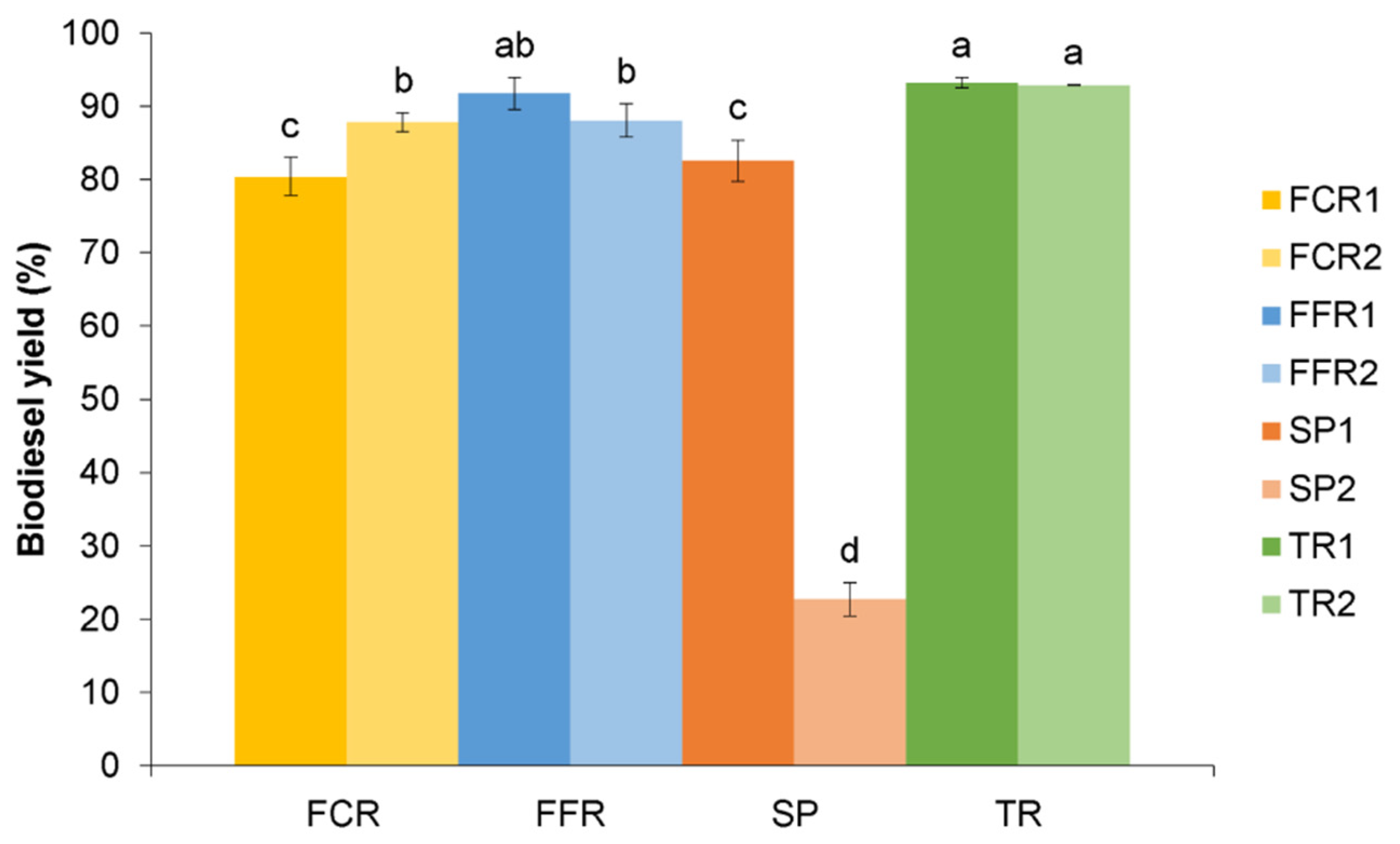
3.2. Biodiesel Yield
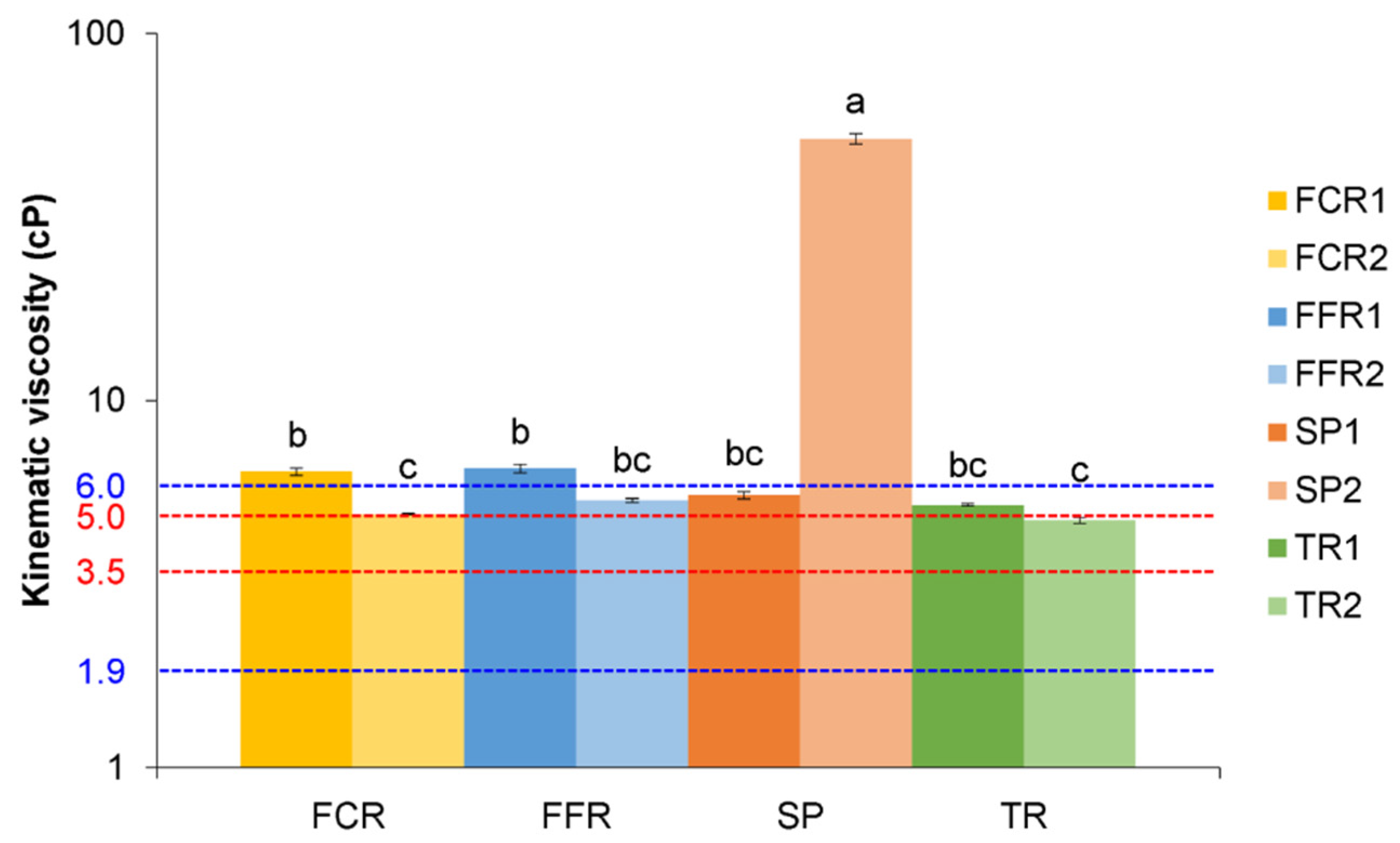
3.3. Viscosity
3.4. Calorific Value
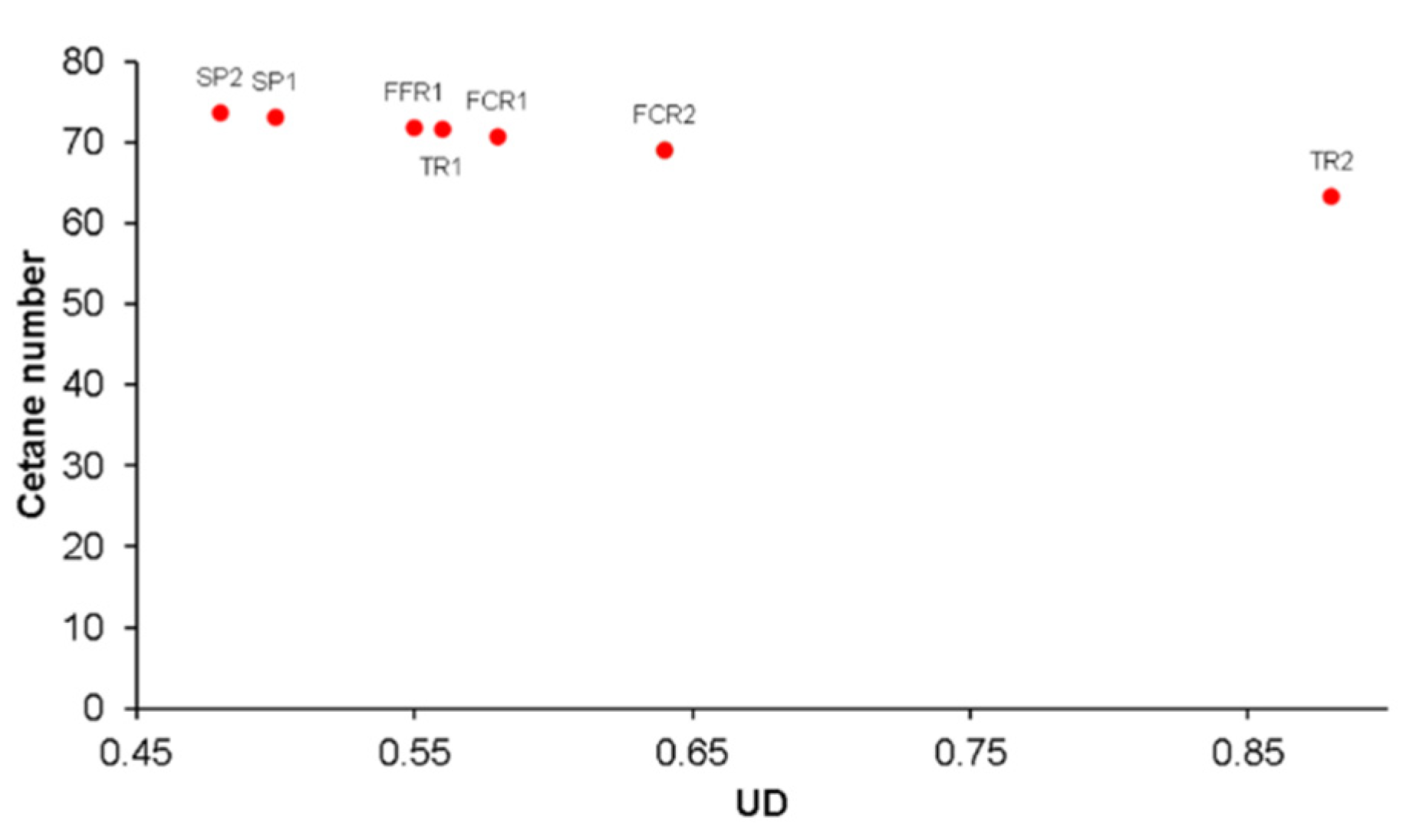
3.5. Cetane Number
3.6. Effect of Type of Restaurant
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Plata, V.; Rojas, O.; Gauthier-Maradei, P. Improvement of palm oil biodiesel filterability by treatment with reactivated. Fuel 2020, 260, 116198. [Google Scholar] [CrossRef]
- Plata, V.; Gauthier-Maradei, P.; Kafarov, V. Influence of minor components on precipitate formation and filterability of palm oil biodiesel. Fuel 2015, 144, 130–136. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. Parametric study of the alkali catalyzed transesterification of waste frying oil for biodiesel production. Energy Convers. Manag. 2014, 79, 246–254. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of biodiesel: Current scenario. Renew. Sust. Energy Rev. 2009, 13, 1646–1651. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; Almeida, V.C.; Da Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from waste cooking oil. Renew. Sust. Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Zhang, Y.; Dubé, M.A.; McLean, D.D.; Kates, M. Biodiesel production from waste cooking oil: 1. Process design and technological assessment. Bioresour. Technol. 2003, 89, 1–16. [Google Scholar] [CrossRef]
- Wyse-Mason, R.R.; Beckles, D.M. An investigation of restaurant waste oil characteristics for biodiesel production in Trinidad and Tobago. Energy Sustain. Dev. 2012, 16, 515–519. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Chemistry of deep-fat frying oils. J. Food Sci. 2007, 72, R77–R86. [Google Scholar] [CrossRef]
- Dogan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- AOAC. Fatty acids (free) in crude and refined oils. In Official Methods of Analysis, 21st ed.; Latimer, G.W., Ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- AOAC. Peroxide Value. In Official Methods of Analysis, 21st ed.; Latimer, G.W., Ed.; AOAC International: Rockville, MD, USA, 2019. [Google Scholar]
- ASTM. Standard test methods for rheological properties of non-newtonian materials by rotational viscometer. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- ISO. Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids; ISO Publications: Geneva, Switzerland, 1990. [Google Scholar]
- ISO. Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids; ISO Publications: Geneva, Switzerland, 2000. [Google Scholar]
- Carmona-Cabello, M.; Leiva-Candia, D.; Castro-Cantarero, J.L.; Pinzi, S.; Dorado, M.P. Valorization of food waste from restaurants by transesterification of the lipid fraction. Fuel 2018, 215, 492–498. [Google Scholar] [CrossRef]
- Mba, O.I.; Dumont, M.J.; Ngadi, M. Palm oil: Processing, characterization and utilization in the food industry: A review. Food Biosci. 2015, 10, 26–41. [Google Scholar] [CrossRef]
- Mendoza, L.; Plata, V.; Gauthier-Maradei, P. Effect of minor components on chemical composition, thermal behavior, and morphology of biodiesel precipitate. Fuel 2018, 228, 323–331. [Google Scholar] [CrossRef]
- Clark, W.L.; Serbia, G.W. Safety aspects of frying fats and oils. Food Technol. 1991, 45, 84–89. [Google Scholar]
- Abbasi, K.S.; Qayyum, A.; Mehmood, A.; Mahmood, T.; Khan, S.U.; Liaquat, M.; Sohail, A.; Ahmad, A. Analysis of selective potato varieties and their functional assessment. Food Sci. Technol. 2019, 39, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Andersson, R.; Westerlund, E.; Tilly, A.C.; Aman, P. Natural variations in the chemical composition of white flour. J. Cereal Sci. 1993, 17, 183–189. [Google Scholar] [CrossRef]
- ASTM. Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2012. [Google Scholar]
- CEN. Liquid Petroleum Products—Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications—Requirements and Test Methods; CEN Publications: Brussels, Belgium, 2012. [Google Scholar]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef] [Green Version]
- Encinar, J.M.; Gonzalez, J.F.; Rodriguez-Reinares, A. Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Shalaby, E.A.; El Gendy, N.S. Two steps alkaline transesterification of waste cooking oil and quality assessment of the produced biodiesel. Int. J. Chem. Biochem. Sci. 2012, 1, 30–35. [Google Scholar]
- Sahar, S.S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Ivaniš, G.R.; Radovic, I.R.; Veljkovic, V.B.; Kijevcanin, M.L. Thermodynamic properties of biodiesel and petro-diesel blends at high pressures and temperatures. Experimental and modeling. Fuel 2016, 184, 277–288. [Google Scholar] [CrossRef]
- Lapuerta, M.; Rodríguez-Fernández, J.; Armas, O. Correlation for the estimation of the density of fatty acid esters fuels and its implications. A proposed biodiesel cetane index. Chem. Phys. Lipids 2010, 163, 720–727. [Google Scholar] [CrossRef] [PubMed]
- Knothe, G. A comprehensive evaluation of the cetane numbers of fatty acid methyl esters. Fuel 2014, 119, 6–13. [Google Scholar] [CrossRef]
- Pinzi, S.; Garcia, I.L.; Lopez-Gimenez, F.J.; Luque de Castro, M.D.; Dorado, G.; Dorado, M.P. The ideal vegetable oil-based biodiesel composition: A review of social, economical and technical implications. Energy Fuels 2009, 23, 2325–2341. [Google Scholar] [CrossRef]
- Schumacher, L.G.; Van Gerpen, J.; Adams, B. Biodiesel fuels. In Encyclopedia of Energy, 1st ed.; Cleveland, C.J., Ed.; Elsevier: Boston, MA, USA, 2004; Volume 1, pp. 151–162. [Google Scholar]
- Knothe, G.; Steidley, K.R. A comparison of used cooking oils: A very heterogeneous feedstock for biodiesel. Bioresour. Technol. 2009, 100, 5796–5801. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.R.; Khoshandam, B.; Nasiri, M.; Ghazvini, M. Biodiesel production via alkali-catalyzed transesterification of Malaysian RBD palm oil—Characterization, kinetics model. J. Taiwan Inst. Chem. Eng. 2012, 43, 504–510. [Google Scholar] [CrossRef]




| WCO | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | ∑SFA a | ∑UFA b |
|---|---|---|---|---|---|---|---|---|---|
| FCR1 | 40.36 | 3.92 | 2.78 | 51.68 | 0.65 | 0.31 | 0.30 | 43.44 | 56.56 |
| FCR2 | 34.62 | 2.30 | 1.79 | 60.31 | 0.50 | 0.22 | 0.26 | 36.67 | 63.33 |
| FFR1 | 43.47 | 1.15 | 2.55 | 51.53 | 0.63 | 0.22 | 0.44 | 46.47 | 53.53 |
| FFR2 | 33.98 | 0.98 | 2.44 | 59.87 | 2.08 | 0.22 | 0.42 | 36.84 | 63.16 |
| SP1 | 48.47 | 1.33 | 2.49 | 46.69 | 0.14 | 0.47 | 0.40 | 51.37 | 48.63 |
| SP2 | 50.97 | 1.82 | 2.60 | 43.02 | 0.20 | 0.92 | 0.46 | 54.04 | 45.96 |
| TR1 | 42.34 | 2.40 | 3.03 | 50.87 | 0.64 | 0.33 | 0.39 | 45.76 | 54.24 |
| TR2 | 13.93 | 0.52 | 2.57 | 78.43 | 3.75 | 0.40 | 0.41 | 16.91 | 83.09 |
| Average | 41.44 | 58.56 | |||||||
| WCO | Acid Value mg KOH/g | Peroxide Value meq O2/kg | Dynamic Viscosity cP |
|---|---|---|---|
| FCR1 | 5.17 | 6.62 | 42.4 |
| FCR2 | 2.71 | 2.19 | 48.8 |
| FFR1 | 1.31 | 2.84 | 45.8 |
| FFR2 | 3.14 | 5.91 | 47.7 |
| SP1 | 1.26 | 12.47 | 48.3 |
| SP2 | 8.79 | 6.53 | 72.5 |
| TR1 | 1.04 | 6.53 | 46.7 |
| TR2 | 1.87 | 2.04 | 34.1 |
| WCO | Cooking Temperature (°C) | Time of Use (h/Day) | Length of Reuse (Day) | Food Cooked |
|---|---|---|---|---|
| FCR1 | >300 | 3–5 | 3–7 | Chicken, fries, plantain slices |
| FCR2 | >300 | 8–13 | 1–3 | Chicken, fries |
| FFR1 | 250–300 | 8–13 | 3–7 | Fries |
| FFR2 | 200–250 | 5–8 | 3–7 | Fries, sausages |
| SP1 | 150–200 | 5–8 | 1–3 | Potato chips |
| SP2 | 150–200 | >13 | 3–7 | Empanadas |
| TR1 | <150 | 3–5 | 1–3 | Meat, fries |
| TR2 | <150 | 8–13 | 1–3 | Meat, fries, plantain slices |
| Property | FCR | FFR | SP | TR |
|---|---|---|---|---|
| Biodiesel yield (%) | 84.1 ± 5.3 a | 89.9 ± 2.6 | 52.6 ± 42.3 | 93.1 ± 0.2 |
| Kinematic viscosity (mm2/s) | 5.7 ± 1.1 | 5.9 ± 0.8 | 28.6 ± 32.6 | 5.0 ± 0.3 |
| Calorific value (MJ/kg) | 39.7 ± 0.2 | 39.9 ± 0.2 | 37.7 ± 2.9 | 39.9 ± 0.1 |
| Density (kg/m3) | 932 ± 7 | 935 ± 14 | 962 ± 69 | 919 ± 9 |
| Cetane number | 69.9 | 70.4 | 73.3 | 67.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plata, V.; Ferreira-Beltrán, D.; Gauthier-Maradei, P. Effect of Cooking Conditions on Selected Properties of Biodiesel Produced from Palm-Based Waste Cooking Oils. Energies 2022, 15, 908. https://doi.org/10.3390/en15030908
Plata V, Ferreira-Beltrán D, Gauthier-Maradei P. Effect of Cooking Conditions on Selected Properties of Biodiesel Produced from Palm-Based Waste Cooking Oils. Energies. 2022; 15(3):908. https://doi.org/10.3390/en15030908
Chicago/Turabian StylePlata, Vladimir, Deyanira Ferreira-Beltrán, and Paola Gauthier-Maradei. 2022. "Effect of Cooking Conditions on Selected Properties of Biodiesel Produced from Palm-Based Waste Cooking Oils" Energies 15, no. 3: 908. https://doi.org/10.3390/en15030908





