Can Xylose Be Fermented to Biofuel Butanol in Continuous Long-Term Reactors: If Not, What Options Are There?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microbial Strain, Culture Maintenance & Propagation
2.2. Batch Fermentations
2.3. Continuous Fermentations
2.4. Quantitation of ABE Losses
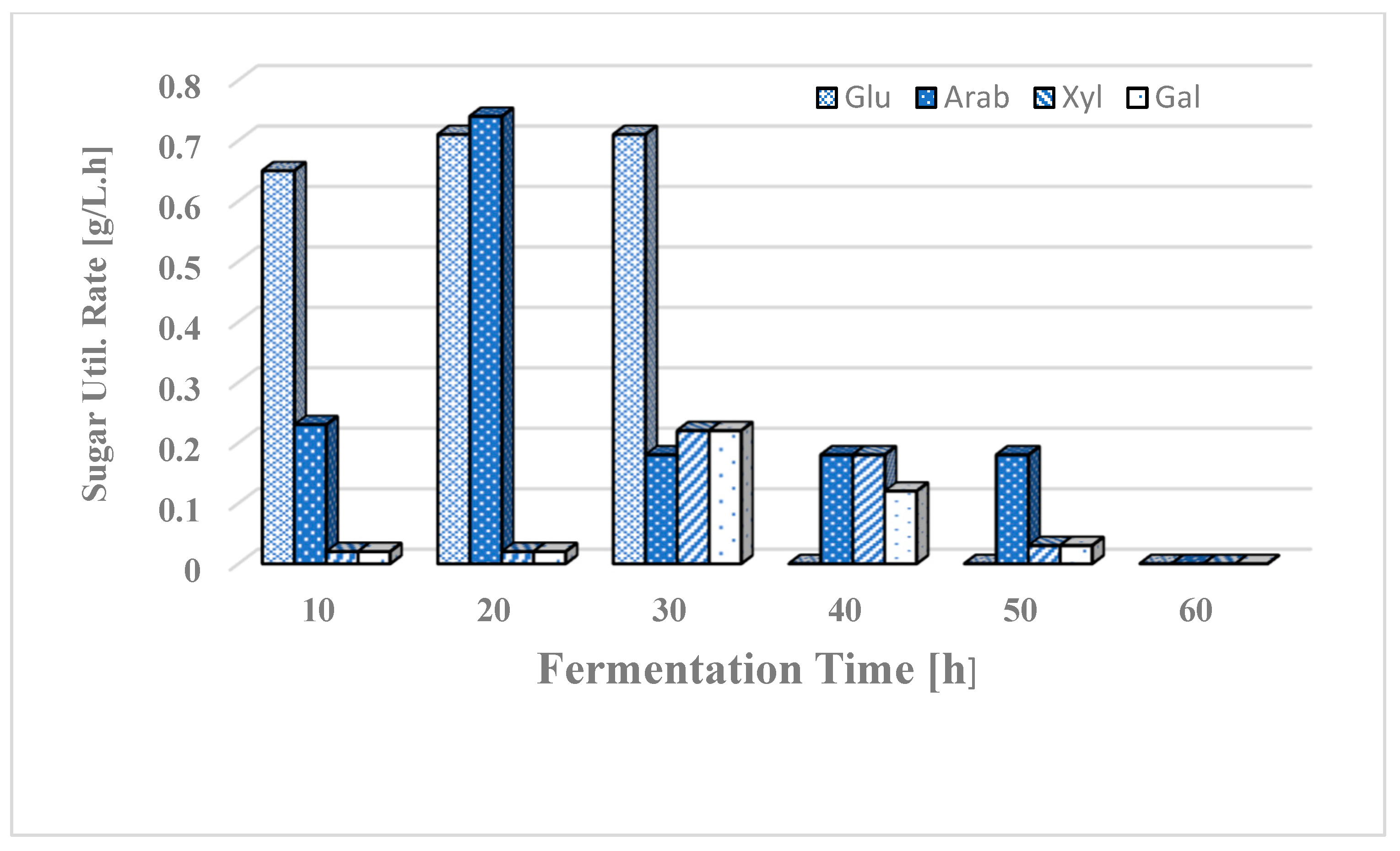
2.5. Measuring Rates of Sugar Utilization
2.6. Analyses
3. Results and Discussion
3.1. Control Glucose Batch Fermentation
3.2. Xylose Fermentation in Batch Reactor
3.3. Simultaneous Use of Glucose and Xylose
3.4. Xylose Fermentation and Simultaneous Product Recovery
3.5. Osmotic Stress Due to Concentrated Xylose
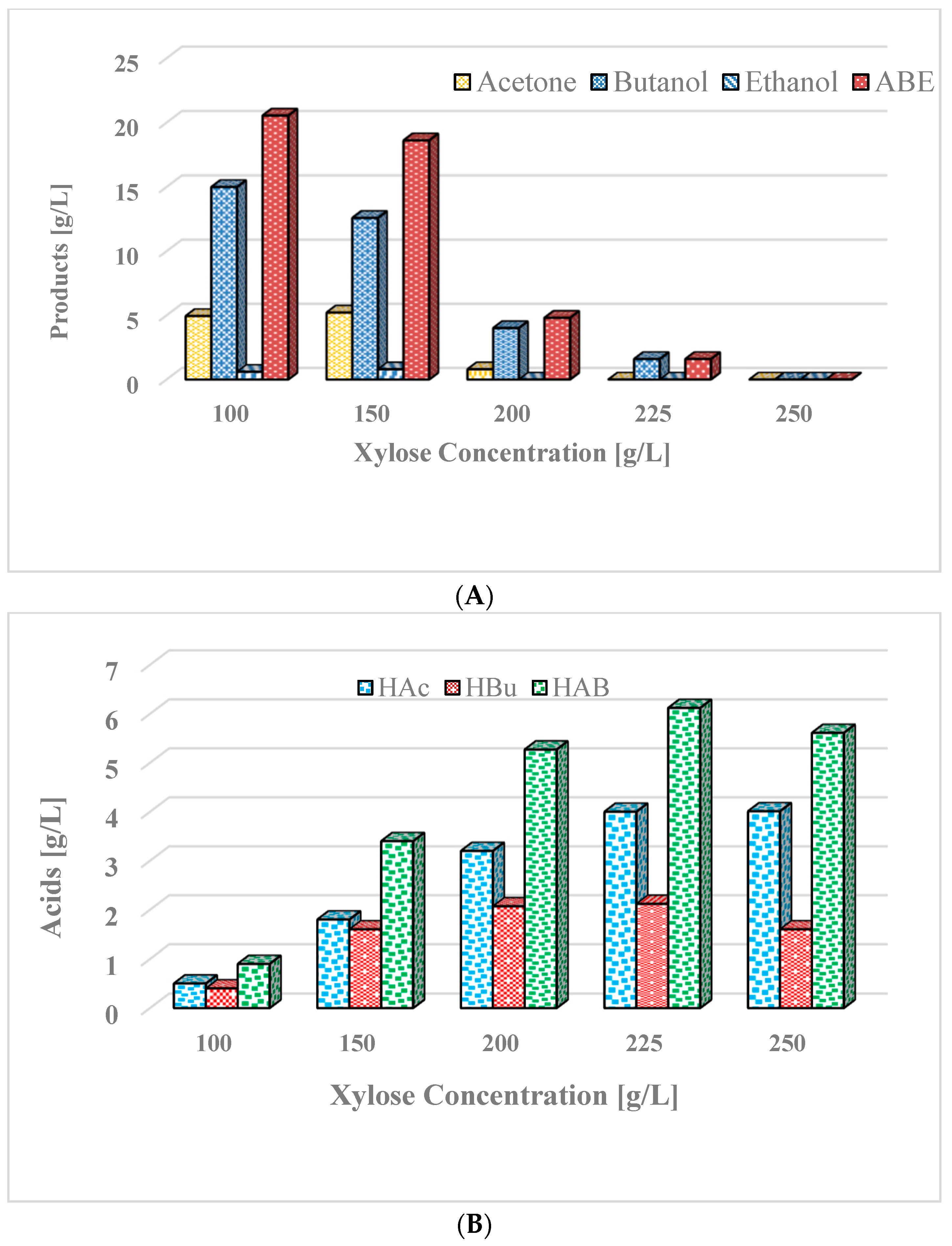
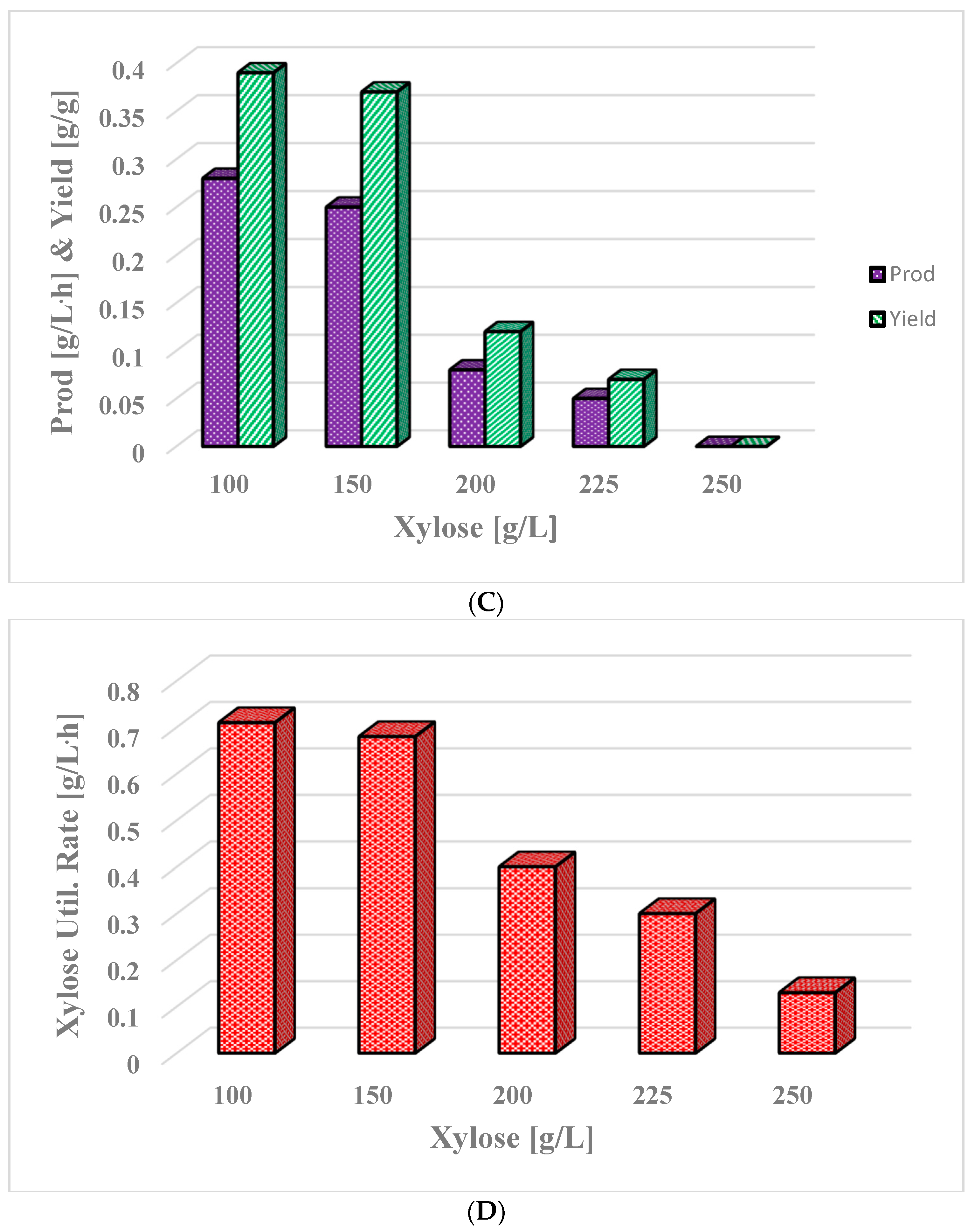
3.6. Butanol Production in Integrated Batch Reactor from Concentrated Xylose
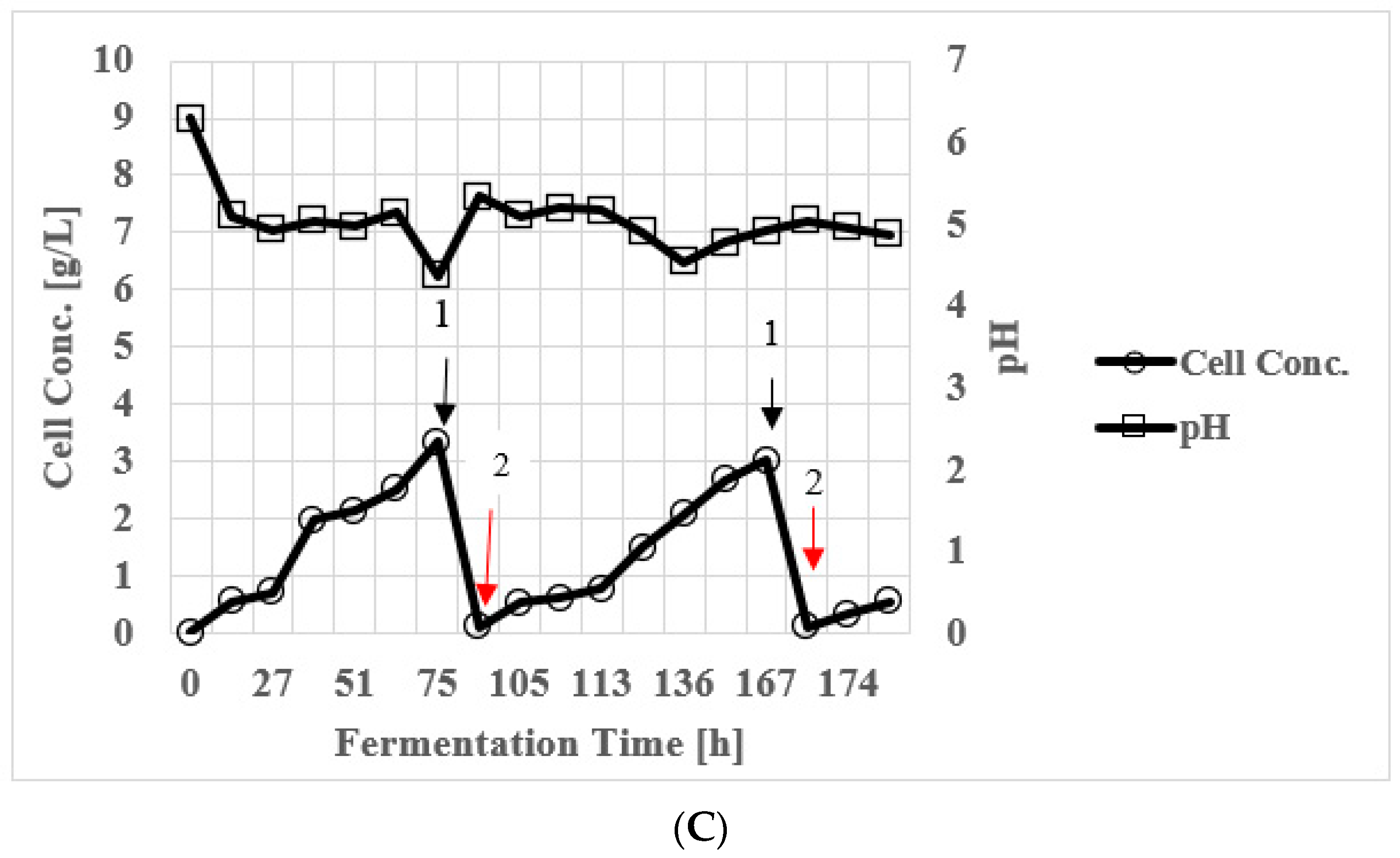
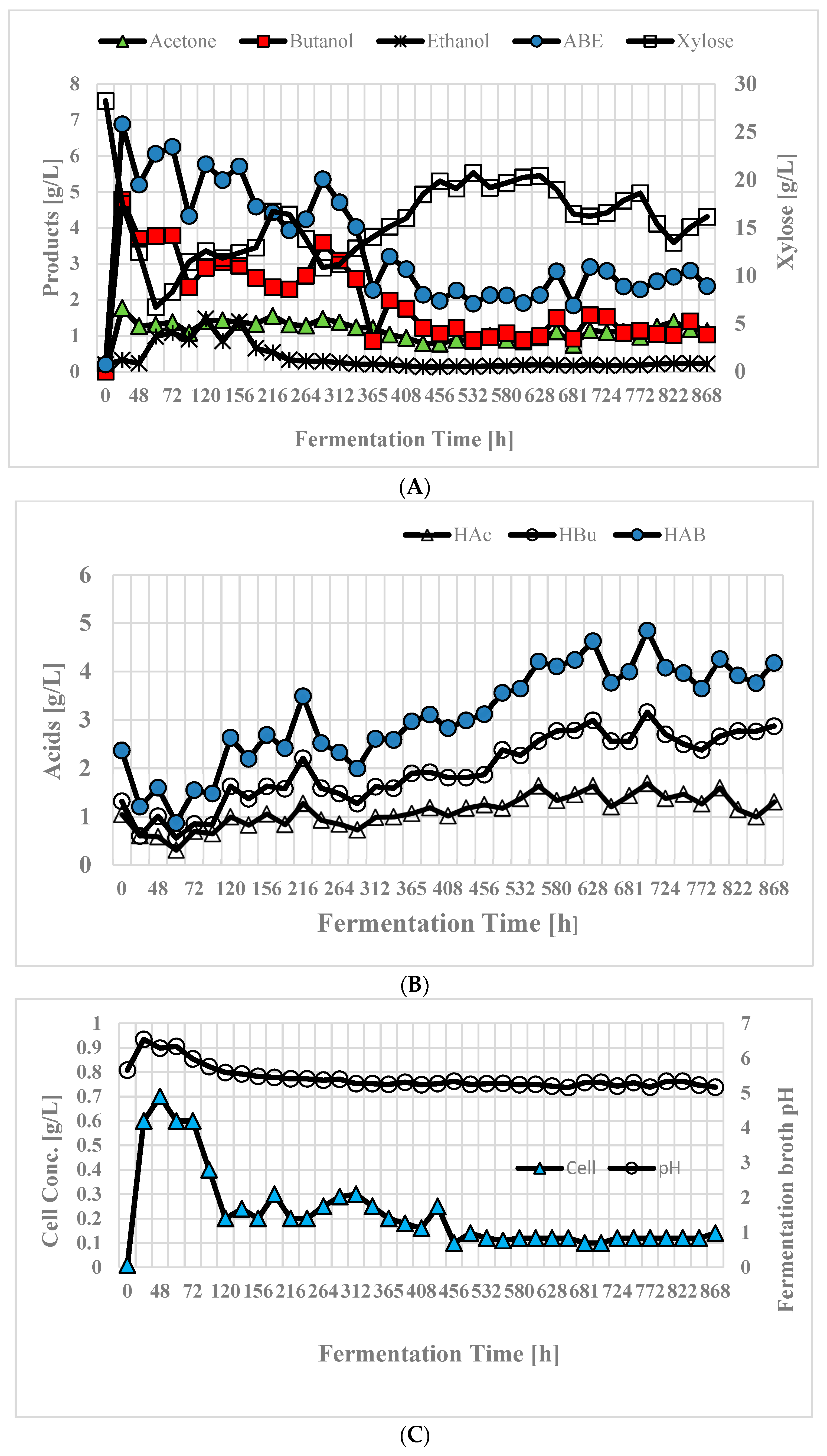
3.7. Butanol Production in Continuous Stirred Tank Reactor (CSTR)
3.8. Comparison with Other Xylose Fermentation Systems
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chow, C.-F.; Lam, C.-S.; Lau, K.-C.; Gong, C.-B. Waste-to-Energy: Production of fuel gases from plastic waste. Polymers 2021, 13, 3672. [Google Scholar] [CrossRef] [PubMed]
- AbdElhafez, S.E.; Taha, T.; Mansy, A.E.; El-Desouky, E.; Abu-Saied, M.A.; Eltaher, K.; Hamdy, A.; Fawal, G.L.; Gamal, A.; Hashim, A.M.; et al. Experimental optimization with the emphasis on techno-economic analysis of production and purification of high value-added bioethanol from sustainable corn stover. Energies 2022, 15, 6131. [Google Scholar] [CrossRef]
- Elsagan, Z.A.; Ali, R.M.; El-Naggar, M.A.; El-Ashtoukhy, E.-S.Z.; AbdElhafez, S.E. New perspectives for maximizing sustainable bioethanol production from corn stover. Ren. Energy 2023, 209, 608–618. [Google Scholar] [CrossRef]
- Qureshi, N. Solvent (Acetone-butanol: AB) production. In Reference Module in Life Sciences; Roitberg, B., Ed.; Elsevier: Oxford, UK, 2017; pp. 1–20. [Google Scholar]
- Buchanan, D.; Martindale, W.; Romeih, E.; Hebishy, E. Recent advances in whey processing and valorisation: Technological and environmental perspectives. Int. J. Dairy Technol. 2023, 76, 291–312. [Google Scholar] [CrossRef]
- Zheng, X.; Xian, X.; Hu, L.; Tao, S.; Zhang, X.; Liu, Y. Efficient short-time hydrothermal depolymerization of sugarcane bagasse in one-pot for cellulosic ethanol production without separation, water washing, and detoxification. Bioresour. Technol. 2021, 339, 125575. [Google Scholar] [CrossRef]
- Liu, Y.; Zheng, X.; Tao, S.; Hu, L.; Zhang, X.; Lin, X. Process optimization for deep eutectic solvent pretreatment and enzymatic hydrolysis of sugar cane bagasse for cellulosic ethanol fermentation. Renew. Energy 2021, 177, 259–267. [Google Scholar] [CrossRef]
- Mukherjee, M.; Goswami, G.; Mondal, P.K.; Das, D. Biobutanol as a potential alternative to petroleum fuel: Sustainable bioprocess and cost analysis. Fuel 2020, 278, 118403. [Google Scholar] [CrossRef]
- Kushwaha, D.; Srivastava, N.; Prasad, D.; Mishra, P.K.; Upadhyay, S.N. Biobutanol production from hydrolysates of cyanobacteria Lyngbya limnetica and Oscillatoria obscura. Fuel 2020, 271, 117583. [Google Scholar] [CrossRef]
- Dürre, P. New insights and novel developments in clostridial acetone/butanol/isopropanol fermentation. Appl. Microbiol. Biotechnol. 1998, 49, 639–648. [Google Scholar] [CrossRef]
- Zverlov, V.V.; Berezina, O.; Velikodvorskaya, G.A.; Schwarz, W.H. Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: Use of hydrolyzed agricultural waste for biorefinery. Appl. Microbiol. Biotechnol. 2006, 71, 587–597. [Google Scholar] [CrossRef]
- Jin, Q.; An, Z.H.; Damle, A.; Poe, N.; Wu, J.; Wang, H.J.; Wang, Z.W.; Huang, H.B. High Acetone-Butanol-Ethanol production from food waste by recombinant Clostridium saccharoperbutylacetonicum in batch and continuous immobilized-cell fermentation. ACS Sustain. Chem. Eng. 2020, 8, 9822–9832. [Google Scholar] [CrossRef]
- Jones, D.; Woods, D. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986, 50, 484–524. [Google Scholar] [CrossRef] [PubMed]
- Balat, M.; Ayar, G. Biomass Energy in the world, use of biomass and potential trends. Energy Sources 2005, 27, 931–940. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw hydrolysate using Clostridium beijerinckii. Bioprocess Biosyst. Eng. 2007, 30, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Biores. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Morais, A.R.C.; Zhang, J.; Dong, H.; Otto, W.G.; Mokomele, T.; Hodge, D.; Balan, V.; Dale, B.E.; Lukasik, R.M.; da Costa Sousa, L. Development of an ammonia pretreatment that creates synergies between biorefineries and advanced biomass logistics models. Green Chem. 2022, 24, 4443–4462. [Google Scholar] [CrossRef]
- Qureshi, N.; Klasson, K.T.; Saha, B.C.; Liu, S. Butanol production from sweet sorghum bagasse (SSB) with high solids content: Part I—Comparison of hot water pretreatment with dilute sulfuric acid. Biotechnol. Prog. 2018, 34, 960–966. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Liu, S.; Ezeji, T.C.; Nichols, N.N. Cellulosic butanol biorefinery: Production of biobutanol from high solid loadings of sweet sorghum bagasse—Simultaneous saccharification, fermentation and recovery. Fermentation 2021, 7, 310. [Google Scholar] [CrossRef]
- Qureshi, N.; Saha, B.C.; Cotta, M.A. Butanol production from wheat straw by simultaneous saccharification and fermentation using Clostridium beijerinckii: Part II-Fed batch fermentation. Biomass Bioenergy 2008, 32, 176–183. [Google Scholar] [CrossRef]
- Friedl, A.; Qureshi, N.; Maddox, I.S. Continuous acetone-butanol-ethanol (ABE) fermentation using immobilised cells of Clostridium acetobutylicum in a packed bed reactor and integration with product removal by pervaporation. Biotechnol. Bioeng. 1991, 38, 518–527. [Google Scholar] [CrossRef]
- Mawson, A.J. Consideration of the fermentation of concentrated whey solution for ethanol production. In Fermentation Technologies: Industrial Applications; Yu, P.L., Ed.; Elsevier Applied Science: London, UK, 1990; pp. 264–270. [Google Scholar]
- Aquino, R.D. Biobutanol on the horizon. Chem. Eng. Prog. 2007, 103, 10. [Google Scholar]
- Ennis, B.; Marshall, C.; Maddox, I.S.; Paterson, A. Continuous product recovery by in-situ gas stripping/condensation during solvent production from whey permeate using Clostridium acetobutylicum. Biotechnol. Lett. 1986, 8, 725–730. [Google Scholar] [CrossRef]
- Maddox, I.S. The acetone-butanol-ethanol fermentation: Recent progress in technology. Biotechnol. Genet. Eng. Rev. 1989, 7, 189–220. [Google Scholar] [CrossRef]
- Groot, W.; van der Lans, R.; Luyben, K.C.A.M. Technologies for butanol recovery integrated with fermentations. Process Biochem. 1992, 27, 61–75. [Google Scholar] [CrossRef]
- Garcia, A.; Innotti, E.L.; Fischer, J.L. Butanol fermentation liquor production and separation by reverse osmosis. Biotechnol. Bioeng. 1986, 28, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tsai, G.-J.; Tsao, G.T. Enhancement of in situ adsorption on the acetone-butanol fermentation by Clostridium acetobutylicum. Sep. Technol. 1994, 4, 81–92. [Google Scholar] [CrossRef]
- Roffler, S.; Blanch, H.; Wilke, C. In-situ recovery of butanol during fermentation—Part 1: Batch extractive fermentation. Bioprocess Eng. 1987, 2, 1–12. [Google Scholar] [CrossRef]
- Alsaker, K.V.; Spitzer, T.R.; Papoutsakis, E.T. Transcriptional analysis of spo0A overexpression in Clostridium acetobutylicum and its effect on the cell’s response to butanol stress. J. Bacteriol. 2004, 186, 1959–1971. [Google Scholar] [CrossRef] [Green Version]
- Annous, B.; Blaschek, H. Isolation and characterization of α-amylase derived from starchgrown Clostridium acetobutylicum ATCC 824. J. Ind. Microbiol. Biotechnol. 1994, 13, 10–16. [Google Scholar]
- Formanek, J.; Mackie, R.; Blaschek, H. Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl. Environ. Microbiol. 1997, 63, 2306–2310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zong, W.; Hong, W.; Zhang, Z.-T.; Wang, Y. Exploiting CRISPR-Cas system for multiplex genome editing in Clostridium tyrobutyricum and engineer the strain for high-level butanol production. Metab. Eng. 2018, 47, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Blaschek, H.P. Butanol recovery from model solution/fermentation broth by pervaporation: Evaluation of membrane performance. Biomass Bioenergy 1999, 17, 175–184. [Google Scholar] [CrossRef]
- Qureshi, N.; Li, X.; Hughes, S.R.; Saha, B.C.; Cotta, M.A. Production of acetone butanol ethanol from corn fiber xylan using Clostridium acetobutylicum. Biotechnol. Prog. 2006, 22, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.; Liu, S.; Harry-O’kuru, R. Production of acetone–butanol–ethanol (ABE) from concentrated yellow top presscake using Clostridium beijerinckii P260. J. Chem. Technol. Biotechnol. 2020, 95, 614–620. [Google Scholar] [CrossRef]
- Qureshi, N.; Paterson, A.H.J.; Maddox, I.S. Model for continuous production of solvents from whey permeate in a packed bed reactor using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Appl. Microbiol. Biotechnol. 1988, 29, 323–328. [Google Scholar] [CrossRef]
- Huang, W.-C.; Ramey, D.E.; Yang, S.-T. Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed bioreactor. Appl. Biochem. Biotechnol. 2004, 113–116, 887–898. [Google Scholar] [CrossRef] [PubMed]
- Xin, F.; Wu, Y.R.; He, J. Simultaneous fermentation of glucose and xylose to butanol by Clostridium sp. strain BOH3. Appl. Environ. Microbiol. 2014, 80, 4771–4778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Tashiro, Y.; Yoshida, T.; Gao, M.; Wang, Q.; Sonomoto, K. Continuous butanol fermentation from xylose with high cell density by cell recycling system. Bioresour. Technol. 2013, 129, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Li, Z.; Jiang, Y.; Yang, Y.; Jiang, W.; Gu, Y.; Yang, S. Metabolic engineering of D-xylose pathway in Clostridium beijerinckii to optimize solvent production from xylose mother liquid. Metab. Eng. 2012, 14, 569–578. [Google Scholar] [CrossRef]

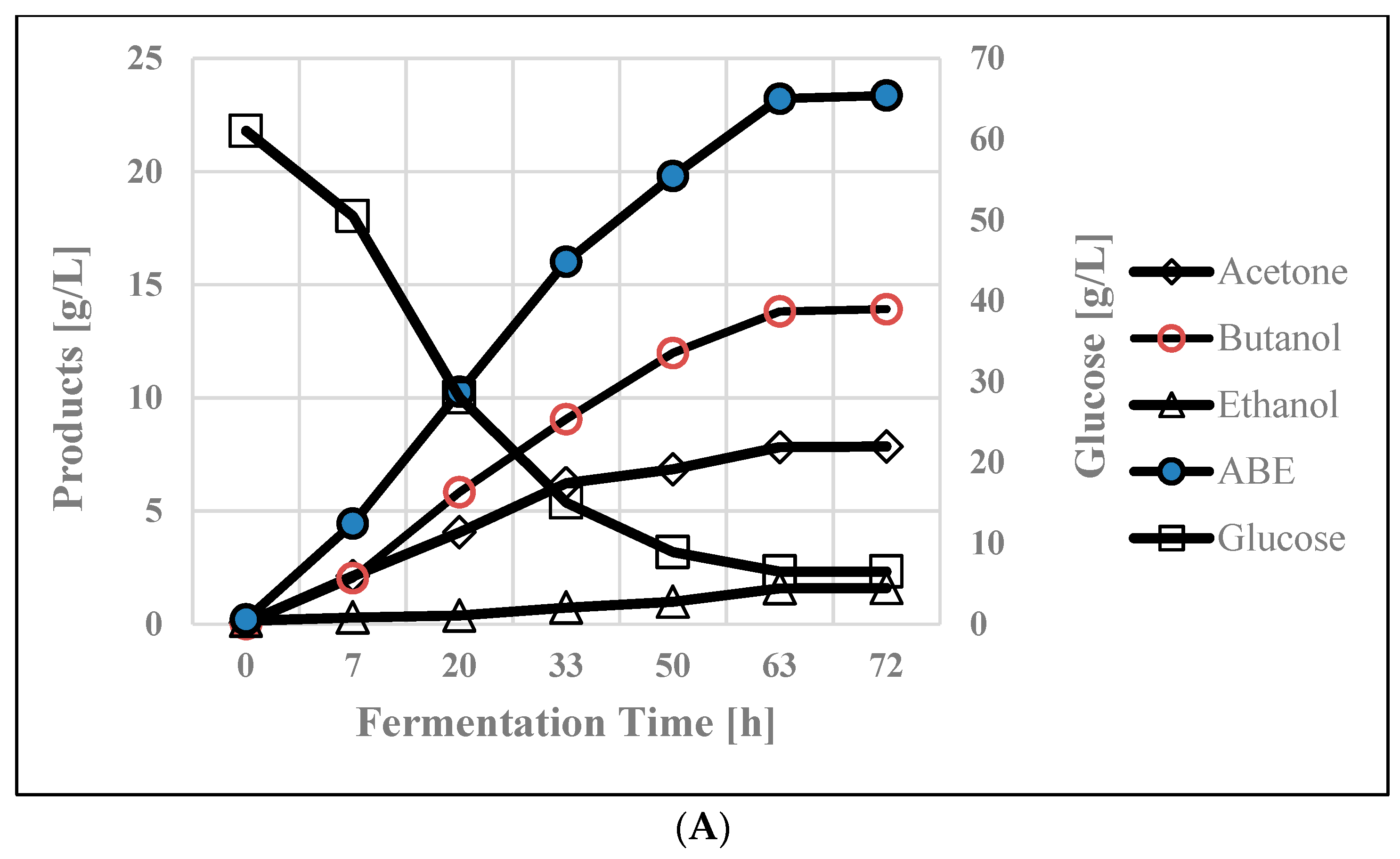
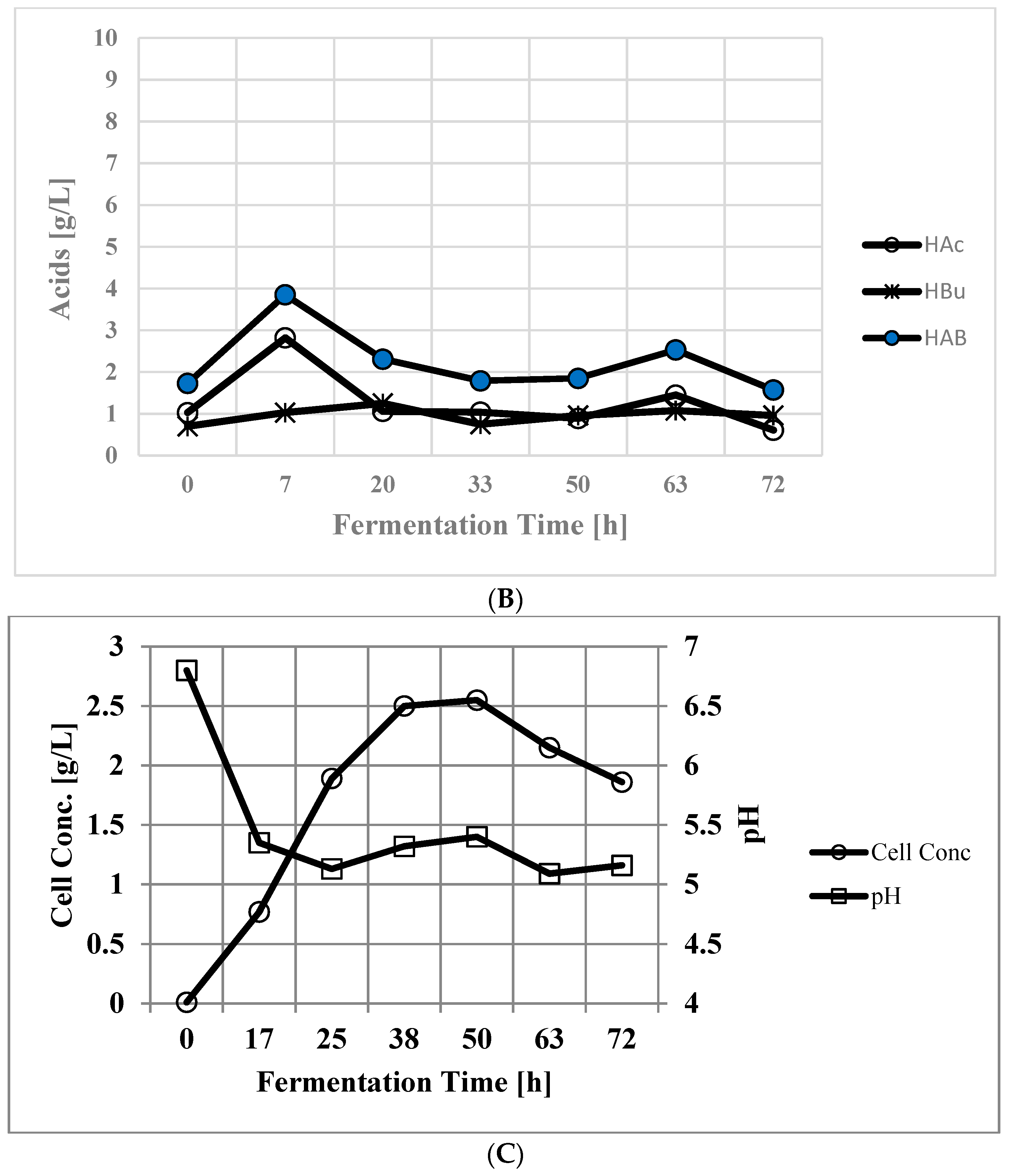
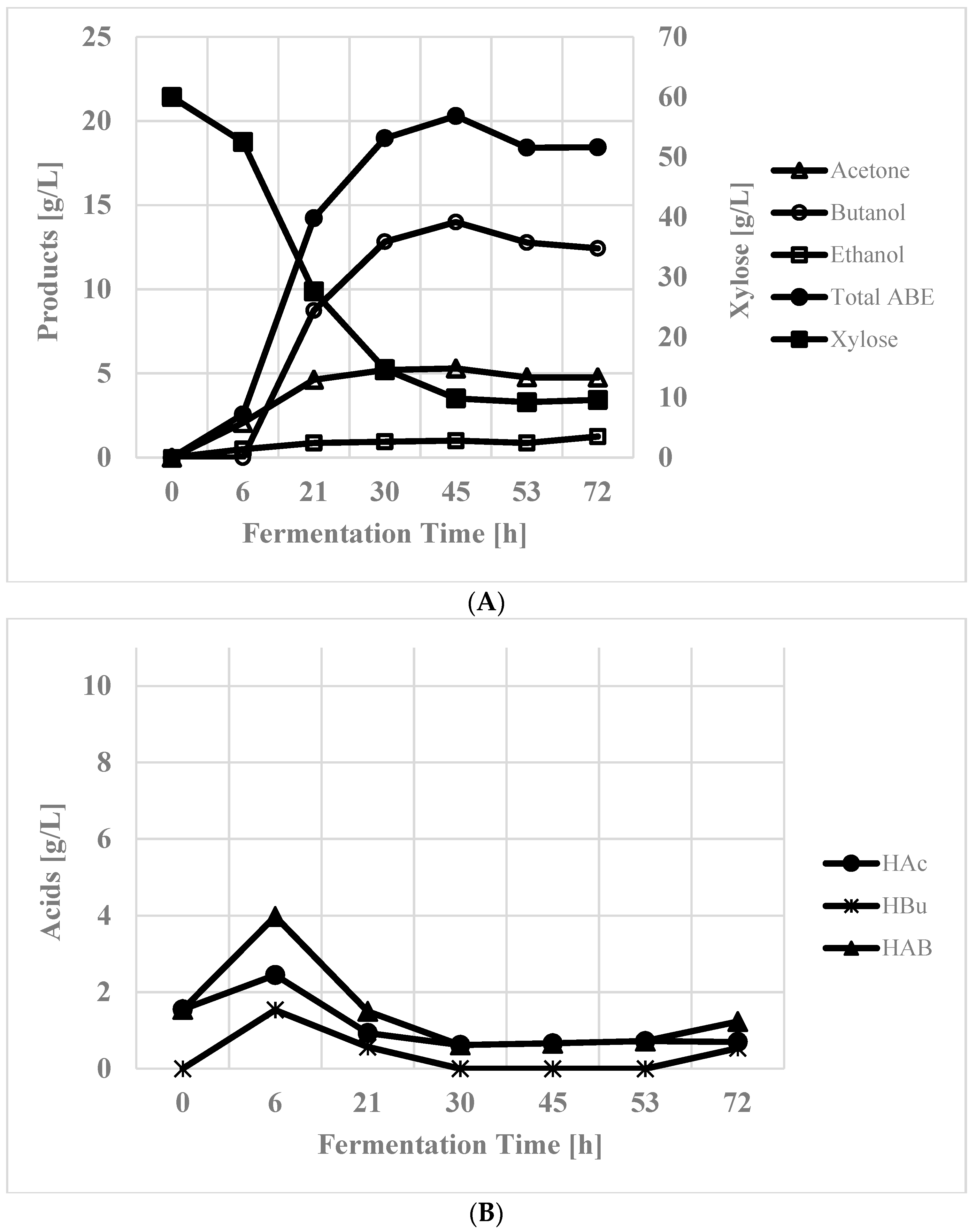
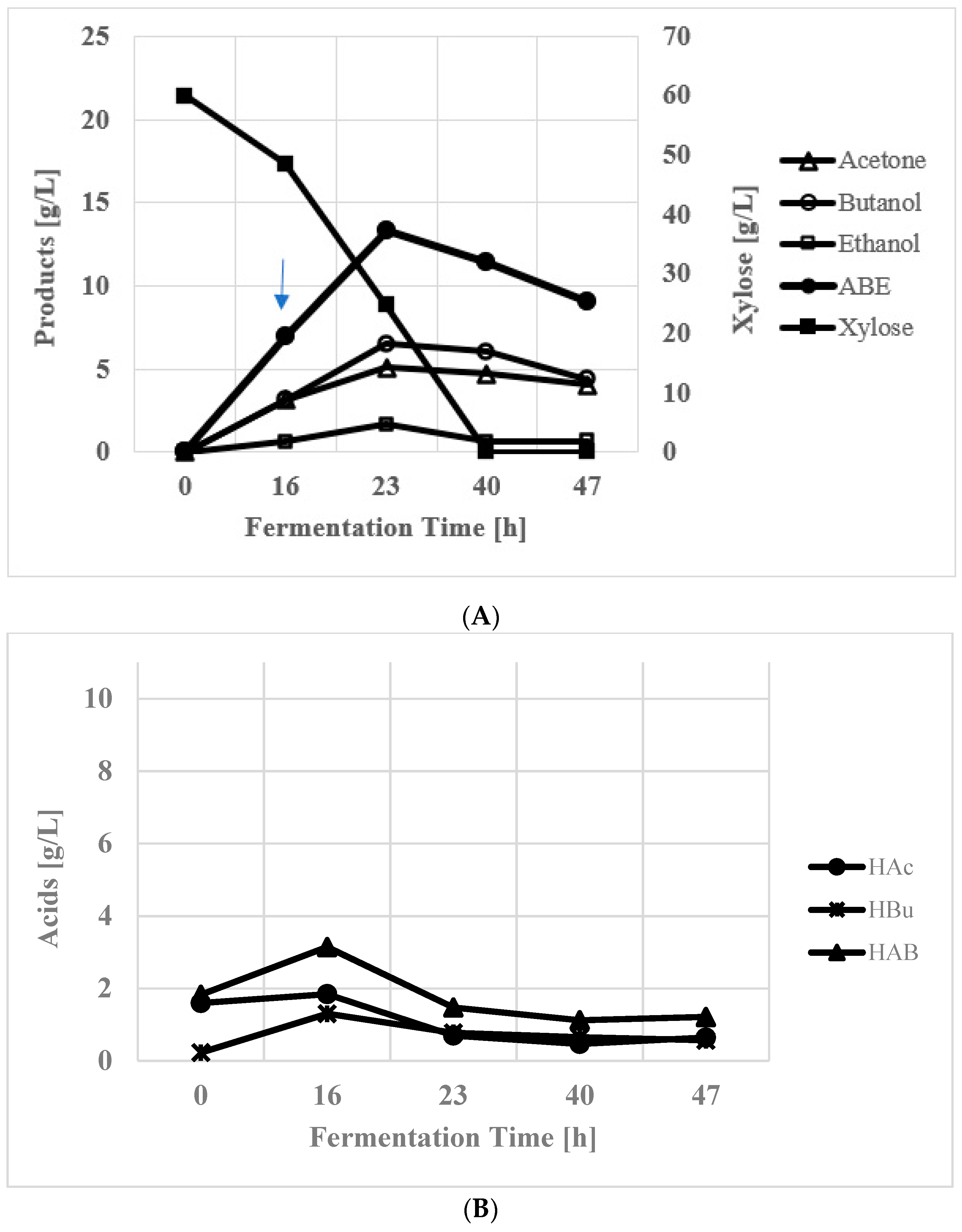

| Time [h] | Cond. vol [mL] | Acetone [g/L] | Butanol [g/L] | Ethanol [g/L] | ABE [g/L] |
|---|---|---|---|---|---|
| 23 | 24 | 23.00 | 59.00 | 3.84 | 85.84 |
| 40 | 103 | 27.59 | 68.31 | 1.69 | 97.59 |
| 47 | 44 | 16.00 | 35.55 | 2.10 | 53.65 |
| Exp. | Volume [mL] | Centrifuged | pH Adjusted | YE Added [g/L] | Stocks Added [mL/L] | Anaerobic Condition Created | Inoculated with New Inoculum | ABE Produced [g/L] |
|---|---|---|---|---|---|---|---|---|
| Processing after 51 h when fermentation ceased | ||||||||
| A | 10 | No | 5.2 | No | No | Yes | Yes | 4.64 |
| B | 10 | Yes 1 | 6.0 | Yes, 1.0 | Yes, 10 | Yes | Yes | 25.65 |
| Processing after 167 h when fermentation ceased | ||||||||
| C | 10 | No | 5.2 | No | No | Yes | Yes | 5.16 |
| D | 10 | Yes 1 | 6.0 | Yes, 1.0 | Yes, 10 | Yes | Yes | 23.21 |
| E | 10 | Yes 1 | 6.0 | Yes, 0.5 | Yes, 5 | Yes | Yes | 19.56 |
| F | 10 | Yes 1 | 6.0 | No | No | Yes | Yes | 5.62 |
| Time [h] | Cond. vol. [mL] | Acetone [g/L] | Butanol [g/L] | Ethanol [g/L] | ABE [g/L] |
|---|---|---|---|---|---|
| 43 | 93 | 21.07 | 31.80 | 2.11 | 54.98 |
| 51 | 48 | 22.84 | 30.71 | 1.83 | 55.38 |
| 68 | 109 | 19.98 | 24.02 | 1.75 | 45.75 |
| 75 | 44 | 14.78 | 13.92 | 1.07 | 29.77 |
| 92 | 99 | 6.84 | 5.85 | 0.00 | 12.69 |
| 113 | 32 | 22.33 | 25.09 | 0.55 | 47.97 |
| 129 | 100 | 33.30 | 53.49 | 0.94 | 87.73 |
| 136 | 50 | 19.31 | 23.81 | 0.50 | 43.62 |
| 153 | 91 | 11.98 | 16.18 | 0.85 | 29.01 |
| Experiment/ Substrate | Initial xyl [g/L] | Residual xyl [g/L] | ABE Produced [g/L] | Productivity [g/L·h] | Yield [g/g] | Simultaneous Product Recovery |
|---|---|---|---|---|---|---|
| Control glu/ Experiment 1 | 61.0 * | 6.5 * | 23.40 | 0.33 | 0.43 | No |
| Experiment 2/ xylose | 60.0 | 9.8 | 18.44 b | 0.26 b | 0.40 | No |
| Experiment 3/ xylose | 60.0 | 0.0 | 26.50 b | 0.66 b | 0.44 | Yes |
| Experiment 4/ xylose | 100.0–250.0 | 52.5–243.0 | 20.4–0.00 | 0.28–0.00 | 0.43–0.00 | No |
| Experiment 5/ xylose | 150.0 | 0.00 | 66.30 b | 0.44 b | 0.44 | Yes |
- Significant F-tests from ANOVA were obtained indicating differences between the runs for total ABE (p < 0.0001) and ABE productivity (p < 0.0001).
- b Experiment comparisons within a column that are significantly different than the control at the 0.05 level based on t-tests. Total ABE produced in experiment 5 is different than experiment 1 (control). Productivity in experiment 3 and 5 are different than experiment 1.
- a Some of the results presented in this summary table were taken from tables and figures.
- * glu—glucose was used in the control experiment.
- xyl—xylose.
- Experiment 1 was glucose control. Experiment 2 was xylose fermentation without recovery. Experiment 3 xylose fermentation with simultaneous product recovery. Experiment 4 was 100–250 g/L xylose fermentation (no product recovery) and Experiment 5 was 150 g/L xylose fermentation with simultaneous product recovery.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qureshi, N.; Lin, X.; Tao, S.; Liu, S.; Huang, H.; Nichols, N.N. Can Xylose Be Fermented to Biofuel Butanol in Continuous Long-Term Reactors: If Not, What Options Are There? Energies 2023, 16, 4945. https://doi.org/10.3390/en16134945
Qureshi N, Lin X, Tao S, Liu S, Huang H, Nichols NN. Can Xylose Be Fermented to Biofuel Butanol in Continuous Long-Term Reactors: If Not, What Options Are There? Energies. 2023; 16(13):4945. https://doi.org/10.3390/en16134945
Chicago/Turabian StyleQureshi, Nasib, Xiaoqing Lin, Shunhui Tao, Siqing Liu, Haibo Huang, and Nancy N. Nichols. 2023. "Can Xylose Be Fermented to Biofuel Butanol in Continuous Long-Term Reactors: If Not, What Options Are There?" Energies 16, no. 13: 4945. https://doi.org/10.3390/en16134945







