Comparison and Evaluation of Transport Property Prediction Performance of Supercritical Hydrocarbon Aviation Fuels and Their Pyrolyzed Products via Endothermic Reactions
Abstract
:1. Introduction
2. Prediction Methods
3. Prediction Condition and Hydrocarbon Fuels
3.1. Prediction Condition and Comparison Method
3.2. Hydrocarbon Fuels
4. Results and Discussion
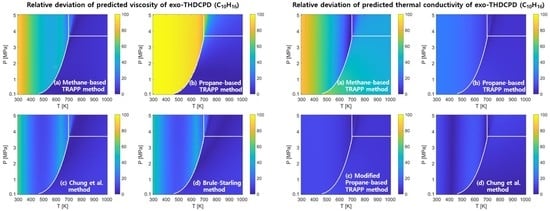
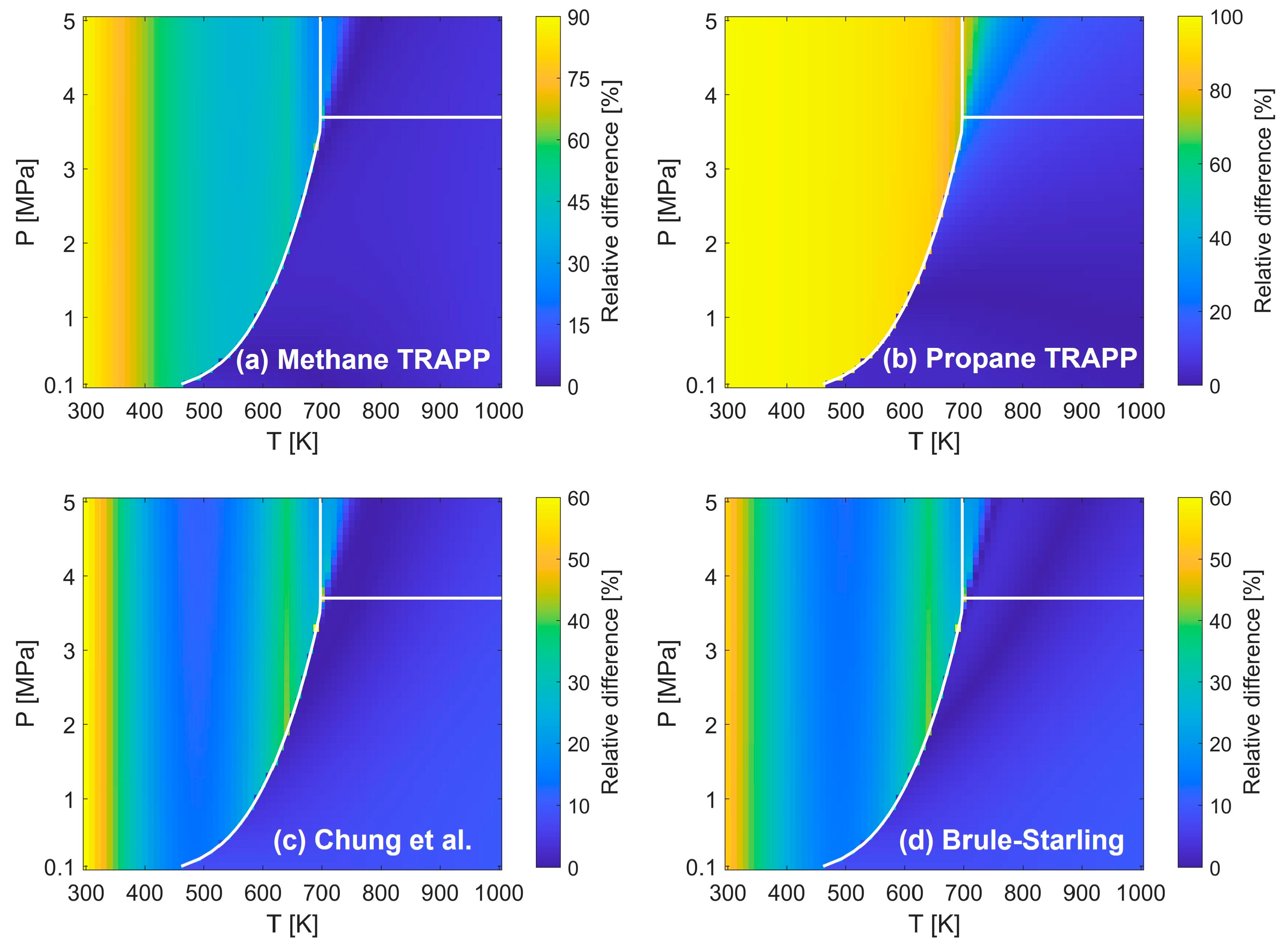
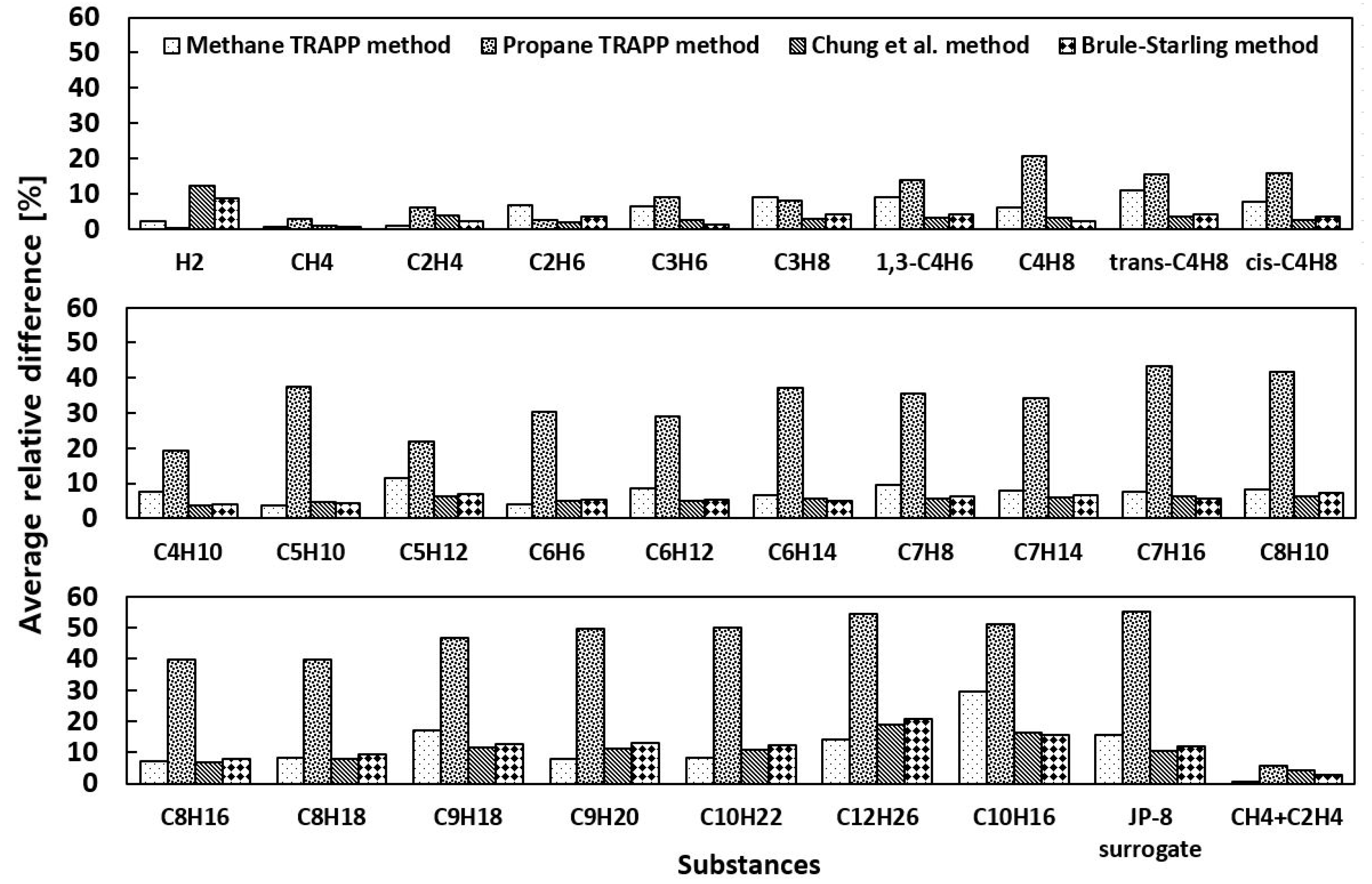
4.1. Viscosity
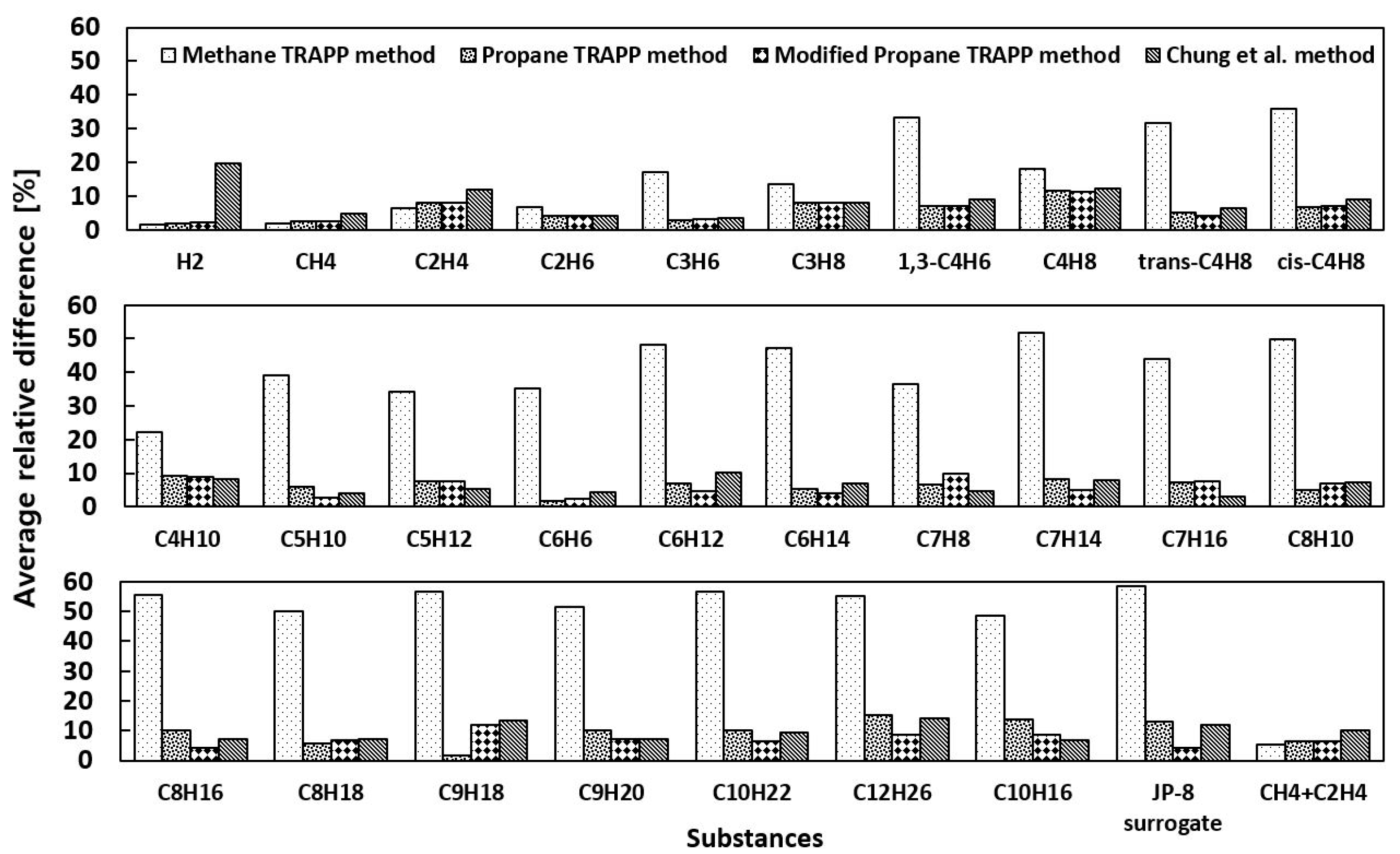
4.2. Thermal Conductivity
4.3. Discussion
5. Summary and Conclusions
- The viscosity of low molecular weight substances, including hydrogen, methane, ethylene, and their mixture, is predicted much more accurately by the TRAPP methods, while that of most high molecular weight hydrocarbons is estimated accurately by both the Chung et al. and Brule-Starling methods.
- The comparison of the total average values concludes that the Chung et al. and Brule-Starling methods are best for the prediction of the viscosity of all substances, ranging from hydrogen to the low and high molecular weight hydrocarbons, in the temperature and pressure ranges specified in the present study.
- The quantified comparison by total average difference from the NIST values confirms that Modified Propane TRAPP best predicts the thermal conductivity of all of the 29 substances over the temperature and pressure ranges, and the Propane TRAPP and Chung et al. methods show very little difference.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| P | Pressure |
| Pc | Critical pressure |
| T | Temperature |
| Tc | Critical temperature |
| Tr | Reduced temperature |
| v | Specific volume |
| Xλ | Translational correction factor for thermal conductivity |
| Zc | Critical compressibility factor |
| Greek symbols | |
| η | Viscosity |
| λ | Thermal conductivity |
| κ | Association parameter |
| μr | Dimensionless dipole moment |
| ω | Acentric factor |
References
- Monkam, L.K.; Schweinitz, A.G.; Friedrichs, J.; Gao, X. Feasibility analysis of a new thermal insulation concept of cryogenic fuel tanks for hydrogen fuel cell powered commercial aircraft. Int. J. Hydrogen Energy 2022, 47, 31395–31408. [Google Scholar] [CrossRef]
- Melo, S.P.; Toghyani, S.; Cerdas, F.; Liu, X.; Gao, X.; Lindner, L.; Barke, A.; Thies, C.; Spengler, T.S.; Hermann, C. Model-based assessment of the environmental impacts of fuel cell systems designed for eVTOLs. J. Hydrogen Energy 2023, 48, 3171–3187. [Google Scholar] [CrossRef]
- Keller, M.; Hulinska, S.; Kraus, J. Integration of UAM into Cities-The Public View. Transp. Res. Procedia 2021, 59, 137–143. [Google Scholar] [CrossRef]
- Sziroczak, D.; Smith, H. A review of design issues specific to hypersonic flight vehicles. Prog. Aerosp. Sci. 2016, 84, 1–28. [Google Scholar] [CrossRef] [Green Version]
- Van Wie, D.M.; D’Alessio, S.M.; White, M.E. Hypersonic Airbreathing Propulsion. Johns Hopkins APL Tech. Dig. 2005, 4, 430–437. [Google Scholar]
- Glass, D.E. Ceramic Matrix Composite (CMC) Thermal Protection Systems (TPS) and Hot Structures for Hypersonic Vehicles. In Proceedings of the AIAA International Space Planes and Hypersonic Systems and Technologies Conference, Dayton, OH, USA, 28 April–1 May 2008. [Google Scholar]
- Gasner, J.A.; Foster, R.C.; Fujimura, C. Evaluation of thermal management for a Mach 5.5 hypersonic vehicle. In Proceedings of the AIAA 28th Joint Propulsion Conference and Exhibit, Nashville, TN, USA, 6–8 July 1992. AIAA 92-3721. [Google Scholar]
- Edwards, T. USAF Supercritical hydrocarbon fuels interest. In Proceedings of the AIAA 31st Aerospace Sciences Meeting, Reno, NV, USA, 11–14 January 1993. AIAA 93-0807. [Google Scholar]
- Cooper, M.; Shepherd, J.E. Experiments studying thermal cracking, catalytic cracking, and pre-mixed partial oxidation of JP-10. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003. AIAA 2003-4687. [Google Scholar]
- Rao, P.N.; Kunzru, D. Thermal cracking of JP-10: Kinetics and products distribution. J. Anal. Appl. Pyrolysis 2004, 76, 154–160. [Google Scholar]
- Huang, D.; Wu, Z.; Sunden, B.; Li, W. A brief review on convection heat transfer of fluids at supercritical pressures in tubes and the recent progress. Appl. Energy 2016, 162, 494–505. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Zuo, J.; Qin, J.; Cheng, K.; Feng, Y.; Bao, W. Research progress on active thermal protection for hypersonic vehicles. Prog. Aerosp. Sci. 2020, 119, 100646. [Google Scholar] [CrossRef]
- Feng, Y.; Qin, J.; Zhang, S.; Bao, W.; Cao, Y.; Huang, H. Modeling and analysis of heat and mass transfers of supercritical hydrocarbon fuel with pyrolysis in mini-channel. Int. J. Heat Mass Transf. 2015, 91, 520–531. [Google Scholar] [CrossRef]
- Jiang, R.; Liu, G.; Zhang, X. Thermal cracking of hydrocarbon aviation fuels in regenerative cooling microchannels. Energy Fuels 2013, 27, 2563–2577. [Google Scholar] [CrossRef]
- Guo, Y.; Bi, Q.; Liu, Z.; Yang, Z.; Jiang, L. Experimental investigation of thermal-hydraulic characteristics of endothermic hydrocarbon fuel in 1mm and 2mm diameter mini-channels. Appl. Therm. Eng. 2017, 122, 420–428. [Google Scholar] [CrossRef]
- Li, S.; Wang, Y.; Dong, M.; Pu, H.; Jiao, S.; Shang, Y. Experimental investigation on flow and heat transfer instabilities of RP-3 aviation kerosene in a vertical miniature tube under supercritical pressures. Appl. Therm. Eng. 2019, 149, 73–84. [Google Scholar] [CrossRef]
- Fan, X.; Yu, G.; Li, J.; Lu, X.; Zhang, X.; Sung, C.-J. Combustion and ignition of thermally cracked kerosene in supersonic model combustors. J. Propuls. Power 2007, 23, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Yu, G.; Li, J.; Zhang, X.; Sung, C.-J. Investigation of vaporized kerosene injection and combustion in a supersonic model combustor. J. Propuls. Power 2006, 22, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Z.; Wang, Z.; Sun, M. Effects of fuel cracking on combustion characteristics of a supersonic model combustor. Acta Astronaut. 2015, 110, 1–8. [Google Scholar] [CrossRef]
- Lemmon, E.W.; Bell, I.H.; Huber, M.L.; McLinden, M.O. REFPROP Documentation. Release 10.0; NIST: Gaithersburg, MD, USA, 2018.
- Huber, M.L. NIST Thermophysical Properties of Hydrocarbon Mixtures Database (SUPERTRAPP) Version 3.2 User’s Guide; NIST: Gaithersburg, MD, USA, 2007.
- Pu, H.; Li, S.; Jiao, S.; Dong, M.; Shang, Y. Numerical investigation on convective heat transfer to aviation kerosene flowing in vertical tubes at supercritical pressures. Int. J. Heat Mass Transf. 2018, 118, 857–871. [Google Scholar] [CrossRef]
- Dinda, S.; Vuchuru, K.; Konda, S.; Uttatavalli, A.N. Heat management in supersonic/hypersonic vehicles using endothermic fuel: Perspective and challenges. ACS Omega 2021, 6, 26741–26755. [Google Scholar] [CrossRef]
- Luo, S.; Xu, D.; Song, J.; Liu, J. A review of regenerative cooling technologies for scramjets. Appl. Therm. Eng. 2021, 190, 116754. [Google Scholar] [CrossRef]
- Zhu, J.; Tao, Z.; Deng, H.; Wang, K.; Yu, X. Numerical investigation of heat transfer characteristics and flow resistance of kerosene RP-3 under supercritical pressure. Int. J. Heat Mass Transf. 2015, 91, 330–341. [Google Scholar] [CrossRef]
- Kim, J.S.; Seo, J.; Han, D.; Kim, K.H. Prediction of thermochemical and transport properties of hydrocarbon aviation fuel in supercritical state with thermal decomposition. Fuel 2022, 325, 124805. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, H.J.; Park, W. Prediction of Transport Properties of Hydrocarbon Aviation Fuels Using TRAPP Methods. Int. J. Aeronaut. Space Sci. 2023, in press. [Google Scholar] [CrossRef]
- Assael, M.J.; Trusler, J.P.M.; Tsolakis, T.F. Thermophysical Properties of Fluids: An Introduction to their Prediction; Imperial College Press: London, UK, 1996. [Google Scholar]
- Seo, J.; Kim, J.S.; Kim, K.H. Improved prediction of thermochemical properties of JP-10 using an extended RK-PR equation of state. J. Ind. Eng. Chem. 2023, 123, 88–103. [Google Scholar] [CrossRef]
- Kim, S.K.; Choi, H.S.; Kim, Y. Thermodynamic modeling based on a generalized cubic equation of state for kerosene/LOx rocket combustion. Combust. Flame 2012, 159, 1351–1365. [Google Scholar] [CrossRef]
- Bao, W.; Zhang, S.; Qin, J.; Zhou, W.; Xie, K. Numerical analysis of flowing cracked hydrocarbon fuel inside cooling channels in view of thermal management. Energy 2014, 67, 149–161. [Google Scholar] [CrossRef]
- Li, H.; Qin, J.; Jiang, Y.; Zhang, D.; Cheng, K.; Bao, W.; Huang, H. Experimental and theoretical investigation of power generation scheme driven by thermal cracked gaseous hydrocarbon fuel for hypersonic vehicle. Energy Convers. Manag. 2018, 165, 334–343. [Google Scholar] [CrossRef]
- Yu, W.; Zhou, W.; Jia, Z.; Han, Z. Characteristics of scramjet regenerative cooling with endothermic chemical reactions. Acta Astronaut. 2022, 195, 1–11. [Google Scholar] [CrossRef]
- Cismondi, M.; Mollerup, J. Development and application of a three-parameter RK-PR equation of state. Fluid Phase Equilib. 2005, 232, 74–89. [Google Scholar] [CrossRef]
- Hwang, S.; Lee, H.J. Investigation on a prediction methodology of thermodynamic properties of supercritical hydrocarbon aviation fuels. J. ILASS-Korea 2021, 26, 171–181. [Google Scholar]
- Li, W.; Huang, D.; Xu, G.; Tao, Z.; Wu, Z.; Zhu, H. Heat transfer to aviation kerosene flowing upward in smooth tubes at supercritical pressures. Int. J. Heat Mass Transf. 2015, 85, 1084–1094. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; Jing, K.; Wang, L.; Li, Y.; Zhang, X.; Liu, G. Kinetics and modeling of supercritical pyrolysis of endothermic hydrocarbon fuels in regenerative cooling channels. Chem. Eng. Sci. 2019, 207, 202–214. [Google Scholar] [CrossRef]
- Poling, B.E.; Prausnitz, J.M.; O’Connel, J.P. The Properties of Gases and Liquids, 5th ed.; McGraw-Hill Education Press: New York, NY, USA, 2001. [Google Scholar]
- Ely, J.F.; Hanley, H.J.M. Prediction of transport properties. 1. Viscosity of fluids and mixtures. Ind. Eng. Chem. Fundam. 1981, 20, 323–332. [Google Scholar] [CrossRef]
- Ely, J.F.; Hanley, H.J.M. Prediction of transport properties. 2. Thermal conductivity of pure fluids and mixtures. Ind. Eng. Chem. Fundam. 1983, 22, 90–97. [Google Scholar] [CrossRef]
- Huber, M.L.; Ely, J.F. Prediction of viscosity of refrigerants and refrigerant mixtures. Fluid Phase Equilib. 1992, 80, 239–248. [Google Scholar] [CrossRef]
- Huber, M.L.; Friend, D.G.; Ely, J.F. Prediction of the thermal conductivity of refrigerants and refrigerant mixtures. Fluid Phase Equilib. 1992, 80, 249–261. [Google Scholar] [CrossRef]
- Milat, J.; Dymond, J.H.; Nieto de Castro, C.A. Transport Properties of Fluids, Their Correlation, Prediction and Estimation; Cambridge University Press: Cambridge, UK, 1996. [Google Scholar]
- Hwang, S.; Lee, H.J. A study on the prediction of transport properties of hydrocarbon aviation fuels using the methane-based TRAPP method. J. ILASS-Korea 2022, 27, 66–76. [Google Scholar]
- Chung, T.-H.; Ajlan, M.; Lee, L.L.; Starling, K.E. Generalized multiparameter correlation for nonpolar and polar fluid transport properties. Ind. Eng. Chem. Res. 1988, 27, 671–679. [Google Scholar] [CrossRef]
- Brule, M.R.; Starling, K.E. Thermophysical properties of complex systems: Applications of multiproperty analysis. Ind. Eng. Chem. Process Des. Dev. 1984, 23, 833–845. [Google Scholar] [CrossRef]
- Ely, J.F. A predictive, exact shape factor extended corresponding states model for mixtures. Adv. Cryog. Eng. 1990, 35, 1511–1520. [Google Scholar]
- Yang, C.; Quan, Z.; Chen, Y.; Zhu, Q.; Wang, J.; Li, X. A Comprehensive Investigating of the Pyrolysis Effect on Heat Transfer Characteristics for n-Decane in the Horizon Mini-Channel. Energy Fuels 2020, 34, 199–210. [Google Scholar] [CrossRef]
- Cooke, J.A.; Bellucci, M.; Smooke, M.D.; Gomez, A.; Violi, A.; Faravelli, T.; Ranzi, E. Computational and experimental study of JP-8, a surrogate, and its components in counterflow diffusion flames. Proc. Combust. Inst. 2005, 30, 439–446. [Google Scholar] [CrossRef]
- Ruan, B.; Meng, H.; Yang, V. Simplification of pyrolytic reaction mechanism and turbulent heat transfer of n-decane at supercritical pressures. Int. J. Heat Mass Transf. 2014, 69, 455–463. [Google Scholar] [CrossRef]
- Zhang, D.; Hou, L.; Gao, M.; Zhang, X. Experiment and modeling on thermal cracking of n-dodecane at supercritical pressure. Energy Fuels 2018, 32, 12426–12434. [Google Scholar] [CrossRef]
- Huang, B.; Shrestha, U.; Davis, R.J.; Chelliah, H.K. Endothermic pyrolysis of JP-10 with and without zeolite catalyst for hypersonic applications. AIAA J. 2018, 56, 1616–1626. [Google Scholar] [CrossRef]



| Name | Formula | M (kg/kmol) | Pc (MPa) | Tc (K) |
|---|---|---|---|---|
| hydrogen | H2 | 2.016 | 1.29 | 32.98 |
| methane | CH4 | 16.04 | 4.60 | 190.6 |
| ethylene | C2H4 | 28.05 | 5.04 | 282.3 |
| ethane | C2H6 | 30.07 | 4.87 | 305.3 |
| propene | C3H6 | 42.08 | 4.60 | 364.9 |
| propane | C3H8 | 44.10 | 4.25 | 369.8 |
| 1,3-butadiene | 1,3-C4H6 | 54.09 | 4.32 | 425.0 |
| butene | C4H8 | 56.11 | 4.02 | 419.5 |
| trans-2-butene | trans-C4H8 | 56.11 | 4.10 | 428.6 |
| cis-2-butene | cis-C4H8 | 56.11 | 4.21 | 435.5 |
| butane | C4H10 | 58.12 | 3.80 | 425.1 |
| 1-pentene | C5H10 | 70.13 | 3.56 | 464.8 |
| pentane | C5H12 | 72.15 | 3.37 | 469.7 |
| benzene | C6H6 | 78.11 | 4.90 | 562.1 |
| 1-hexene | C6H12 | 84.16 | 3.14 | 504.0 |
| hexane | C6H14 | 86.18 | 3.03 | 507.6 |
| toluene | C7H8 | 92.14 | 4.11 | 591.8 |
| 1-heptene | C7H14 | 98.19 | 2.92 | 537.3 |
| heptane | C7H16 | 100.2 | 2.74 | 540.2 |
| ethylbenzene | C8H10 | 106.2 | 3.61 | 617.2 |
| 1-octene | C8H16 | 112.2 | 2.68 | 567.0 |
| octane | C8H18 | 114.2 | 2.49 | 568.7 |
| 1-nonene | C9H18 | 126.2 | 2.33 | 594.0 |
| nonane | C9H20 | 128.3 | 2.29 | 594.6 |
| decane | C10H22 | 142.3 | 2.11 | 617.7 |
| dodecane | C12H26 | 170.3 | 1.82 | 658.0 |
| exo-THDCPD | C10H16 | 136.2 | 3.73 | 698.0 |
| JP-8 surrogate | Mixture | 144.6 | 2.19 | 628.4 |
| methane + ethylene | Mixture | 23.63 | 4.91 | 248.5 |
| Substance | Methane TRAPP | Propane TRAPP | Chung et al. | Brule-Starling |
|---|---|---|---|---|
| H2 | 2.40 | 0.46 | 12.2 | 8.78 |
| CH4 | 0.71 | 2.91 | 1.06 | 0.54 |
| C2H4 | 0.83 | 6.16 | 3.99 | 2.38 |
| C2H6 | 6.89 | 2.73 | 2.08 | 3.56 |
| C3H6 | 6.39 | 9.00 | 2.45 | 1.41 |
| C3H8 | 9.08 | 8.20 | 2.96 | 4.17 |
| 1,3-C4H6 | 9.02 | 14.0 | 3.31 | 4.31 |
| C4H8 | 6.28 | 20.7 | 3.15 | 2.29 |
| trans-C4H8 | 10.9 | 15.4 | 3.66 | 4.08 |
| cis-C4H8 | 7.60 | 15.9 | 2.54 | 3.45 |
| C4H10 | 7.46 | 19.2 | 3.62 | 3.85 |
| C5H10 | 3.81 | 37.3 | 4.80 | 4.45 |
| C5H12 | 11.4 | 21.7 | 6.24 | 7.04 |
| C6H6 | 4.09 | 30.2 | 4.98 | 5.24 |
| C6H12 | 8.41 | 28.9 | 4.83 | 5.13 |
| C6H14 | 6.58 | 37.2 | 5.60 | 4.84 |
| C7H8 | 9.50 | 35.4 | 5.59 | 6.22 |
| C7H14 | 7.90 | 34.2 | 5.85 | 6.70 |
| C7H16 | 7.65 | 43.3 | 6.37 | 5.75 |
| C8H10 | 8.12 | 41.6 | 6.33 | 7.34 |
| C8H16 | 6.97 | 39.6 | 6.72 | 7.92 |
| C8H18 | 8.36 | 39.9 | 8.06 | 9.41 |
| C9H18 | 17.0 | 46.6 | 11.5 | 12.8 |
| C9H20 | 7.91 | 49.7 | 11.3 | 12.9 |
| C10H22 | 8.11 | 50.0 | 10.6 | 12.2 |
| C12H26 | 14.1 | 54.3 | 18.9 | 20.7 |
| C10H16 | 29.5 | 51.2 | 16.1 | 15.7 |
| JP-8 surrogate | 15.6 | 55.2 | 10.5 | 11.8 |
| CH4 + C2H4 | 0.39 | 5.54 | 4.25 | 2.70 |
| Total average | 8.38 | 28.2 | 6.54 | 6.82 |
| Substance | Methane TRAPP | Propane TRAPP | Modified Propane TRAPP | Chung et al. |
|---|---|---|---|---|
| H2 | 1.74 | 1.99 | 2.23 | 19.6 |
| CH4 | 1.92 | 2.68 | 2.57 | 4.84 |
| C2H4 | 6.33 | 8.07 | 8.12 | 12.0 |
| C2H6 | 6.88 | 4.33 | 4.24 | 4.35 |
| C3H6 | 17.2 | 3.04 | 3.06 | 3.67 |
| C3H8 | 13.7 | 8.17 | 8.17 | 8.11 |
| 1,3-C4H6 | 33.3 | 7.04 | 7.06 | 9.06 |
| C4H8 | 18.2 | 11.6 | 11.4 | 12.4 |
| trans-C4H8 | 31.6 | 5.06 | 4.27 | 6.42 |
| cis-C4H8 | 35.9 | 6.74 | 7.23 | 8.95 |
| C4H10 | 22.3 | 9.34 | 8.72 | 8.33 |
| C5H10 | 39.0 | 5.87 | 2.76 | 3.84 |
| C5H12 | 34.3 | 7.60 | 7.62 | 5.26 |
| C6H6 | 35.3 | 1.63 | 2.45 | 4.18 |
| C6H12 | 48.1 | 7.05 | 4.68 | 10.1 |
| C6H14 | 47.2 | 5.29 | 4.11 | 7.07 |
| C7H8 | 36.6 | 6.56 | 9.88 | 4.57 |
| C7H14 | 51.9 | 8.18 | 5.05 | 7.80 |
| C7H16 | 44.0 | 7.27 | 7.44 | 3.07 |
| C8H10 | 50.0 | 4.94 | 6.82 | 7.18 |
| C8H16 | 55.3 | 10.1 | 4.10 | 7.20 |
| C8H18 | 50.0 | 5.71 | 6.64 | 7.20 |
| C9H18 | 56.6 | 1.55 | 12.0 | 13.5 |
| C9H20 | 51.6 | 10.1 | 7.05 | 7.07 |
| C10H22 | 56.5 | 10.1 | 6.49 | 9.22 |
| C12H26 | 55.0 | 15.3 | 8.52 | 14.0 |
| C10H16 | 48.4 | 13.8 | 8.61 | 6.93 |
| JP-8 surrogate | 58.5 | 12.9 | 4.28 | 11.8 |
| CH4 + C2H4 | 5.31 | 6.47 | 6.45 | 9.90 |
| Total average | 34.9 | 7.19 | 6.28 | 8.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, S.-r.; Lee, H.J. Comparison and Evaluation of Transport Property Prediction Performance of Supercritical Hydrocarbon Aviation Fuels and Their Pyrolyzed Products via Endothermic Reactions. Energies 2023, 16, 5195. https://doi.org/10.3390/en16135195
Hwang S-r, Lee HJ. Comparison and Evaluation of Transport Property Prediction Performance of Supercritical Hydrocarbon Aviation Fuels and Their Pyrolyzed Products via Endothermic Reactions. Energies. 2023; 16(13):5195. https://doi.org/10.3390/en16135195
Chicago/Turabian StyleHwang, Sung-rok, and Hyung Ju Lee. 2023. "Comparison and Evaluation of Transport Property Prediction Performance of Supercritical Hydrocarbon Aviation Fuels and Their Pyrolyzed Products via Endothermic Reactions" Energies 16, no. 13: 5195. https://doi.org/10.3390/en16135195