Effect of Magnesium Additives on Phosphorous Recovery during Sewage Sludge Combustion and Further Improvement of Bioavailable Phosphorous
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Combustion Experiments
2.3. Determination of Total Phosphorous and Bioavailable Phosphorous
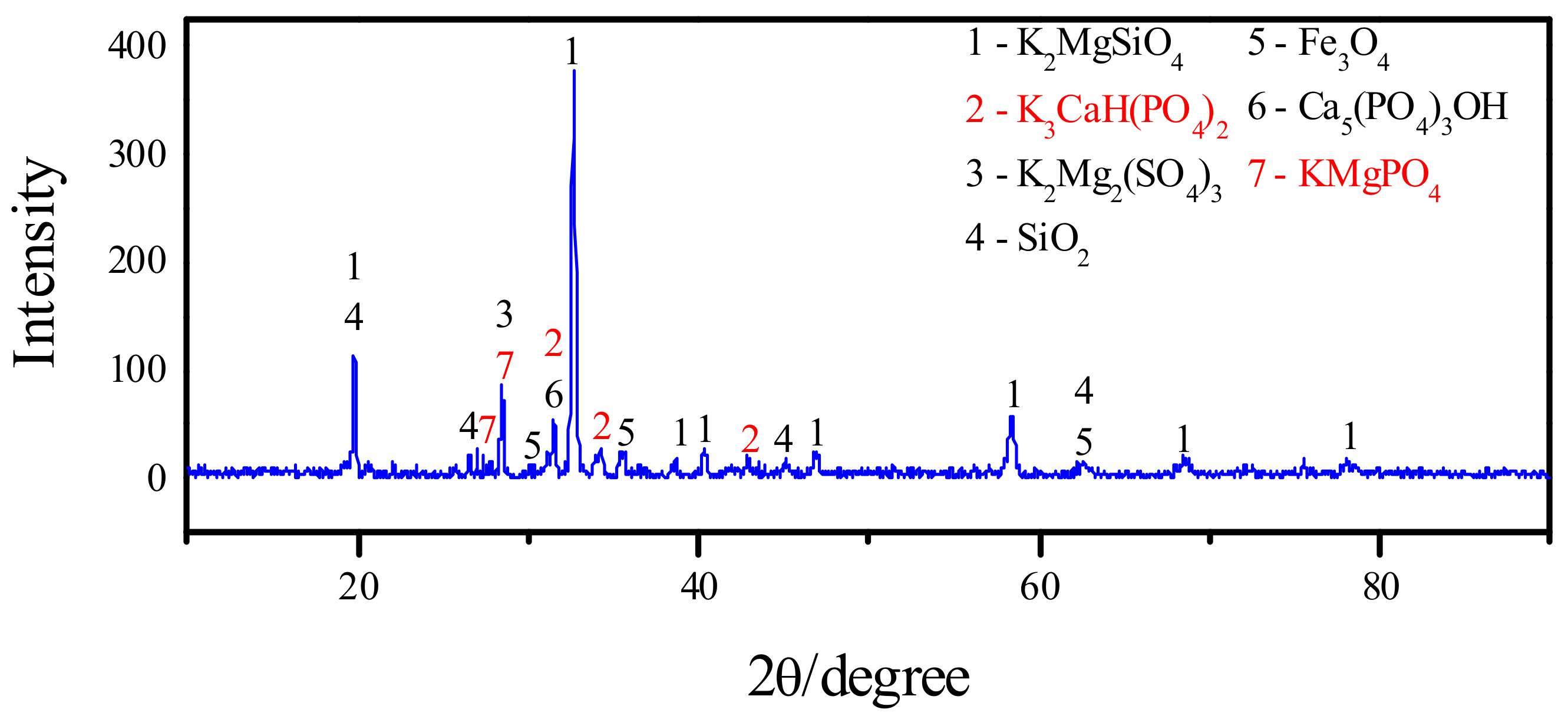
2.4. Thermochemical Modification by K2CO3
3. Results and Discussions
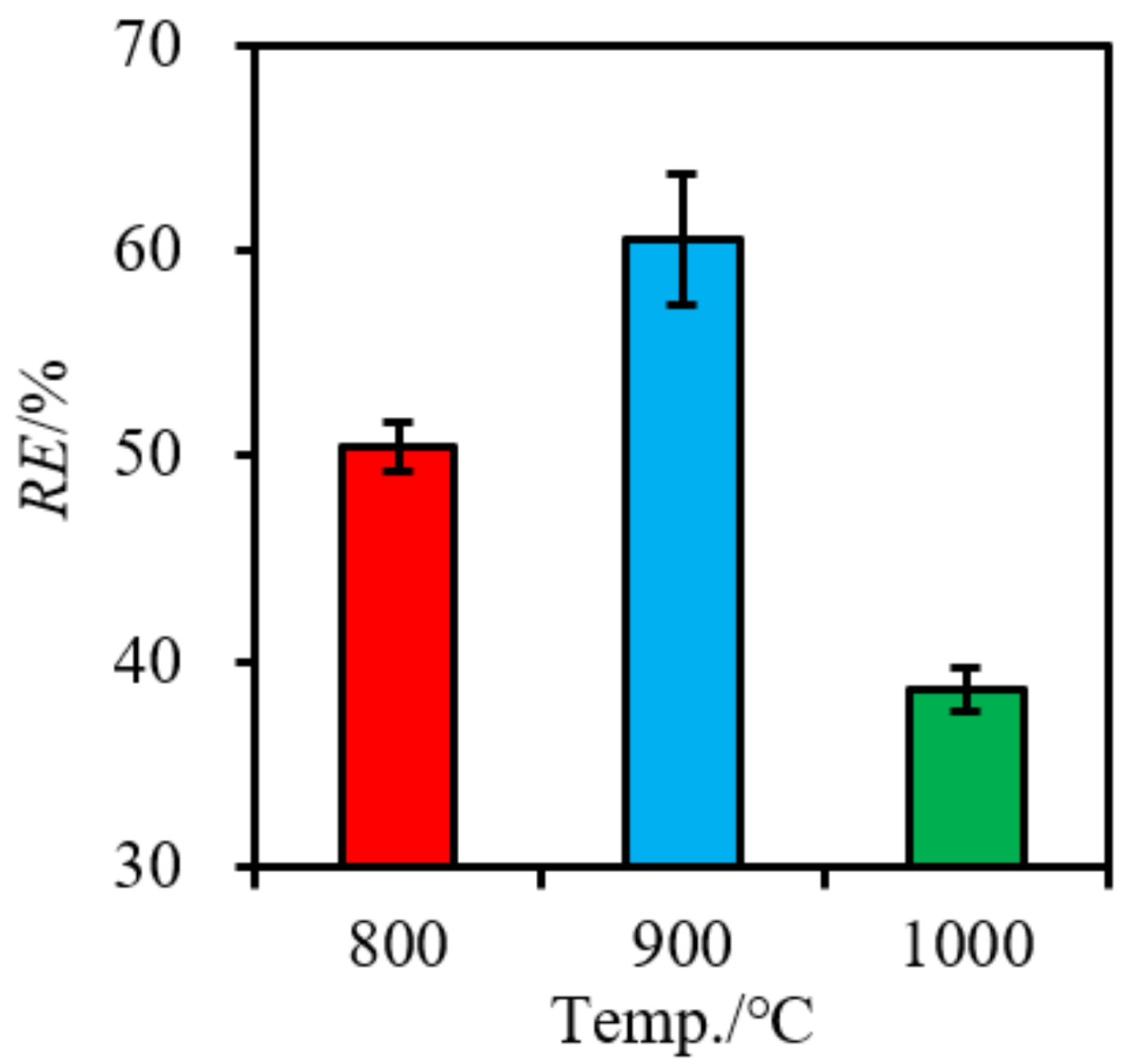
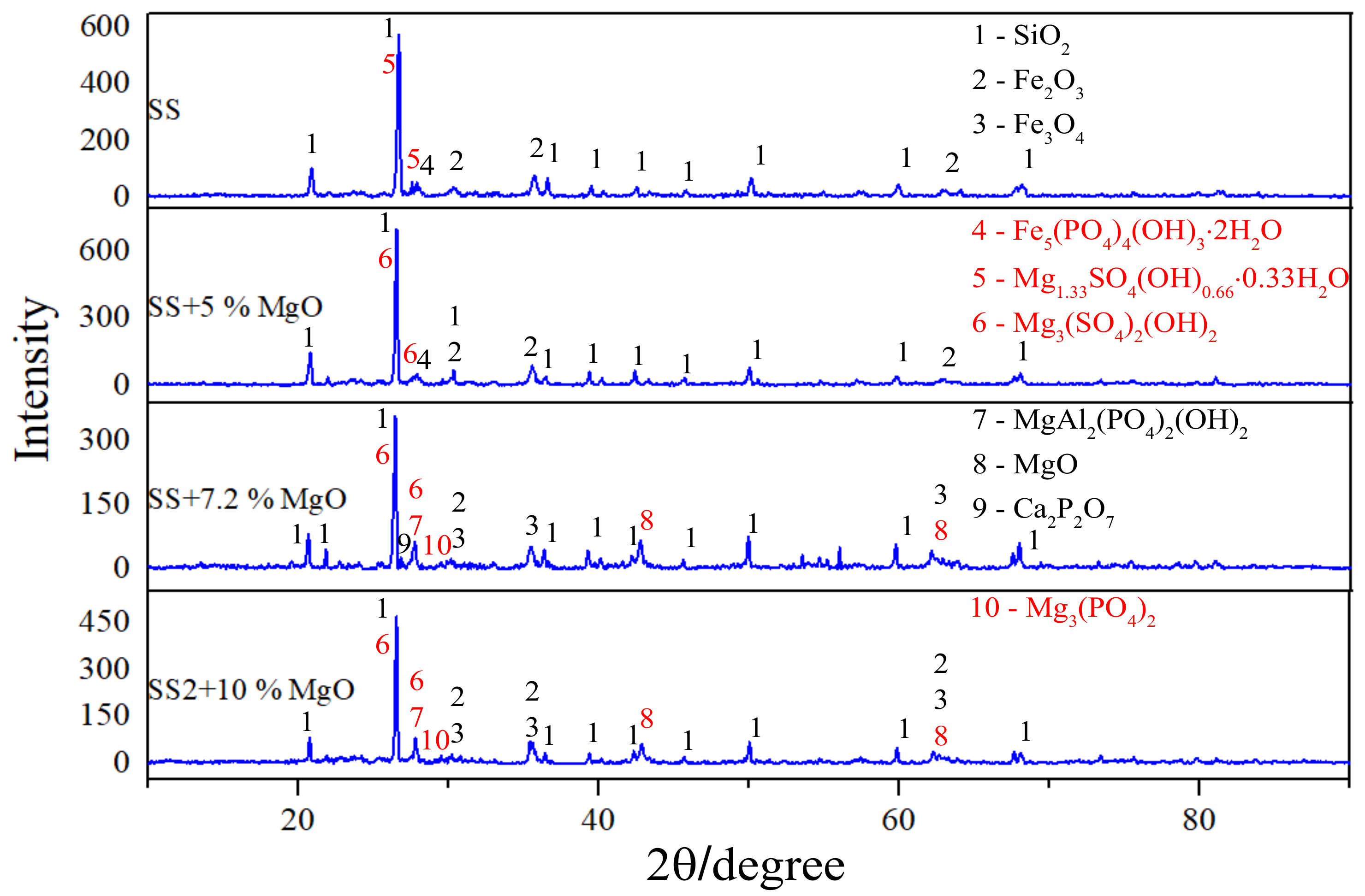
3.1. Phosphorous Migration during Mono-Combustion of SS
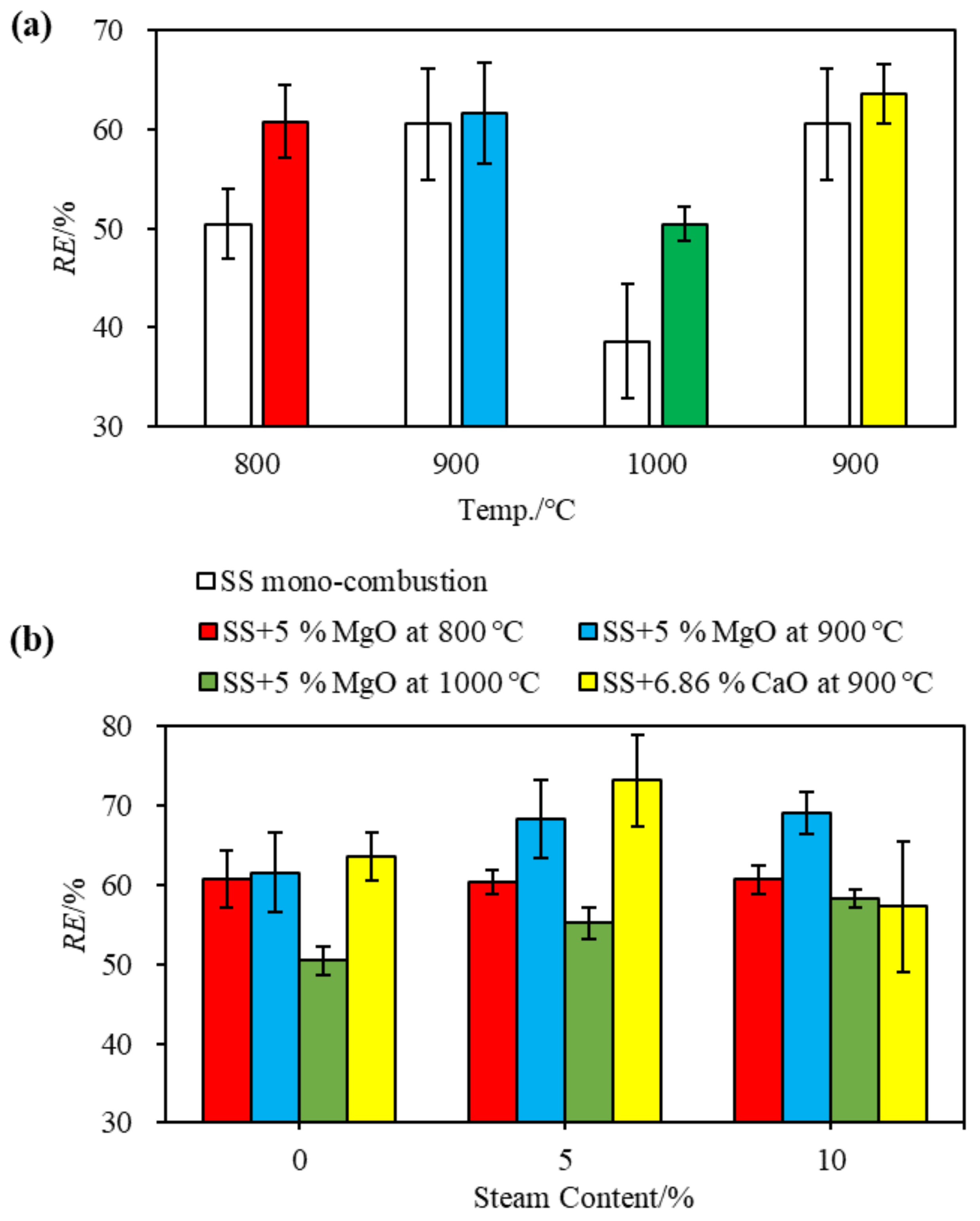
3.2. Phosphorous Capture Improved by MgO during Combustion with Various Moisture Content at 800–1000 °C
3.3. Further Phosphorous Capture by Adding More MgO or Other Magnesian Minerals at 900 °C
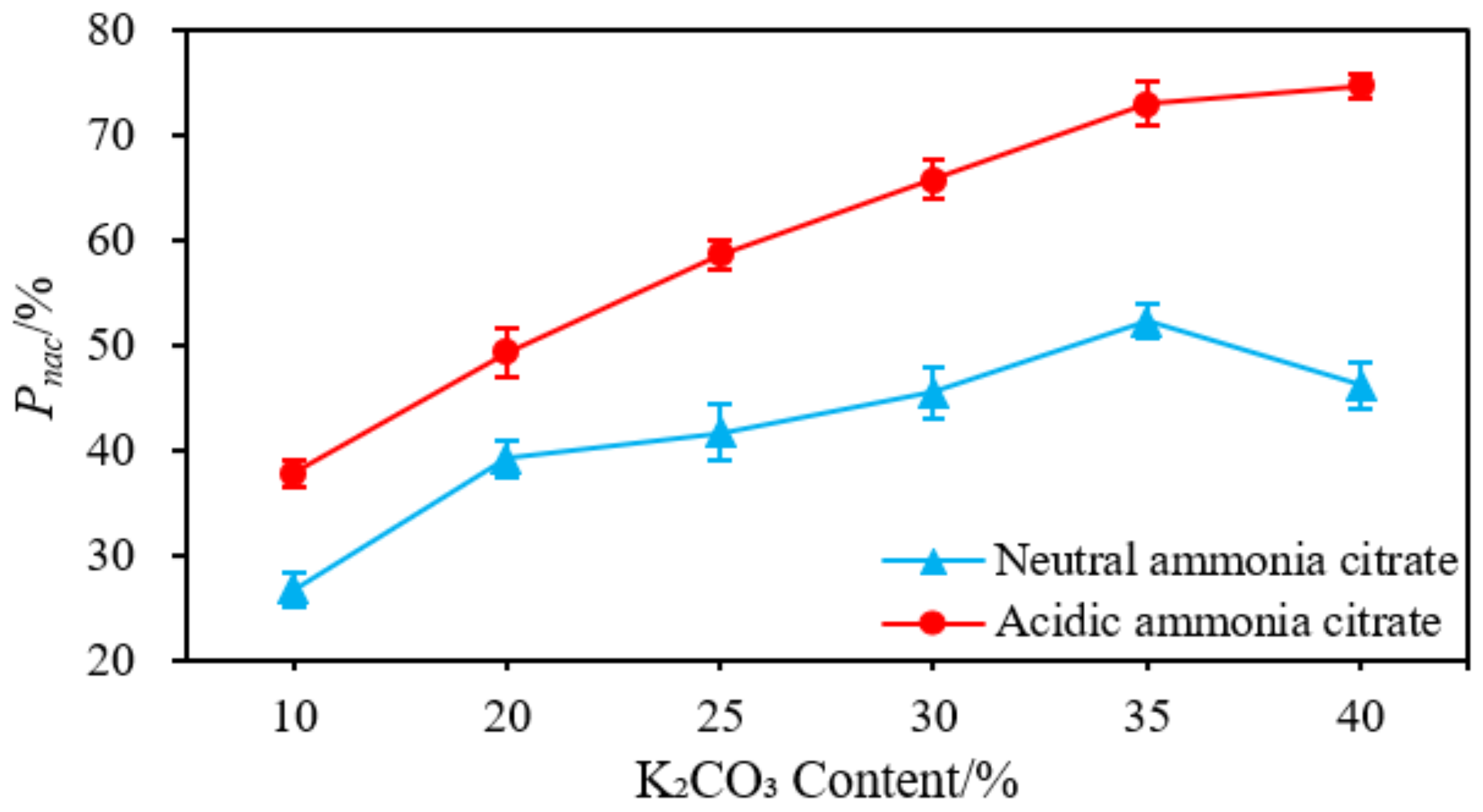
3.4. Evaluation and Improvement of Bioavailable Phosphorous in Ashes of SS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Bureau of Statistics of China. Available online: http://www.stats.gov.cn/tjsj/ndsj/2020/indexch.htm (accessed on 30 December 2020).
- Duan, N.; Dong, B.; Wu, B.; Dai, X. High-solid anaerobic digestion of sewage sludge under mesophilic conditions: Feasibility study. Bioresour. Technol. 2012, 104, 150–156. [Google Scholar] [CrossRef]
- Ministry of Housing and Urban-Rural Development of the People’s Republic of China. Available online: http://www.mohurd.gov.cn/xytj/tjzljsxytjgb/jstjnj/ (accessed on 31 December 2020).
- Dai, X.; Duan, N.; Dong, B.; Dai, L. High-solids anaerobic co-digestion of sewage sludge and food waste in comparison with mono digestions: Stability and performance. Waste Manag. 2013, 33, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Soria-Verdugo, A.; Garcia-Hernando, N.; Garcia-Gutierrez, L.M.; Ruiz-Rivas, U. Analysis of biomass and sewage sludge devolatilization using the distributed activation energy model. Energy Convers. Manag. 2013, 65, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Murakami, T.; Suzuki, Y.; Nagasawa, H.; Yamamoto, T.; Koseki, T.; Hirose, H.; Okamoto, S. Combustion characteristics of sewage sludge in an incineration plant for energy recovery. Fuel Process. Technol. 2009, 90, 778–783. [Google Scholar] [CrossRef]
- Chen, H.; Yan, S.; Ye, Z.; Meng, H.; Zhu, Y. Utilization of urban sewage sludge: Chinese perspectives. Environ. Sci. Pollut. Res. 2012, 19, 1454–1463. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wei, Q.; Zhang, B.; Bi, J. Life cycle GHG emissions of sewage sludge treatment and disposal options in Tai Lake Watershed, China. Sci. Total Environ. 2013, 447, 361–369. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Kelessidis, A.; Stasinakis, A.S. Comparative study of the methods used for treatment and final disposal of sewage sludge in European countries. Waste Manag. 2012, 32, 1186–1195. [Google Scholar] [CrossRef]
- Krüger, O.; Adam, C. Recovery potential of German sewage sludge ash. Waste Manag. 2014, 45, 400–406. [Google Scholar] [CrossRef]
- Wong, J.W.C.; Li, K.; Fang, K.; Su, D.C. Toxicity evaluation of sewage sludges in Hong Kong. Environ. Int. 2001, 27, 373–380. [Google Scholar] [CrossRef]
- Beck, J.; Brandenstein, J.; Unterberger, S.; Hein, K.R.G. Effects of sewage sludge and meat and bone meal Co-combustion on SCR catalysts. Appl. Catal. B Environ. 2004, 49, 15–25. [Google Scholar] [CrossRef]
- Chen, J.P.; Buzanowski, M.A.; Yang, R.T.; Cichanowicz, J.E. Deactivation of the vanadia catalyst in the selective catalytic reduction process. J. Air Waste Manag. Assoc. 1990, 40, 1403–1409. [Google Scholar] [CrossRef]
- Blanco, J.; Avila, P.; Barthelemy, C.; Bahamonde, A.; Odriozola, J.A.; De La Banda, J.F.G.; Heinemann, H. Influence of phosphorus in vanadium-containing catalysts for NOx removal. Appl. Catal. 1989, 55, 151–164. [Google Scholar] [CrossRef]
- Beck, J.; Unterberger, S. The behaviour of phosphorus in the flue gas during the combustion of high-phosphate fuels. Fuel 2006, 85, 1541–1549. [Google Scholar] [CrossRef]
- Niu, X.; Shen, L. Release and transformation of phosphorus in chemical looping combustion of sewage sludge. Chem. Eng. J. 2018, 335, 621–630. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Ahrenfeldt, J.; Henriksen, U.B.; Frandsen, F.J.; Müller-Stöver, D.S. Changes imposed by pyrolysis, thermal gasification and incineration on composition and phosphorus fertilizer quality of municipal sewage sludge. J. Environ. Manag. 2017, 198, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Qian, T.T.; Jiang, H. Migration of phosphorus in sewage sludge during different thermal treatment processes. ACS Sustain. Chem. Eng. 2014, 2, 1411–1419. [Google Scholar] [CrossRef]
- Li, R.; Teng, W.; Li, Y.; Wang, W.; Cui, R.; Yang, T. Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. J. Clean. Prod. 2017, 140, 964–970. [Google Scholar] [CrossRef]
- Lim, B.H.; Kim, D.J. Selective acidic elution of Ca from sewage sludge ash for phosphorus recovery under pH control. J. Ind. Eng. Chem. 2017, 46, 62–67. [Google Scholar] [CrossRef]
- Vogel, C.; Exner, R.M.; Adam, C. Heavy metal removal from sewage sludge ash by thermochemical treatment with polyvinylchloride. Environ. Sci. Technol. 2013, 47, 563–567. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Krüger, O.; Grabner, A.; Adam, C. Complete survey of German sewage sludge ash. Environ. Sci. Technol. 2014, 48, 11811–11818. [Google Scholar] [CrossRef] [PubMed]
- Sharpley, A.N.; McDowell, R.W.; Kleinman, P.J.A. Amounts, Forms, and Solubility of Phosphorus in Soils Receiving Manure. Soil Sci. Soc. Am. J. 2004, 68, 2048–2057. [Google Scholar] [CrossRef] [Green Version]
- McDowell, R.W.; Condron, L.M.; Mahieu, N.; Brookes, P.C.; Poulton, P.R.; Sharpley, A.N. Analysis of Potentially Mobile Phosphorus in Arable Soils Using Solid State Nuclear Magnetic Resonance. J. Environ. Qual. 2002, 31, 450. [Google Scholar] [CrossRef] [Green Version]
- Mcdowell, R.W.; Condron, L.M. Chemical nature and potential mobility of phosphorus in fertilized grassland soils. Nutr. Cycl. Agroecosystems 2000, 57, 225–233. [Google Scholar] [CrossRef]
- Steckenmesser, D.; Vogel, C.; Adam, C.; Steffens, D. Effect of various types of thermochemical processing of sewage sludges on phosphorus speciation, solubility, and fertilization performance. Waste Manag. 2017, 62, 194–203. [Google Scholar] [CrossRef]
- Stemann, J.; Peplinski, B.; Adam, C. Thermochemical treatment of sewage sludge ash with sodium salt additives for phosphorus fertilizer production—Analysis of underlying chemical reactions. Waste Manag. 2015, 45, 385–390. [Google Scholar] [CrossRef]
- Vogel, C.; Adam, C. Heavy metal removal from sewage sludge ash by thermochemical treatment with gaseous hydrochloric acid. Environ. Sci. Technol. 2011, 45, 7445–7450. [Google Scholar] [CrossRef]
- Jeon, S.; Kim, D.J. Enhanced phosphorus bioavailability and heavy metal removal from sewage sludge ash through thermochemical treatment with chlorine donors. J. Ind. Eng. Chem. 2018, 58, 216–221. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus recovery from wastewater by struvite crystallization: A review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef] [Green Version]
- Arslanoğlu, H.; Tümen, F. Potassium struvite (slow release fertilizer) and activated carbon production: Resource recovery from vinasse and grape marc organic waste using thermal processing. Process Saf. Environ. Prot. 2021, 147, 1077–1087. [Google Scholar] [CrossRef]
- Stratful, I.; Brett, S.; Scrimshaw, M.B.; Lester, J.N. Biological phosphorus removal, its role in phosphorus recycling. Environ. Technol. 1999, 20, 681–695. [Google Scholar] [CrossRef]
- Chen, J.; Jiao, F.; Zhang, L.; Yao, H.; Ninomiya, Y. Elucidating the mechanism of Cr(VI) formation upon the interaction with metal oxides during coal oxy-fuel combustion. J. Hazard. Mater. 2013, 261, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dong, H.; Chen, J.; Lu, C.; Luo, G.; Yao, H. Transformation of Organically Bound Chromium during Oxy-coal Combustion: The Influence of Steam and Mineral. Energy Fuels 2018, 32, 1992–1998. [Google Scholar] [CrossRef]
- Xie, C.; Xu, J.; Tang, J.; Baig, S.A.; Xu, X. Comparison of Phosphorus Determination Methods by Ion Chromatography and Molybdenum Blue Methods. Commun. Soil Sci. Plant Anal. 2013, 44, 2535–2545. [Google Scholar] [CrossRef]
- Ni, Y.; Zhang, H.; Fan, S.; Zhang, X.; Zhang, Q.; Chen, J. Emissions of PCDD/Fs from municipal solid waste incinerators in China. Chemosphere 2009, 75, 1153–1158. [Google Scholar] [CrossRef]
- European Parliament, Council of the European Union. Regulation (EC) No 2003/2003 of the European Parliament and of the Council of 13 October 2003 Relating to Fertilisers (Text with EEA Relevance). Available online: http://data.europa.eu/eli/reg/2003/2003/oj (accessed on 21 November 2003).
- Balks, M.R.; Bond, W.J.; Smith, C.J. Effects of sodium accumulation on soil physical properties under an effluent-irrigated plantation. Aust. J. Soil Res. 1998, 36, 821–830. [Google Scholar] [CrossRef]
- He, D.; Ou, Z.; Qin, C.; Deng, T.; Yin, J.; Pu, G. Understanding the catalytic acceleration effect of steam on CaCO3 decomposition by density function theory. Chem. Eng. J. 2020, 379, 122348. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhao, R.; Xiong, Z.; Li, X.; Chen, J.; Yao, H. Transformation of phosphorous during incineration of sewage sludge: Influence of steam and mineral. Fuel 2021, 303, 121307. [Google Scholar] [CrossRef]
- Liu, J.; Zhuo, Z.; Xie, W.; Kuo, J.; Lu, X.; Buyukada, M.; Evrendilek, F. Interaction effects of chlorine and phosphorus on thermochemical behaviors of heavy metals during incineration of sulfur-rich textile dyeing sludge. Chem. Eng. J. 2018, 351, 897–911. [Google Scholar] [CrossRef]
- Huang, J.; Wu, X.; Liu, J.; Chang, K.; Evrendilek, F.; Liang, G. Flue gas-to-ash desulfurization of combustion of textile dyeing sludge: Its dependency on temperature, lignocellulosic residue, and CaO. Chem. Eng. J. 2021, 417, 127906. [Google Scholar] [CrossRef]
- Wang, H.; Guo, S.; Liu, D.; Yang, L.; Wei, X.; Wu, S. Understanding the Impacts of Water Vapor on CaO Sulfurization in a Laboratory-Scale Fluidized Bed. Energy Fuels 2016, 30, 7108–7117. [Google Scholar] [CrossRef]





| Sample | Proximate Analysis | Ultimate Analysis | Phosphorous | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| M | V | A | FC | C | H | O * | N | S | ||
| SS | 3.97 | 37.50 | 55.18 | 3.35 | 17.78 | 1.91 | 17.58 | 2.05 | 1.53 | 3.01 |
| Sample | SiO2 | Al2O3 | Fe2O3 | P2O5 | CaO | MgO | SO3 | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| SS | 43.95 | 17.98 | 12.48 | 12.09 | 6.16 | 2.65 | 1.54 | 2.42 | 0.73 |
| Additives | Combustion Temp./°C | Content of H2O/% | Pnac/% |
|---|---|---|---|
| / | / | / | 39.23 * |
| None | 800 | 0 | 13.39 ± 0.82 |
| 5 | 15.64 ± 1.85 | ||
| 10 | 15.37 ± 1.77 | ||
| None | 900 | 0 | 13.30 ± 1.20 |
| 5 | 14.37 ± 0.52 | ||
| 10 | 13.26 ± 0.61 | ||
| None | 1000 | 0 | 9.99 ± 1.32 |
| 5 | 13.06 ± 0.51 | ||
| 10 | 11.90 ± 1.30 | ||
| 5% MgO | 800 | 0 | 20.06 ± 1.20 |
| 5 | 19.44 ± 0.49 | ||
| 10 | 9.67 ± 0.29 | ||
| 5% MgO | 900 | 0 | 15.94 ± 1.90 |
| 5 | 16.16 ± 2.20 | ||
| 10 | 17.33 ± 2.00 | ||
| 5% MgO | 1000 | 0 | 11.82 ± 0.41 |
| 5 | 13.69 ± 0.50 | ||
| 10 | 13.64 ± 0.27 | ||
| 6% MgO | 800 | 5 | 18.98 ± 1.54 |
| 900 | 15.67 ± 1.13 | ||
| 1000 | 13.93 ± 1.55 | ||
| 7.2% MgO | 800 | 5 | 12.24 ± 1.22 |
| 900 | 11.78 ± 0.85 | ||
| 1000 | 7.04 ± 0.72 | ||
| 15.06% hydromagnesite | 900 | 0 | 14.66 ± 1.14 |
| 5 | 19.99 ± 3.75 | ||
| 10 | 20.78 ± 1.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, Y.; Ren, X.; Chen, J. Effect of Magnesium Additives on Phosphorous Recovery during Sewage Sludge Combustion and Further Improvement of Bioavailable Phosphorous. Energies 2022, 15, 909. https://doi.org/10.3390/en15030909
Xiao Y, Ren X, Chen J. Effect of Magnesium Additives on Phosphorous Recovery during Sewage Sludge Combustion and Further Improvement of Bioavailable Phosphorous. Energies. 2022; 15(3):909. https://doi.org/10.3390/en15030909
Chicago/Turabian StyleXiao, Yi, Xiaohan Ren, and Juan Chen. 2022. "Effect of Magnesium Additives on Phosphorous Recovery during Sewage Sludge Combustion and Further Improvement of Bioavailable Phosphorous" Energies 15, no. 3: 909. https://doi.org/10.3390/en15030909






