Evaluation of the Optimal Conditions for Oxygen-Rich and Oxygen-Lean Torrefaction of Forestry Byproduct as a Fuel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thermogravimetric Analysis
2.2. Torrefaction Experiments
2.3. Fuel Characteristic Evaluation
2.3.1. Elemental Composition Changes
2.3.2. Proximate Composition Changes
2.3.3. Calorific Value and Energy Yield
2.3.4. Decarbonization, Dehydrogenation, and Deoxygenation
2.3.5. Combustion Indices
3. Results and Discussion
3.1. Results of the Thermogravimetric Analysis
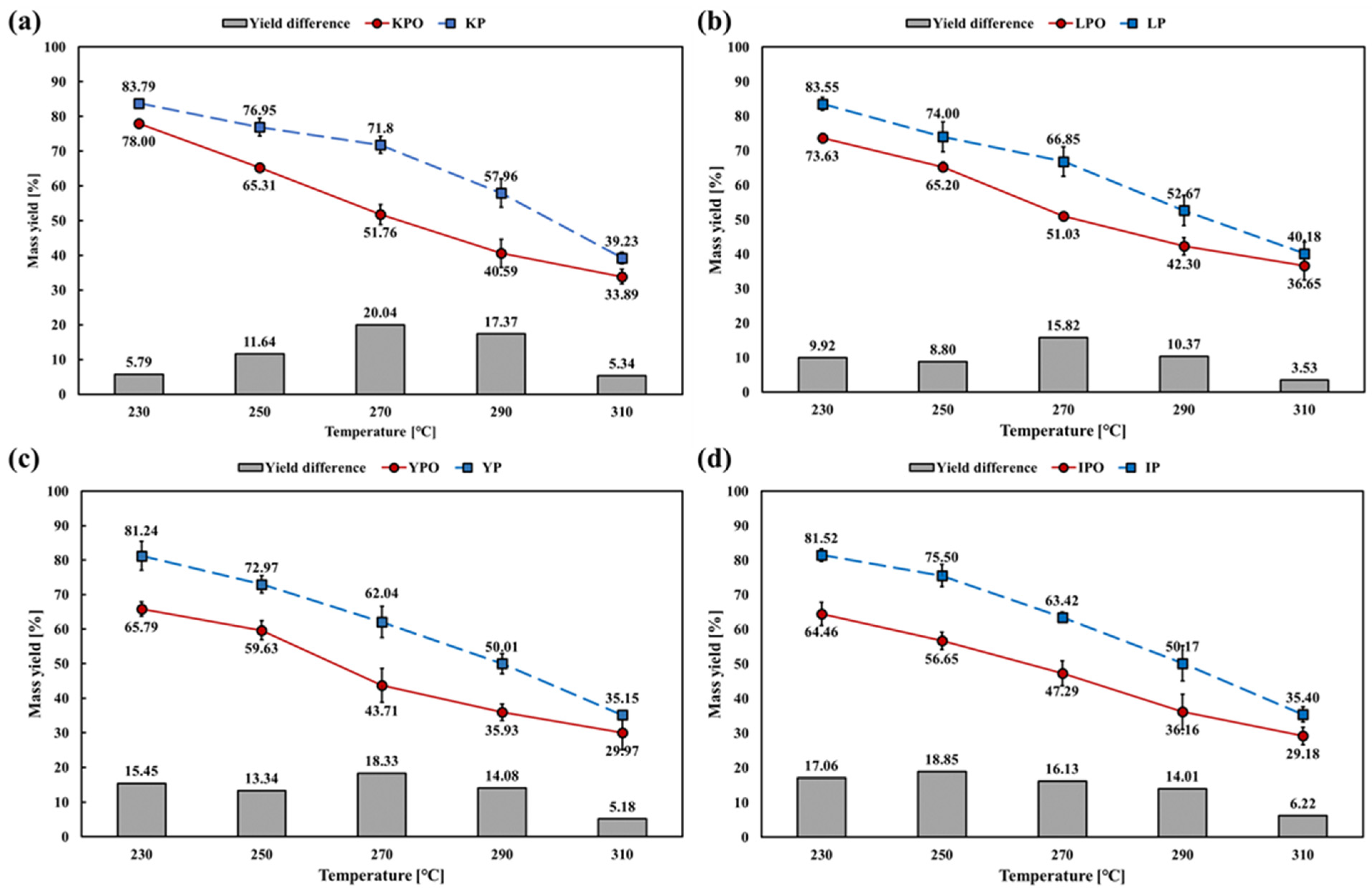
3.2. Effect on Mass Yield
3.3. Effect on Fuel Characteristic
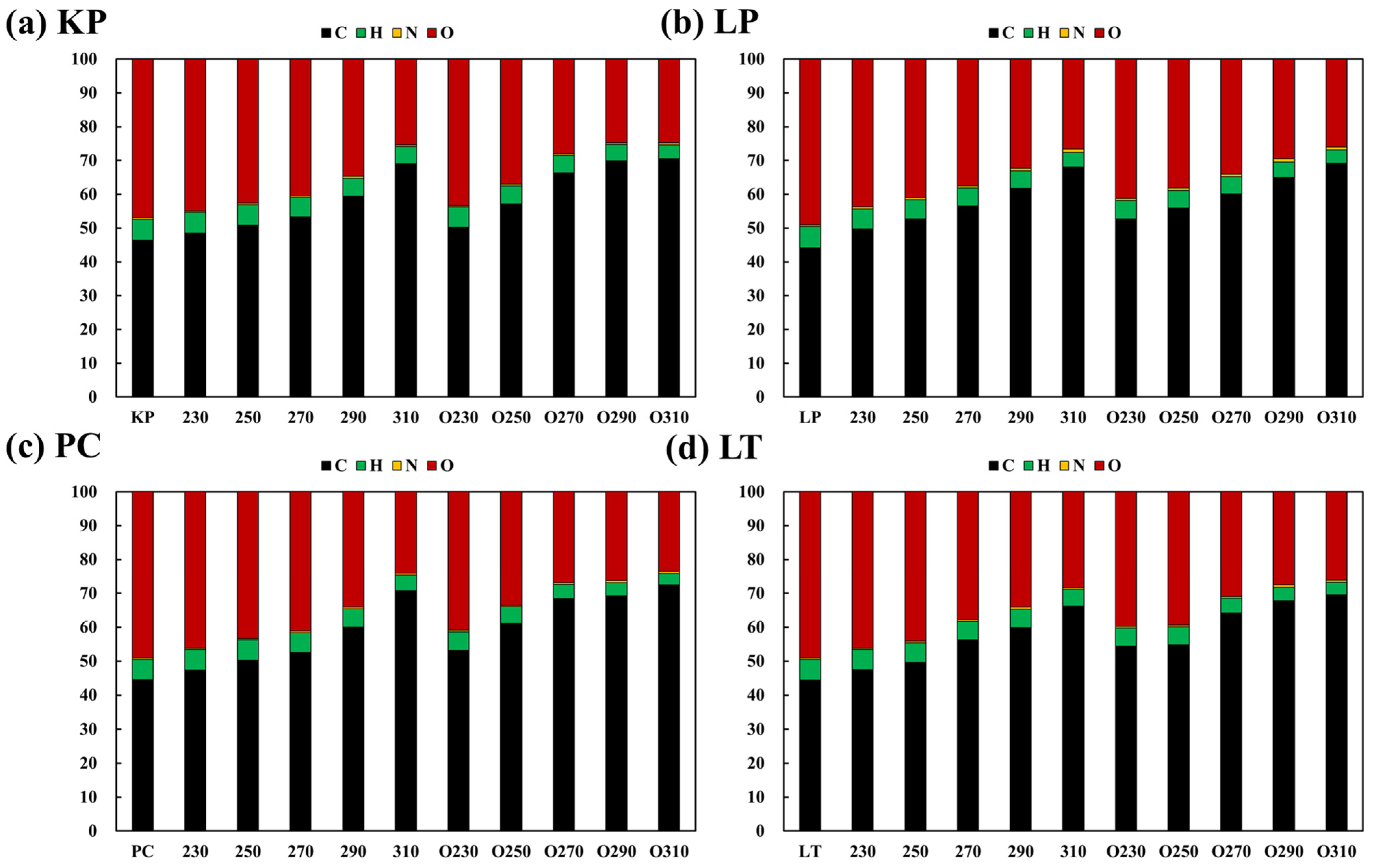
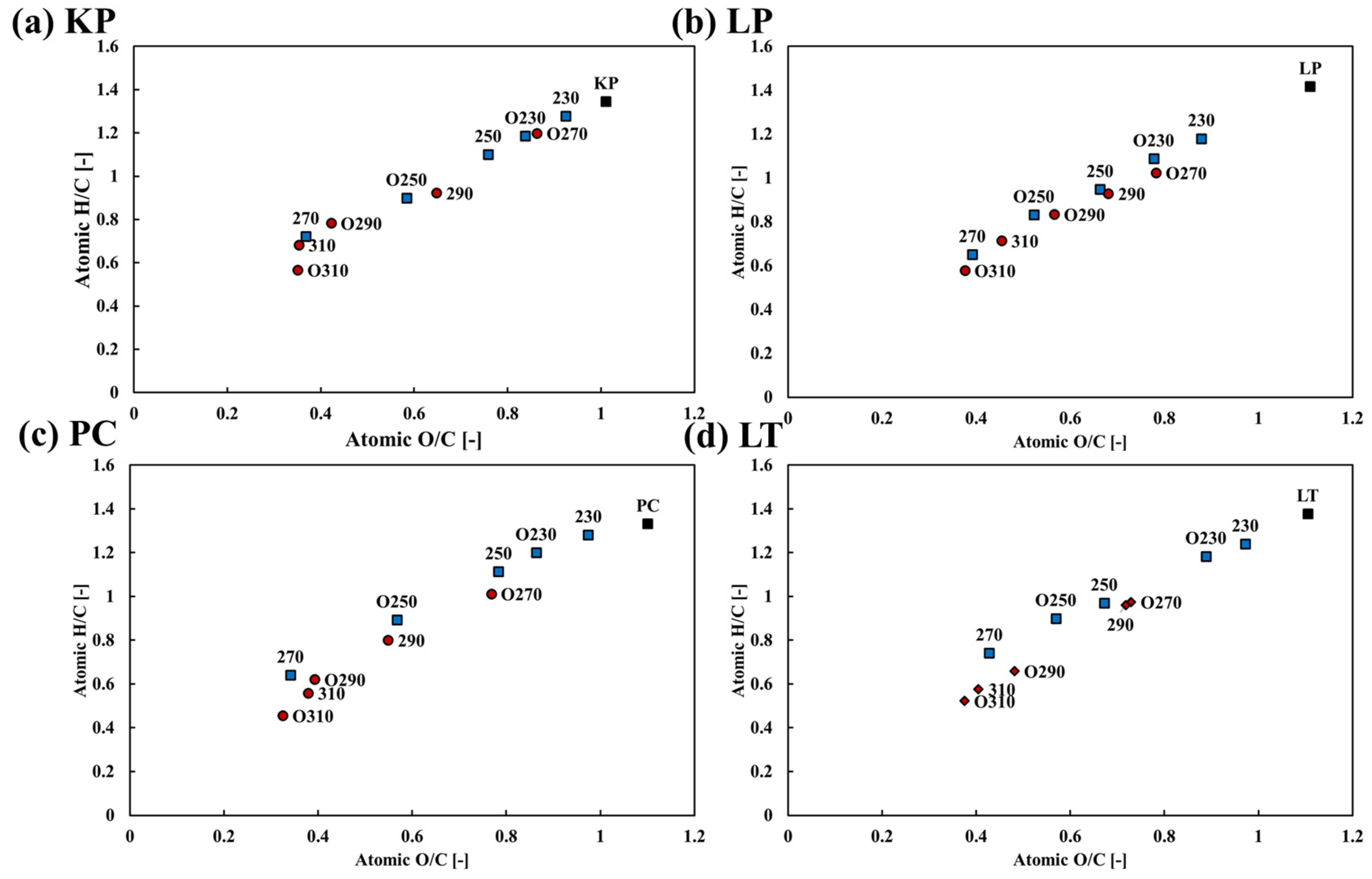
3.3.1. Effect on the Elemental Composition Ratio
3.3.2. Effect on the Proximate Composition Ratio
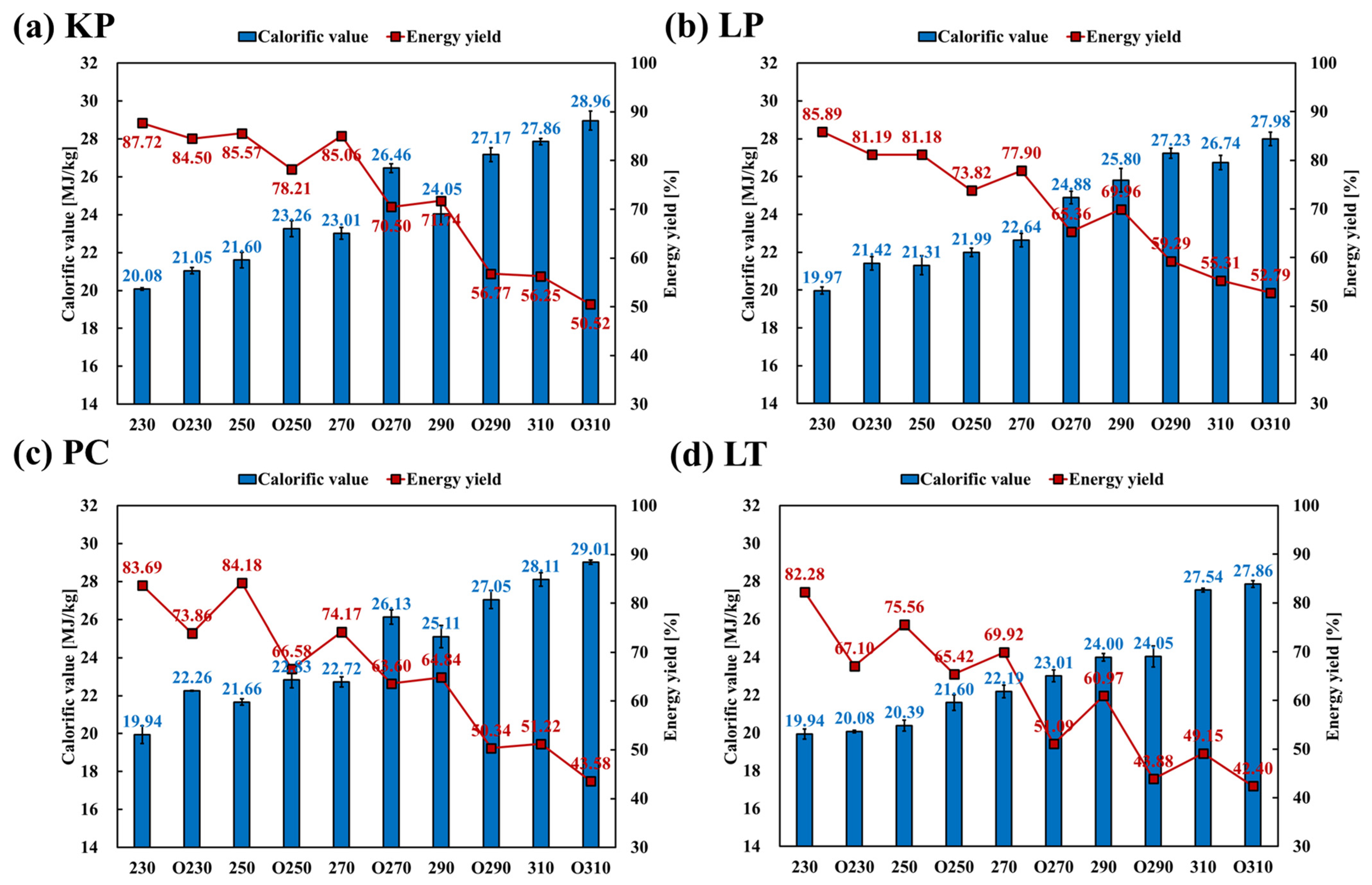
3.3.3. Effect on calorific Value and Energy Yield
3.3.4. Effect on DC, DH, and DO
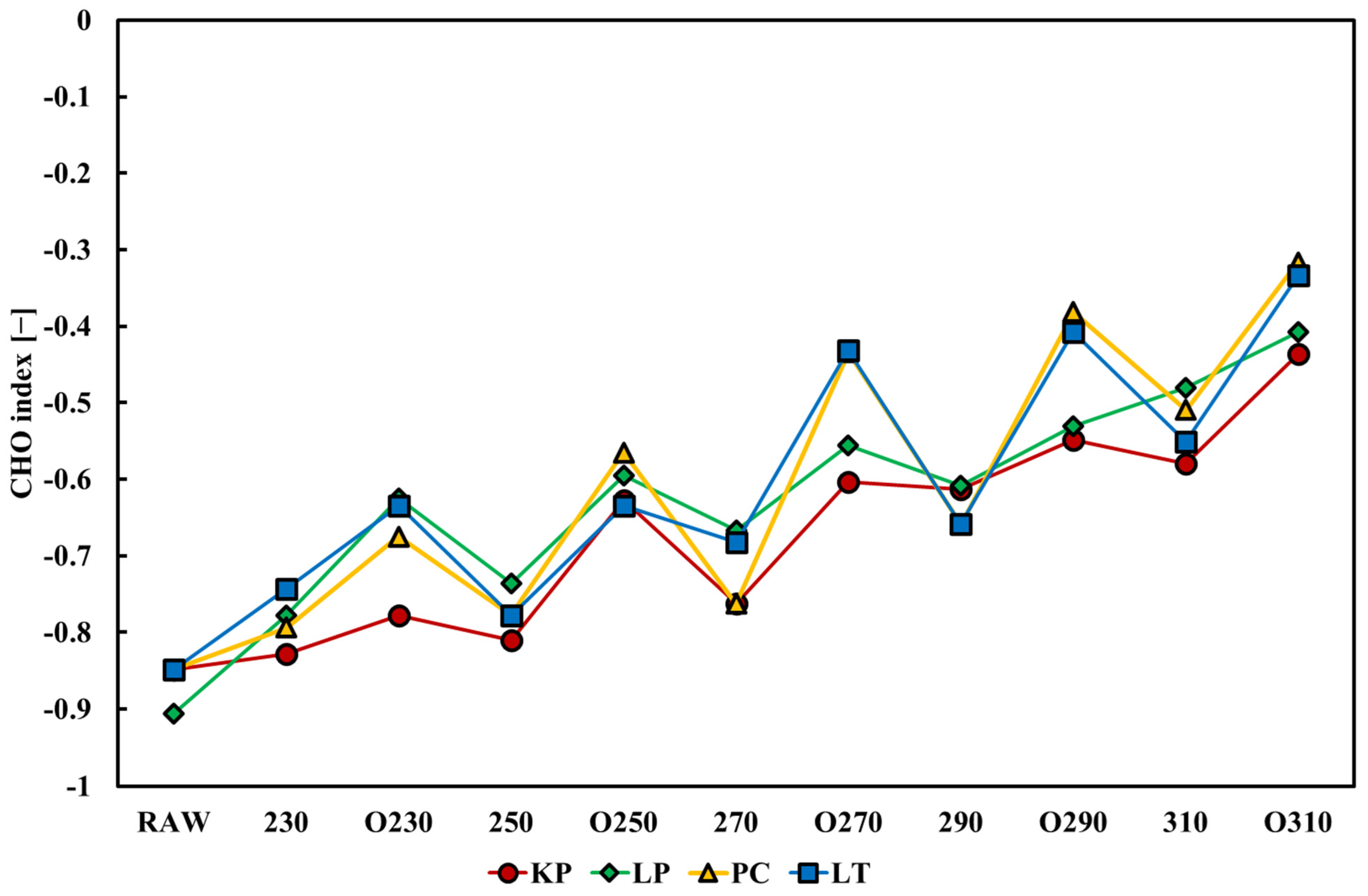
3.3.5. Effect on Combustion Indices
3.4. Optimal Conditions
4. Conclusions
- (1).
- In this study, the process temperature and oxygen conditions remarkably affected the torrefaction of unused forestry byproduct wood chips. Hardwood and softwood showed 5.18–18.85% p and 3.53–20.04% p of mass yield difference between oxygen-rich and oxygen-lean conditions, respectively.
- (2).
- Under oxygen-rich conditions, the carbon composition ratios of hardwood and softwood increased by 62.88% and 56.45%, respectively. The maximum increase in the relative carbon proportion of each wood type under oxygen-lean conditions were 49.21% and 53.97%, respectively.
- (3).
- A maximum CV increase of 50.95% under oxygen-rich conditions (from 19.18 MJ/kg to 28.96 MJ/kg) and 48.48% under oxygen-lean conditions (from 19.18 MJ/kg to 27.98 MJ/kg) was observed.
- (4).
- For hardwoods, KP and LP, the energy yields ranged from 59.25–87.72% and 55.31–85.89%, and from 50.52–84.50% and 52.79–81.19% under oxygen-lean and oxygen-rich conditions, respectively. In contrast, for softwoods, PC and LT, the energy yields were lower compared to those of hardwoods, showing 51.22–83.69% and 49.15–82.28%, and 43.58–73.86% and 42.40–67.10% under oxygen-lean and oxygen-rich conditions, respectively.
- (5).
- Decarbonization, dehydrogenation, and deoxygenation were observed in the range of 6.35–53.84%, 15.79–84.20%, and 19.51–86.38%, respectively.; these indices increased linearly with the calorific value.
- (6).
- The higher the temperature, the more favorable the CI for coal co-firing, similar to that under oxygen-rich conditions. II was excellent in most cases (excluding LP290, LT250, LTO230, and LTO270).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Energy Agency. World Energy Outlook 2022; International Energy Agency: Paris, France, 2022. [Google Scholar]
- Kim, H. IEA’s Global Energy Demand Forecast; IEA: Ulsan, Republic of Korea, 2022. [Google Scholar]
- Chun, T. Rim Yearbook of Energy Statistics 2021. 2021. Available online: http://www.kesis.net/sub/sub_0003.jsp?M_MENU_ID=M_M_002&S_MENU_ID=S_M_012 (accessed on 16 August 2022).
- Electric Power Statistics Information System. Power Generation Fuel Consumption Trend. Available online: https://epsis.kpx.or.kr/epsisnew/selectEkpoBftChart.do?menuId=020100&locale= (accessed on 12 August 2022).
- Baek, S.; Park, H.; Ko, S. Economic Evaluation of Coals Imported in Last 3 Years for Power Plant Based on Thermal Performance Analysis. J. Korean Soc. Combust. 2013, 18, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Park, J.-H.; Lee, Y.-J.; Song, G.-S.; Park, S.-J.; Kim, J.-G.; Song, K.-H.; Choi, Y.-C. Fuel/Combustion Characterization of Glycerol-Impregnated Hybrid Coal. Proceeding Korea Soc. New Renew. Energy 2016, 178. Available online: https://www.dbpia.co.kr/journal/articleDetail?nodeId=NODE07154924R (accessed on 12 August 2022).
- Oh, K.C.; Park, S.Y.; Kim, S.J.; Choi, Y.S.; Lee, C.G.; Cho, L.H.; Kim, D.H. Development and Validation of Mass Reduction Model to Optimize Torrefaction for Agricultural Byproduct Biomass. Renew. Energy 2019, 139, 988–999. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Han, S.-K. Production of Biofuels and Biochemicals by Biorefinery. Trans. Korean Hydrog. New Energy Soc. 2016, 27, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; Dong, X.; Zhao, P.; Luo, Z.; Sun, Z.; Yang, X.; Li, Q.; Wang, L.; Zhang, Y.; Zhou, H. Decoupled Temperature and Pressure Hydrothermal Synthesis of Carbon Sub-Micron Spheres from Cellulose. Nat. Commun. 2022, 13, 3616. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A Handful of Carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef]
- The Ministry of Trade Industry and Energy. Act on the Promotion of the Development, Use, and Diffusion of New and Renewable Energy; The Ministry of Trade Industry and Energy: Seoul, Republic of Korea, 2022. [Google Scholar]
- Ministry of Trade Industry and Energy. Management and Operation Guidelines for the Mandatory Supply of New and Renewable Energy and the Mandatory Fuel Mixture System; The Ministry of Trade Industry and Energy: Seoul, Republic of Korea, 2022. [Google Scholar]
- Kim, S.J.; Oh, K.C.; Park, S.Y.; Ju, Y.M.; Cho, L.H.; Lee, C.G.; Kim, M.J.; Jeong, I.S.; Kim, D.H. Development and Validation of Mass Reduction Prediction Model and Analysis of Fuel Properties for Agro-Byproduct Torrefaction. Energies 2021, 14, 6125. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Vassilev, V.S. Advantages and Disadvantages of Composition and Properties of Biomass in Comparison with Coal: An Overview. Fuel 2015, 158, 330–350. [Google Scholar] [CrossRef]
- Hwang, S.J.; Kim, J.H.; Nam, J.S. Factorial Experiment for the Collecting Device of an Agricultural By-Product Collector. J. Biosyst. Eng. 2020, 45, 422–431. [Google Scholar] [CrossRef]
- Kim, G.-H.; Kim, K.-D.; Lee, H.-S.; Choi, Y.-S.; Mun, H.-S.; Oh, J.-H.; Shin, B.-S. Development of Wi-Fi-Based Teleoperation System for Forest Harvester. J. Biosyst. Eng. 2021, 46, 206–216. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 2. [Google Scholar]
- Lee, J.-W.; Kim, Y.-H.; Lee, S.-M.; Lee, H.-W. Torrefaction Characteristics of Wood Chip for the Production of High Energy Density Wood Pellet. Korean Chem. Eng. Res. 2012, 50, 385–389. [Google Scholar] [CrossRef] [Green Version]
- da Silva, C.M.S.; Vital, B.R.; Carneiro, A.d.C.O.; Costa, E.V.S.; de Magalhães, M.A.; Trugilho, P.F. Structural and Compositional Changes in Eucalyptus Wood Chips Subjected to Dry Torrefaction. Ind. Crops Prod. 2017, 109, 598–602. [Google Scholar] [CrossRef]
- Brachi, P.; Miccio, F.; Miccio, M.; Ruoppolo, G. Torrefaction of Tomato Peel Residues in a Fluidized Bed of Inert Particles and a Fixed-Bed Reactor. Energy Fuels 2016, 30, 4858–4868. [Google Scholar] [CrossRef]
- Rousset, P.; MacEdo, L.; Commandré, J.M.; Moreira, A. Biomass Torrefaction under Different Oxygen Concentrations and Its Effect on the Composition of the Solid By-Product. J. Anal. Appl. Pyrolysis 2012, 96, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Alahakoon, A.M.Y.W.; Karunarathna, A.K.; Dharmakeerthi, R.S.; Silva, F.H.C.A. Design and Development of a Double-Chamber Down Draft (DcDD) Pyrolyzer for Biochar Production from Rice Husk. J. Biosyst. Eng. 2022, 47, 458–467. [Google Scholar] [CrossRef]
- Kim, S.J.; Park, S.; Oh, K.C.; Ju, Y.M.; hoon Cho, L.; Kim, D.H. Development of Surface Torrefaction Process to Utilize Agro-Byproducts as an Energy Source. Energy 2021, 233, 121192. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, P.; Yang, X.; Li, Q.; Mohamed, B.A.; Saad, J.M.; Zhang, Y.; Zhou, H. Low-Temperature Hydrothermal Carbonization of Pectin Enabled by High Pressure. J. Anal. Appl. Pyrolysis 2022, 166, 105627. [Google Scholar] [CrossRef]
- Sharma, H.B.; Dubey, B.K. Co-Hydrothermal Carbonization of Food Waste with Yard Waste for Solid Biofuel Production: Hydrochar Characterization and Its Pelletization. Waste Manag. 2020, 118, 521–533. [Google Scholar] [CrossRef]
- Hernandez-Mena, L.E.; Pecora, A.A.B.; Beraldo, A.L. Slow Pyrolysis of Bamboo Biomass: Analysis of Biochar Properties. Chem. Eng. Trans. 2014, 37, 115–120. [Google Scholar] [CrossRef]
- Basu, P.; Dhungana, A.; Rao, S.; Acharya, B. Effect of Oxygen Presence in Torrefier. J. Energy Inst. 2013, 86, 171–176. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Chen, W.; Wang, S.; Liang, G.; Li, W.; Ma, Z.; Zhang, W. Effect of Torrefaction Temperature and O2 Concentration on the Pyrolysis Behaviour of Moso Bamboo. Bioresources 2020, 15, 6344–6370. [Google Scholar] [CrossRef]
- Cruz Ceballos, D.C.; Hawboldt, K.; Hellleur, R. Effect of Production Conditions on Self-Heating Propensity of Torrefied Sawmill Residues. Fuel 2015, 160, 227–237. [Google Scholar] [CrossRef]
- Mann, B.F.; Chen, H.; Herndon, E.M.; Chu, R.K.; Tolic, N.; Portier, E.F.; Chowdhury, T.R.; Robinson, E.W.; Callister, S.J.; Wullschleger, S.D.; et al. Indexing Permafrost Soil Organic Matter Degradation Using High-Resolution Mass Spectrometry. PLoS ONE 2015, 10, e0130557. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Kim, S.J.; Oh, K.C.; Cho, L.H.; Kim, D. Developing a Proximate Component Prediction Model of Biomass Based on Element Analysis. Energies 2023, 16, 509. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chen, W.H.; Lin, B.J.; Chang, J.S.; Ong, H.C. Impact of Torrefaction on the Composition, Structure and Reactivity of a Microalga Residue. Appl. Energy 2016, 181, 110–119. [Google Scholar] [CrossRef]
- Makino, H.; Tanno, K. Coal Combustion for Power Production, 1st ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 263–265. ISBN 9780367783303. [Google Scholar]
- Ohm, T.I.; Chae, J.S.; Kim, J.K.; Oh, S.C. Study on the Characteristics of Biomass for Co-Combustion in Coal Power Plant. J. Mater. Cycles Waste Manag. 2015, 17, 249–257. [Google Scholar] [CrossRef]
- Solihull, E.; Williamson, J.; Wigley, F. The Impact of Ash Deposition on Coal Fired Plants. In Proceedings of the Engineering Foundation Conference, Solihull, UK, 20–25 June 1994. [Google Scholar]
- Jo, E.-J.; Jin, Y.-G.; Hyeon, W.-S.; Han, H.-G.; Min, S.-U.; Yeo, W.-H. A Study on the Characteristics of Waste Biomass Fuel by the Conditions of Torrefaction and Biomass Mixing Ratio. J. Korea Org. Resour. Recycl. Assoc. 2018, 26, 75–84. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Torrefaction of Woody Biomass (Acacia Nilotica): Investigation of Fuel and Flow Properties to Study Its Suitability as a Good Quality Solid Fuel. Renew. Energy 2020, 153, 711–724. [Google Scholar] [CrossRef]
- Akogun, O.A.; Waheed, M.A. Property Upgrades of Some Raw Nigerian Biomass through Torrefaction Pre-Treatment—A Review. J. Phys. Conf. Ser. 2019, 1378, 032026. [Google Scholar] [CrossRef]
- Asthana, A.K. Coal Blending and Its Effects on Boiler Performance. 2015. Available online: https://www.scribd.com/document/331240839/Coal-Blending-and-Its-Effects-on-Boiler-Performance (accessed on 13 March 2023).
- Xu, X.; Pan, R.; Chen, R. Comparative Thermal Degradation Behaviors and Kinetic Mechanisms of Typical Hardwood and Softwood in Oxygenous Atmosphere. Processes 2021, 9, 1598. [Google Scholar] [CrossRef]
- Kim, Y.M.; Han, T.U.; Hwang, B.A.; Lee, B.; Lee, H.W.; Park, Y.K.; Kim, S. Pyrolysis Kinetics and Product Properties of Softwoods, Hardwoods, and the Nut Shell of Softwood. Korean J. Chem. Eng. 2016, 33, 2350–2358. [Google Scholar] [CrossRef]
- Cho, H.S.; Sung, Y.J.; Kim, C.H.; Lee, G.S.; Yim, S.J.; Nam, H.G.; Lee, J.Y.; Kim, S. Bin Study of Oil Palm Biomass Resources (Part 3) Torrefaction of Oil Palm Biomass. J. Korea Tech. Assoc. Pulp Pap. Ind. 2014, 46, 18–28. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Riva, L.; Skreiberg, Ø.; Khalil, R.; Bartocci, P.; Yang, Q.; Yang, H.; Wang, X.; Chen, D.; Rudolfsson, M.; et al. Effect of Torrefaction on Properties of Pellets Produced from Woody Biomass. Energy Fuels 2020, 34, 15343–15354. [Google Scholar] [CrossRef]
- Homdoung, N.; Sasujit, K.; Uttharuan, J.; Tippayawong, N. Influence of Torrefaction Temperature and Time on the Yields and Properties of Torrefied Biomass. Eng. Appl. Sci. Res. 2019, 46, 170–175. [Google Scholar] [CrossRef]







| Ultimate Analysis [db%] | Proximate Analysis | Calorific Value [MJ/kg] | |||||||
|---|---|---|---|---|---|---|---|---|---|
| C | H | N | O * | MC [%ar] | VM [%db] | FC [%db] | Ash [%db] | ||
| KP | 46.38 | 6.24 | 0.48 | 46.89 | 15.6 | 81.1 | 17.2 | 1.7 | 19.18 |
| LP | 44.18 | 6.26 | 0.53 | 49.03 | 25.2 | 83.2 | 15.2 | 1.6 | 19.43 |
| PC | 44.53 | 6.10 | 0.44 | 48.93 | 27.6 | 84.6 | 14.4 | 1.0 | 19.32 |
| LT | 44.36 | 6.11 | 0.51 | 49.01 | 13.1 | 87.2 | 12.1 | 0.7 | 19.69 |
| VM [db%] | FC [db%] | Ash [db%] | ||
|---|---|---|---|---|
| KP | Measured [%] | 81.10 | 17.20 | 1.70 |
| Calculated [%] | 77.92 | 19.47 | 2.61 | |
| Difference [%p] | 3.18 | −2.27 | −0.91 | |
| LP | Measured [%] | 83.20 | 15.20 | 1.60 |
| Calculated [%] | 79.92 | 17.20 | 2.88 | |
| Difference [%p] | 3.28 | −2.00 | −1.28 | |
| PC | Measured [%] | 84.60 | 14.40 | 1.00 |
| Calculated [%] | 78.51 | 18.50 | 2.99 | |
| Difference [%p] | 6.09 | −4.10 | −1.99 | |
| LT | Measured [%] | 83.20 | 15.20 | 1.60 |
| Calculated [%] | 78.59 | 18.29 | 3.13 | |
| Difference [%p] | 4.61 | −3.09 | −1.53 |
| Name | CV | Name | CV | Name | CV | Name | CV |
|---|---|---|---|---|---|---|---|
| KPO310 A | 28.96 (S) | LPO310A | 27.98 (S) | PCO310 A | 29.01 (S) | LTO310 A | 27.86 (S) |
| KP310A | 27.86 (S) | LPO290 B | 27.23 (S) | PC310 B | 28.11 (S) | LT310 A | 27.54 (S) |
| KPO290B | 27.17 (S) | LP310 B | 26.74 (S) | PCO290 C | 27.05 (S) | LTO290 B | 24.05 (B) |
| KPO270C | 26.46 (S) | LP290 C | 25.80 (S) | PCO270 D | 26.13 (S) | LT290 B | 24.00 (B) |
| KP290D | 24.05 (B) | LPO270 D | 24.88 (B) | PC290 E | 25.11 (B) | LTO270 C | 23.01 (A) |
| KPO250E | 23.26 (A) | LP270 E | 22.64 (A) | PCO250F | 22.83 (A) | LT270 D | 22.19 (A) |
| KP270E | 23.01 (A) | LPO250 F | 21.99 (A) | PC270 F, G | 22.72 (A) | LTO250 E | 21.60 (A) |
| KP250F | 21.60 (A) | LPO230 G | 21.42 (A) | PCO230 G | 22.26 (A) | LT250 F | 20.39 (-) |
| KPO230G | 21.05 (A) | LP250 G | 21.31 (A) | PC250 H | 21.66 (A) | LTO230 F | 20.08 (-) |
| KP230H | 20.08 (-) | LP230 H | 19.97 (-) | PC230 I | 19.94 (-) | LT230 F | 19.94 (-) |
| Temp (℃) | CV | CI | VI | EY | Result | CV | CI | VI | EY | Result | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RAW | KP | - | - | O | - | - | LT | - | - | O | - | - | |
| Oxygen-lean | 230 | - | - | O | - | - | - | - | O | - | - | ||
| 250 | A | - | O | O | A | - | - | X | - | - | |||
| 270 | A | - | O | X | - | A | - | O | O | A | |||
| 290 | B | - | O | O | B | B | - | O | O | B | |||
| 310 | S | - | O | O | S | S | - | O | O | S | |||
| Oxygen-rich | 230 | A | - | O | O | A | - | - | X | - | - | ||
| 250 | A | - | O | O | - | A | - | O | O | A | |||
| 270 | S | - | O | O | S | A | CO | X | - | - | |||
| 290 | S | - | O | X | - | B | - | O | O | - | |||
| 310 | S | CO | O | X | CO | S | CO | O | - | CO | |||
| RAW | LP | - | - | O | - | PC | - | - | O | - | - | ||
| Oxygen-lean | 230 | - | - | O | - | - | - | O | - | - | |||
| 250 | A | - | O | O | A | A | - | O | O | A | |||
| 270 | A | - | O | X | A | - | O | X | - | ||||
| 290 | S | - | X | X | B | - | O | O | - | ||||
| 310 | S | - | O | O | S | S | CO | O | - | CO | |||
| Oxygen-rich | 230 | A | - | O | O | A | A | - | O | O | |||
| 250 | A | - | O | X | A | - | O | X | - | ||||
| 270 | B | - | O | O | B | B | CO | O | - | CO | |||
| 290 | S | CO | O | X | CO | B | CO | O | - | CO | |||
| 310 | S | CO | O | X | CO | S | CO | O | - | CO | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.Y.; Kim, S.J.; Oh, K.C.; Cho, L.H.; Jeon, Y.K.; Kim, D.H. Evaluation of the Optimal Conditions for Oxygen-Rich and Oxygen-Lean Torrefaction of Forestry Byproduct as a Fuel. Energies 2023, 16, 4763. https://doi.org/10.3390/en16124763
Park SY, Kim SJ, Oh KC, Cho LH, Jeon YK, Kim DH. Evaluation of the Optimal Conditions for Oxygen-Rich and Oxygen-Lean Torrefaction of Forestry Byproduct as a Fuel. Energies. 2023; 16(12):4763. https://doi.org/10.3390/en16124763
Chicago/Turabian StylePark, Sun Yong, Seok Jun Kim, Kwang Cheol Oh, La Hoon Cho, Young Kwang Jeon, and Dae Hyun Kim. 2023. "Evaluation of the Optimal Conditions for Oxygen-Rich and Oxygen-Lean Torrefaction of Forestry Byproduct as a Fuel" Energies 16, no. 12: 4763. https://doi.org/10.3390/en16124763




