Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review
Abstract
:1. Introduction
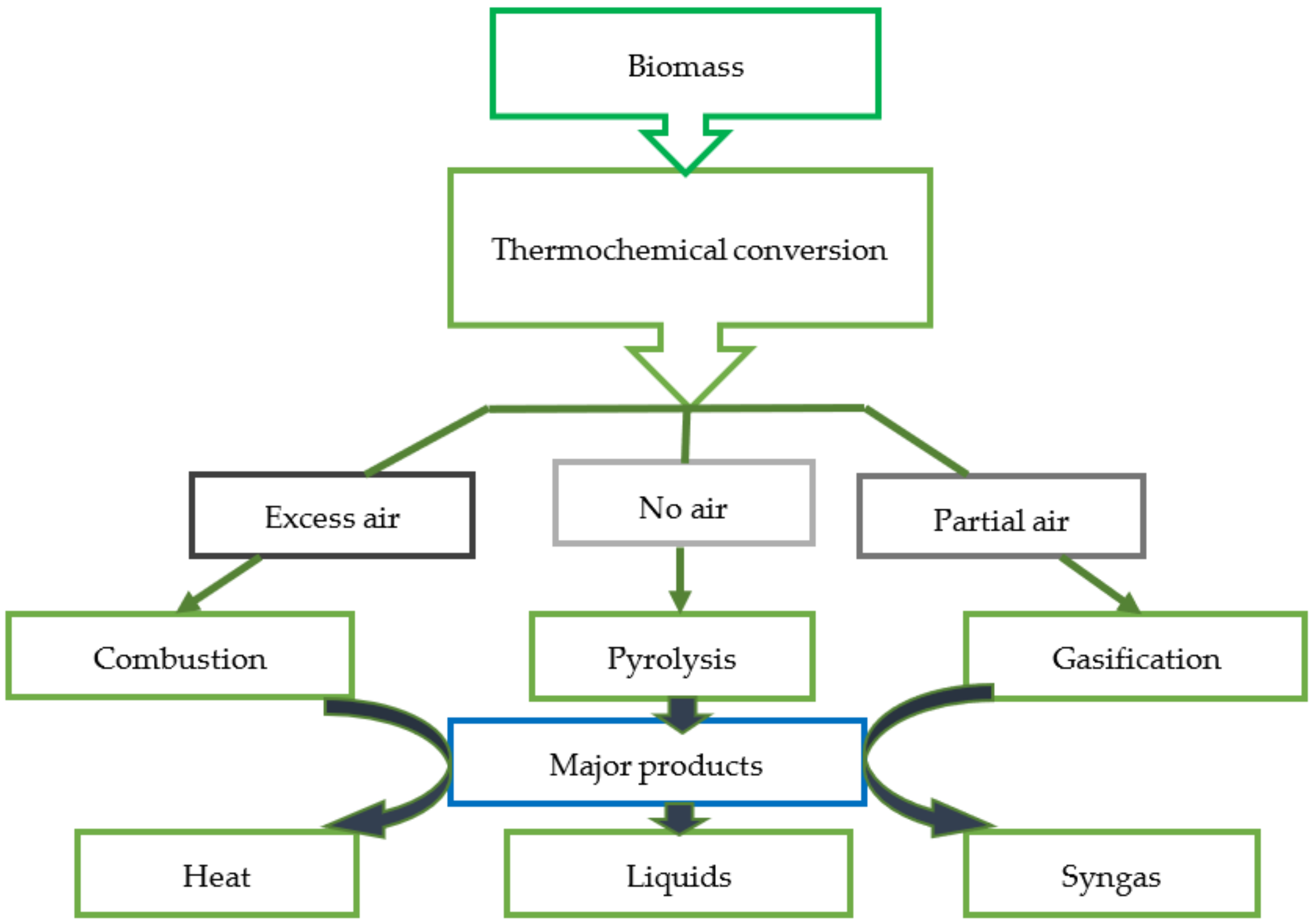
2. Biomass and Thermochemical Conversion of Biomass
2.1. Biomass
2.1.1. Cellulose
2.1.2. Hemicellulose
2.1.3. Lignin
2.1.4. Organic Extractives
2.1.5. Inorganic Matter/Ash
2.2. Thermochemical Conversion of Biomass
2.2.1. Combustion
2.2.2. Gasification
2.2.3. Pyrolysis
3. Pyrolysis of Biomass
3.1. Types of Pyrolysis
3.1.1. Slow Pyrolysis
3.1.2. Intermediate Pyrolysis
3.1.3. Fast Pyrolysis
3.1.4. Flash Pyrolysis
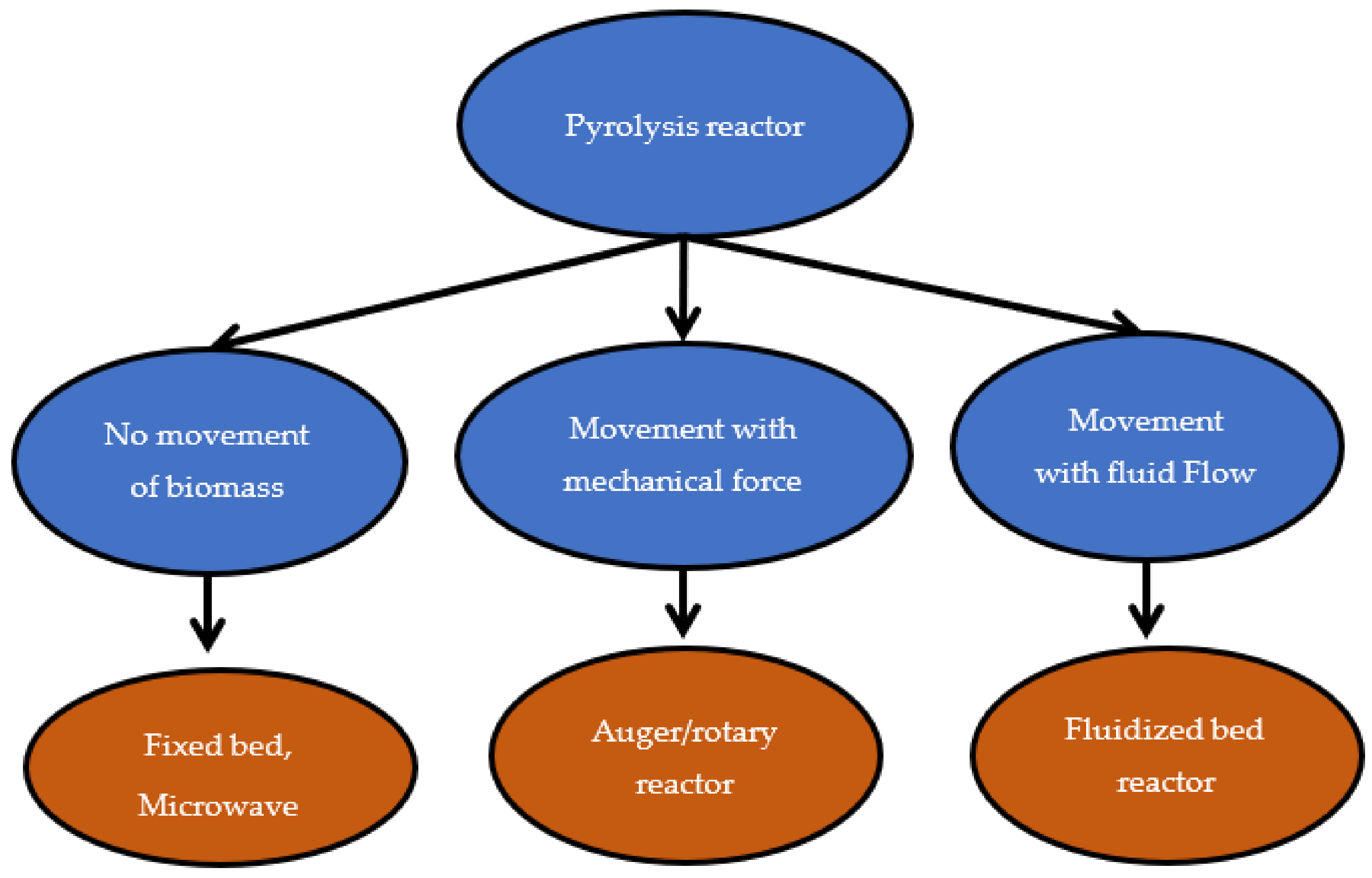
3.2. Reactors for the Pyrolysis Processes
3.2.1. Fixed-Bed Reactor
3.2.2. Auger/Screw Reactor
3.2.3. Fluidized Bed reactor
3.2.4. Microwave Reactor
3.2.5. Other Reactors
3.2.6. Impacts of Pyrolysis and Reactors on the Enhancement of Bio-Oil Quality
3.3. Products from the Pyrolysis Process
3.3.1. Biochar
3.3.2. Bio-Oil
- Physical methods with solvents;
- Catalytic methods with catalysts;
- Non-catalytic methods without catalysts.
3.3.3. Syngas
3.4. Factors Influencing the Pyrolysis Process and Products
3.4.1. Temperature
3.4.2. Heating Rate
3.4.3. Vapor Residence Time
3.4.4. Biomass Particle Size
3.4.5. Presence of Catalysts
4. Catalytic Pyrolysis of Biomass
4.1. Catalytic Pyrolysis
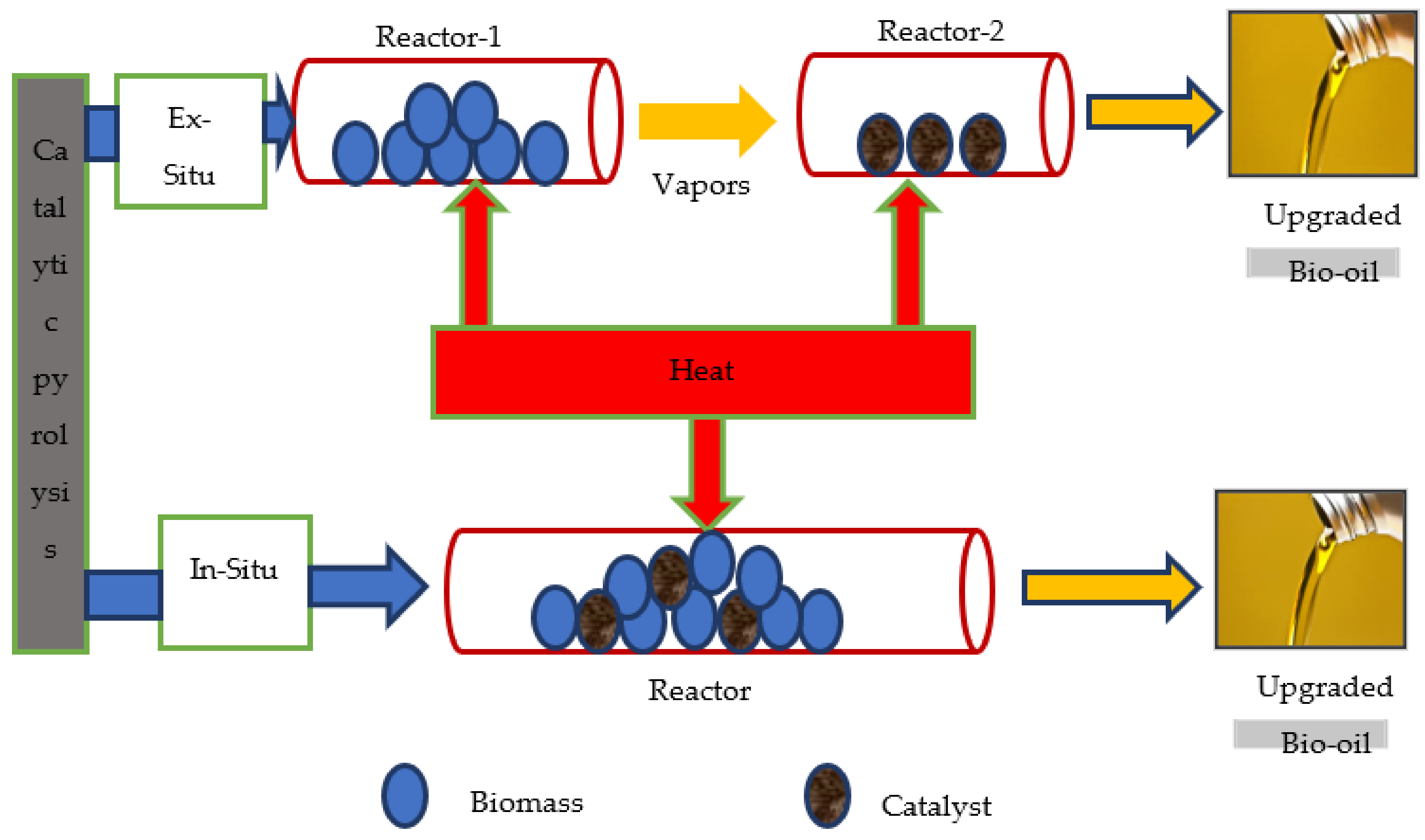
4.1.1. Arrangement of Catalysts
In Situ
Ex Situ
4.1.2. Affecting Factors of Catalyst
Catalyst Position
Ratio of Catalyst and Biomass
Catalyst Deactivation
Catalyst Bed Temperature
5. Effect of Catalyst on the Catalytic Pyrolysis Process
5.1. Catalysts
5.1.1. Zeolite
5.1.2. Natural Catalyst
5.1.3. Activated Carbon
5.1.4. Metal Oxide
5.1.5. Mixture of Catalysts
5.2. Catalytic Effect on Bio-Oil
5.2.1. pH of the Bio-Oil
5.2.2. Water Content
5.2.3. High Heating Value (HHV)
5.2.4. Elemental Analysis
5.2.5. Stability of Bio-Oil
6. Machine Learning and Techno-Economic Analysis of Catalytic Pyrolysis
6.1. Machine Learning
- ➢
- Artificial neural network;
- ➢
- Linear regression;
- ➢
- Decision trees;
- ➢
- Logistic regression;
- ➢
- Gaussian mixture model;
- ➢
- Naive Bayes;
- ➢
- K-means clustering;
- ➢
- Anomaly detection;
- ➢
- Principal component analysis;
- ➢
- K-nearest neighbors algorithm;
- ➢
- Support vector machines
6.2. Techno-Economic Analysis
7. Conclusions and Recommendations
7.1. Conclusions
7.2. Recommendations for Future Works
- The catalytic effect can be observed with different biomass-to-catalyst ratios with in situ and ex situ arrangements in the future;
- Inexpensive materials can be studied as catalysts for catalytic pyrolysis;
- The experiment can be optimized with higher accuracy by changing the operating parameters like heating rate, nitrogen flow rate, etc.;
- The use of regenerated catalysts can be continued several times until satisfactory results are achieved;
- Different mixture ratios can be applied with activated carbon and other low-cost metal oxides to obtain upgraded bio-oils
- The syngas can be analyzed for further applications in the fuel cell to obtain clean energy;
- The bio-oils produced from catalytic pyrolysis can be used as fuel in real-world applications to achieve practical performance;
- A gap between lab-scale studies and the real industrial situation can be thoroughly discussed;
- Standardization of raw materials and process designs for the catalytic pyrolysis process should be performed with optimum techno-economic solutions;
- In order to expedite catalytic pyrolysis research, extensive machine learning processes can be explored due to their capacity to learn nonlinear input and output correlations;
- Future models for process optimization and control ought to strike a balance between determining load and precision. However, ML is not a universal fix for all issues in pyrolysis research; nevertheless, it may work in conjunction with current technologies and provide a great tool for pyrolysis process monitoring, control, and product yield optimization.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Glossary
| Nomenclature | |
| Al | Aluminum |
| Al2O3 | Aluminum oxides |
| Ca | Calcium |
| CaMg(CO3)2 | Dolomite |
| CaO | Calcium oxide |
| CeO2 | Cerium dioxide |
| CO2 | Carbon dioxide |
| COx | Carbon oxides |
| CO | Carbon monoxide |
| (C6H10O5)n | Cellulose |
| (C5H8O4)n | Hemicellulose |
| CH4 | Methane |
| Cr | Chromium |
| Fe | Iron |
| Fe2O3 | Ferric oxide |
| H2 | Hydrogen |
| H2O | Water |
| HCl | Hydrogen chloride |
| K | Potassium |
| KCl | Potassium chloride |
| K2Cl2 | Potassium chloride |
| K3PO4 | Tripotassium phosphate |
| Mg | Magnesium |
| MgO | Magnesium oxide |
| Mn | Manganese |
| MnO2 | Manganese dioxide |
| N2 | Nitrogen |
| Na | Sodium |
| NaCl | Sodium chloride |
| P | Phosphorus |
| R2 | Regression coefficient |
| Si | Silicon |
| SiC | Silicon carbide |
| SiO2 | Silicon dioxide |
| TiO2 | Titanium dioxide |
| ZSM-5 | Zeolite Socony Mobil–5 |
| Zn | Zinc |
| ZnO | Zinc oxide |
| Abbreviations | |
| 3D | Three dimensional |
| AC | Activated carbon |
| AI | Artificial intelligence |
| ANN | Artificial neural network |
| Bentonite | Aluminum phyllosilicate |
| BRHA | Brunei rice husk ash |
| HHV | High heating value |
| ML | Machine learning |
| TEA | Techno-economic analysis |
References
- Mohabeer, C.; Guilhaume, N.; Laurenti, D.; Schuurman, Y. Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energies 2022, 15, 3258. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Albahnasawi, A.; Al Maskari, T.; Abujazar, M.S.S.; Bashir, M.J.K.; Nassani, D.E.; Abu Amr, S.S. Biofuel Production Using Cultivated Algae: Technologies, Economics, and Its Environmental Impacts. Energies 2023, 16, 1316. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Amin, M.R.; Taweekun, J.; Azad, A.K. Progress in Nanomaterials Fabrication and Their Prospects in Artificial Intelligence towards Solid Oxide Fuel Cells: A Review. Int. J. Hydrogen Energy 2022, in press. [Google Scholar] [CrossRef]
- Ferreira, A.P.R.A.; Oliveira, R.C.P.; Mateus, M.M.; Santos, D.M.F.; Ferreira, A.P.R.A.; Oliveira, R.C.P.; Mateus, M.M.; Santos, D.M.F. A Review of the Use of Electrolytic Cells for Energy and Environmental Applications. Energies 2023, 16, 1593. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Haque, M.N.; Islam, S.N.; Hossain, M.A.; Hassan, M.; Roy, H.; et al. Advanced Applications of Carbonaceous Materials in Sustainable Water Treatment, Energy Storage, and CO2 Capture: A Comprehensive Review. Sustainability 2023, 15, 8815. [Google Scholar] [CrossRef]
- Perišić, M.; Barceló, E.; Dimic-Misic, K.; Imani, M.; Spasojević Brkić, V. The Role of Bioeconomy in the Future Energy Scenario: A State-of-the-Art Review. Sustainability 2022, 14, 560. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Syazaidah, I.; Abu Bakar, M.S.; Reza, M.S.; Azad, A.K. Ex-Situ Catalytic Pyrolysis of Chicken Litter for Bio-Oil Production: Experiment and Characterization. J. Environ. Manag. 2021, 297, 113407. [Google Scholar] [CrossRef]
- Dickerson, T.; Soria, J. Catalytic Fast Pyrolysis: A Review. Energies 2013, 6, 514–538. [Google Scholar] [CrossRef]
- van Meerbeek, K.; Appels, L.; Dewil, R.; Calmeyn, A.; Lemmens, P.; Muys, B.; Hermy, M.; Van Meerbeek, K.; Appels, L.; Dewil, R.; et al. Biomass of Invasive Plant Species as a Potential Feedstock for Bioenergy Production. Biofuels Bioprod. Biorefining 2015, 9, 273–282. [Google Scholar] [CrossRef]
- Chen, Y. Biomass to Fuels: Thermo-Chemical or Bio-Chemical Conversion? Ferment. Technol. 2012, 1, 7972. [Google Scholar] [CrossRef]
- Halder, P.; Azad, A.K. Recent Trends and Challenges of Algal Biofuel Conversion Technologies. In Advanced Biofuels: Applications, Technologies and Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–179. ISBN 9780081027912. [Google Scholar]
- Panwar, N.L.; Kothari, R.; Tyagi, V.V. Thermo Chemical Conversion of Biomass—Eco Friendly Energy Routes. Renew. Sustain. Energy Rev. 2012, 16, 1801–1816. [Google Scholar] [CrossRef]
- Zaman, C.Z.; Pal, K.; Yehye, W.A.; Sagadevan, S.; Shah, S.T.; Adebisi, G.A.; Marliana, E.; Rafique, R.F.; Johan, R.B. Pyrolysis: A Sustainable Way to Generate Energy from Waste. In Pyrolysis; InTech: London, UK, 2017; pp. 1–35. ISBN 978-953-51-3312-4. [Google Scholar]
- Chen, W.H.; Lin, B.J. Characteristics of Products from the Pyrolysis of Oil Palm Fiber and Its Pellets in Nitrogen and Carbon Dioxide Atmospheres. Energy 2016, 94, 569–578. [Google Scholar] [CrossRef]
- Wainaina, S.; Awasthi, M.K.; Sarsaiya, S.; Chen, H.; Singh, E.; Kumar, A.; Ravindran, B.; Awasthi, S.K.; Liu, T.; Duan, Y.; et al. Resource Recovery and Circular Economy from Organic Solid Waste Using Aerobic and Anaerobic Digestion Technologies. Bioresour. Technol. 2020, 301, 122778. [Google Scholar] [CrossRef]
- Wang, C.; Huang, R.; Sun, R.; Yang, J.; Sillanpää, M. A Review on Persulfates Activation by Functional Biochar for Organic Contaminants Removal: Synthesis, Characterizations, Radical Determination, and Mechanism. J. Environ. Chem. Eng. 2021, 9, 106267. [Google Scholar] [CrossRef]
- Reza, M.S.; Hasan, A.B.M.K.; Ahmed, A.S.; Afroze, S.; Bakar, M.S.A.; Islam, S.N.; Azad, A.K. COVID-19 Prevention: Role of Activated Carbon. J. Eng. Technol. Sci. 2021, 53, 210404. [Google Scholar] [CrossRef]
- Elliott, D.C. Historical Developments in Hydroprocessing Bio-Oils. Energy Fuels 2007, 21, 1792–1815. [Google Scholar] [CrossRef]
- Afroze, S.; Torino, N.; Reza, M.S.; Radenahmad, N.; Cheok, Q.; Henry, P.F.; Azad, A.K. Structure-Conductivity Relationship of PrBaMnMoO6-δ through in-Situ Measurements: A Neutron Diffraction Study. Ceram. Int. 2021, 47, 541–546. [Google Scholar] [CrossRef]
- Balat, M. An Overview of the Properties and Applications of Biomass Pyrolysis Oils. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 674–689. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.; He, J.; Kumar, R.; Lu, Q. Catalytic Pyrolysis of Lignocellulosic Biomass: A Review of Variations in Process Factors and System Structure. Renew. Sustain. Energy Rev. 2020, 134, 110305. [Google Scholar] [CrossRef]
- Li, P.; Niu, B.; Pan, H.; Zhang, Y.; Long, D. Production of Hydrocarbon-Rich Bio-Oil from Catalytic Pyrolysis of Waste Cooking Oil over Nickel Monoxide Loaded Corn Cob-Derived Activated Carbon. J. Clean. Prod. 2023, 384, 135653. [Google Scholar] [CrossRef]
- Chen, X.; Che, Q.; Li, S.; Liu, Z.; Yang, H.; Chen, Y.; Wang, X.; Shao, J.; Chen, H. Recent Developments in Lignocellulosic Biomass Catalytic Fast Pyrolysis: Strategies for the Optimization of Bio-Oil Quality and Yield. Fuel Process. Technol. 2019, 196, 106180. [Google Scholar] [CrossRef]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic Solid Waste Biorefinery: Sustainable Strategy for Emerging Circular Bioeconomy in China. Ind. Crops Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Taweekun, J.; Ja’afar, F.; Bakar, M.S.A.; Azad, A.K.; Roy, H.; et al. Ex Situ Catalytic Pyrolysis of Invasive Pennisetum Purpureum Grass with Activated Carbon for Upgrading Bio-Oil. Sustainability 2023, 15, 7628. [Google Scholar] [CrossRef]
- Nishu; Liu, R.; Rahman, M.M.; Sarker, M.; Chai, M.; Li, C.; Cai, J. A Review on the Catalytic Pyrolysis of Biomass for the Bio-Oil Production with ZSM-5: Focus on Structure. Fuel Process. Technol. 2020, 199, 106301. [Google Scholar] [CrossRef]
- Jain, A.; Sarsaiya, S.; Kumar Awasthi, M.; Singh, R.; Rajput, R.; Mishra, U.C.; Chen, J.; Shi, J. Bioenergy and Bio-Products from Bio-Waste and Its Associated Modern Circular Economy: Current Research Trends, Challenges, and Future Outlooks. Fuel 2022, 307, 121859. [Google Scholar] [CrossRef]
- Elmaz, F.; Yücel, Ö.; Mutlu, A.Y. Predictive Modeling of Biomass Gasification with Machine Learning-Based Regression Methods. Energy 2020, 191, 116541. [Google Scholar] [CrossRef]
- Jawaid, M.; Paridah, M.T.; Saba, N. Introduction to Biomass and Its Composites. In Lignocellulosic Fibre and Biomass-Based Composite Materials; Woodhead Publishing: Sawston, UK, 2017; pp. 1–11. [Google Scholar] [CrossRef]
- Pampillón-González, L.; Canepa, J.R.L. Biomass as an Alternative for Gas Production. In Advances in Natural Gas Emerging Technologies; InTech: London, UK, 2017. [Google Scholar]
- Tumuluru, J.S.; Wright, C.T.; Boardman, R.D.; Yancey, N.A.; Sokhansanj, S. A Review on Biomass Classification and Composition, Co-Firing Issues and Pretreatment Methods. In Proceedings of the 2011 ASABE Annual International Meeting, Louisville, KY, USA, 7–10 August 2011; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2011. [Google Scholar]
- Mansor, A.M.; Lim, J.S.; Ani, F.N.; Hashim, H.; Ho, W.S. Characteristics of Cellulose, Hemicellulose and Lignin of MD2 Pineapple Biomass. Chem. Eng. Trans. 2019, 72, 79–84. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent Advances in Green Hydrogels from Lignin: A Review. Int. J. Biol. Macromol. 2015, 72, 834–847. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kim, K.H.; Brown, R.C. Catalytic Pyrolysis of Individual Components of Lignocellulosic Biomass. Green Chem. 2014, 16, 727–735. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, J.; Zhang, X.; Xin, J.; Miao, Q.; Wang, J. Ionic Liquid-Based Green Processes for Energy Production. Chem. Soc. Rev. 2014, 43, 7838–7869. [Google Scholar] [CrossRef] [PubMed]
- Preparation and Characterization of Polyvinyl Alcohol-Based Composite Reinforced with Nanocellulose and Nanosilica|Ching|BioResources. Available online: https://ojs.cnr.ncsu.edu/index.php/BioRes/article/view/6992 (accessed on 11 February 2021).
- Sampath, U.G.T.M.; Ching, Y.C.; Chuah, C.H.; Singh, R.; Lin, P.C. Preparation and Characterization of Nanocellulose Reinforced Semi-Interpenetrating Polymer Network of Chitosan Hydrogel. Cellulose 2017, 24, 2215–2228. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating Parameters for Bio-Oil Production in Biomass Pyrolysis: A Review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Huang, W.; Gong, F.; Fan, M.; Zhai, Q.; Hong, C.; Li, Q. Production of Light Olefins by Catalytic Conversion of Lignocellulosic Biomass with HZSM-5 Zeolite Impregnated with 6 wt.% Lanthanum. Bioresour. Technol. 2012, 121, 248–255. [Google Scholar] [CrossRef]
- Peters, B. Prediction of Pyrolysis of Pistachio Shells Based on Its Components Hemicellulose, Cellulose and Lignin. Fuel Process. Technol. 2011, 92, 1993–1998. [Google Scholar] [CrossRef]
- Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Screening Acidic Zeolites for Catalytic Fast Pyrolysis of Biomass and Its Components. J. Anal. Appl. Pyrolysis 2011, 92, 224–232. [Google Scholar] [CrossRef]
- Wendisch, V.F.; Kim, Y.; Lee, J.H. Chemicals from Lignin: Recent Depolymerization Techniques and Upgrading Extended Pathways. Curr. Opin. Green Sustain. Chem. 2018, 14, 33–39. [Google Scholar] [CrossRef]
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef]
- Rahimi, A.; Ulbrich, A.; Coon, J.J.; Stahl, S.S. Formic-Acid-Induced Depolymerization of Oxidized Lignin to Aromatics. Nature 2014, 515, 249–252. [Google Scholar] [CrossRef]
- Cagnon, B.; Py, X.; Guillot, A.; Stoeckli, F.; Chambat, G. Contributions of Hemicellulose, Cellulose and Lignin to the Mass and the Porous Properties of Chars and Steam Activated Carbons from Various Lignocellulosic Precursors. Bioresour. Technol. 2009, 100, 292–298. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Priharto, N.; Ronsse, F.; Yildiz, G.; Heeres, H.J.; Deuss, P.J.; Prins, W. Fast Pyrolysis with Fractional Condensation of Lignin-Rich Digested Stillage from Second-Generation Bioethanol Production. J. Anal. Appl. Pyrolysis 2020, 145, 104756. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A Comprehensive Review on the Pyrolysis of Lignocellulosic Biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Williams, C.L.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Li, C.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Li, C. Sources of Biomass Feedstock Variability and the Potential Impact on Biofuels Production. Bioenergy Res. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Pecha, B.; Garcia-Perez, M. Pyrolysis of Lignocellulosic Biomass. In Bioenergy; Elsevier: London, UK, 2015; pp. 413–442. [Google Scholar]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood/biomass for Bio-Oil: A Critical Review. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Antal, M.J. The Art, Science, and Technology of Charcoal Production. Ind. Eng. Chem. Res. 2003, 42, 1619–1640. [Google Scholar] [CrossRef]
- Pattiya, A. Catalytic Pyrolysis. In Direct Thermochemical Liquefaction for Energy Applications; Elsevier Ltd.: Duxford, UK, 2018; pp. 29–64. ISBN 9780081010259. [Google Scholar]
- Patel, M.; Zhang, X.; Kumar, A. Techno-Economic and Life Cycle Assessment on Lignocellulosic Biomass Thermochemical Conversion Technologies: A Review. Renew. Sustain. Energy Rev. 2016, 53, 1486–1499. [Google Scholar] [CrossRef]
- Bridgwater, T. Biomass for Energy. J. Sci. Food Agric. 2006, 86, 1755–1768. [Google Scholar] [CrossRef]
- Akhtar, A.; Krepl, V.; Ivanova, T. A Combined Overview of Combustion, Pyrolysis, and Gasification of Biomass. Energy Fuels 2018, 32, 7294–7318. [Google Scholar] [CrossRef]
- Lackner, M. Combustion. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Dincer, I.; Zamfirescu, C. Conventional Power Generating Systems. In Advanced Power Generation Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 199–310. [Google Scholar]
- Munawer, M.E. Human Health and Environmental Impacts of Coal Combustion and Post-Combustion Wastes. J. Sustain. Min. 2018, 17, 87–96. [Google Scholar] [CrossRef]
- Jerzak, W.; Kuźnia, M. Examination of Inorganic Gaseous Species and Condensed Phases during Coconut Husk Combustion Based on Thermodynamic Equilibrium Predictions. Renew. Energy 2021, 167, 497–507. [Google Scholar] [CrossRef]
- Radenahmad, N.; Tasfiah, A.; Saghir, M.; Taweekun, J.; Saifullah, M.; Bakar, A.; Reza, S.; Kalam, A. A Review on Biomass Derived Syngas for SOFC Based Combined Heat and Power Application. Renew. Sustain. Energy Rev. 2020, 119, 109560. [Google Scholar] [CrossRef]
- Breault, R.W. Gasification Processes Old and New: A Basic Review of the Major Technologies. Energies 2010, 3, 216–240. [Google Scholar] [CrossRef]
- Afroze, S.; Reza, M.S.; Cheok, Q.; Islam, S.N.; Abdalla, A.M.; Taweekun, J.; Azad, A.K.; Khalilpoor, N.; Issakhov, A. Advanced Applications of Fuel Cells during the COVID-19 Pandemic. Int. J. Chem. Eng. 2021, 2021, 1–9. [Google Scholar] [CrossRef]
- Costa, P.; Pinto, F.; André, R.N.; Marques, P. Integration of Gasification and Solid Oxide Fuel Cells (SOFCs) for Combined Heat and Power (CHP). Processes 2021, 9, 254. [Google Scholar] [CrossRef]
- Reza, M.S.; Taweekun, J.; Afroze, S.; Siddique, S.A.; Islam, M.S.; Wang, C.; Azad, A.K. Investigation of Thermochemical Properties and Pyrolysis of Barley Waste as a Source for Renewable Energy. Sustainability 2023, 15, 1643. [Google Scholar] [CrossRef]
- Jabar, J.M.; Jabar, J.M. Pyrolysis: A Convenient Route for Production of Eco-Friendly Fuels and Precursors for Chemical and Allied Industries. In Recent Perspectives in Pyrolysis Research; IntechOpen: London, UK, 2021; ISBN 978-1-83969-915-3. [Google Scholar]
- Husain, Z.; Ansari, K.B.; Chatake, V.S.; Urunkar, Y.; Pandit, A.B.; Joshi, J.B. Valorisation of Biomass Pellets to Renewable Fuel and Chemicals Using Pyrolysis: Characterisation of Pyrolysis Products and Its Application. Indian Chem. Eng. 2020, 62, 78–91. [Google Scholar] [CrossRef]
- AlNouss, A.; McKay, G.; Al-Ansari, T. Production of Syngas via Gasification Using Optimum Blends of Biomass. J. Clean. Prod. 2020, 242, 118499. [Google Scholar] [CrossRef]
- Bhatia, S.C. Biogas. In Advanced Renewable Energy Systems; Woodhead Publishing: Sawston, UK, 2014; pp. 426–472. ISBN 978-1-78242-269-3. [Google Scholar]
- Tripathi, M.; Sahu, J.N.; Ganesan, P. Effect of Process Parameters on Production of Biochar from Biomass Waste through Pyrolysis: A Review. Renew. Sustain. Energy Rev. 2016, 55, 467–481. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for Pyrolysis Technologies in the Bioenergy Sector: A Review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Pecha, M.B.; Garcia-Perez, M. Pyrolysis of Lignocellulosic Biomass: Oil, Char, and Gas. In Bioenergy; Elsevier: London, UK, 2020; pp. 581–619. [Google Scholar]
- Sarangi, P.K.; Nanda, S.; Mohanty, P. Recent Advancements in Biofuels and Bioenergy Utilization; Springer: Singapore, 2018; ISBN 9789811313073. [Google Scholar]
- Torri, I.D.V.; Paasikallio, V.; Faccini, C.S.; Huff, R.; Caramão, E.B.; Sacon, V.; Oasmaa, A.; Zini, C.A. Bio-Oil Production of Softwood and Hardwood Forest Industry Residues through Fast and Intermediate Pyrolysis and Its Chromatographic Characterization. Bioresour. Technol. 2016, 200, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Acosta, R.; Tavera, C.; Gauthier-Maradei, P.; Nabarlatz, D. Production of Oil and Char by Intermediate Pyrolysis of Scrap Tyres: Influence on Yield and Product Characteristics. Int. J. Chem. React. Eng. 2015, 13, 189–200. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An Overview of Fast Pyrolysis of Biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Funke, A.; Tomasi Morgano, M.; Dahmen, N.; Leibold, H. Experimental Comparison of Two Bench Scale Units for Fast and Intermediate Pyrolysis. J. Anal. Appl. Pyrolysis 2017, 124, 504–514. [Google Scholar] [CrossRef]
- Liang, J.; Shan, G.; Sun, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass: Critical Role of Zeolite Catalysts. Renew. Sustain. Energy Rev. 2021, 139, 110707. [Google Scholar] [CrossRef]
- Li, L.; Rowbotham, J.S.; Christopher Greenwell, H.; Dyer, P.W. An Introduction to Pyrolysis and Catalytic Pyrolysis: Versatile Techniques for Biomass Conversion. In New and Future Developments in Catalysis: Catalytic Biomass Conversion; Elsevier B.V.: Amsterdam, Netherlands, 2013; pp. 173–208. ISBN 9780444538789. [Google Scholar]
- Hornung, A. Intermediate Pyrolysis of Biomass. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing Limited: Sawston, UK, 2013; pp. 172–186. ISBN 9780857091314. [Google Scholar]
- Garcia-Perez, M.; Garcia-Nunez, J.A.; Lewis, T.; Kruger, C.; Kantor, S. Methods for Producing Biochar and Advanced Bio-Fuels in Washington State; Washington State University: Pullman, WA, USA, 2010; ISBN 3604076900. [Google Scholar]
- Bridgwater, A.V.; Peacocke, G.V.C. Fast Pyrolysis Processes for Biomass. Renew. Sustain. Energy Rev. 2000, 4, 1–73. [Google Scholar] [CrossRef]
- Warnecke, R. Gasification of Biomass: Comparison of Fixed Bed and Fluidized Bed Gasifier. Biomass Bioenergy 2000, 18, 489–497. [Google Scholar] [CrossRef]
- Cornelissen, G.; Pandit, N.R.; Taylor, P.; Pandit, B.H.; Sparrevik, M.; Schmidt, H.P. Emissions and Char Quality of Flame-curtain “Kon Tiki” kilns for Farmer-Scale Charcoal/biochar Production. PLoS ONE 2016, 11, e0154617. [Google Scholar] [CrossRef]
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger Reactors for Pyrolysis of Biomass and Wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from Microwave Pyrolysis of Biomass: A Review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Jahirul, M.I.; Rasul, M.G.; Chowdhury, A.A.; Ashwath, N. Biofuels Production through Biomass Pyrolysis—A Technological Review. Energies 2012, 5, 4952–5001. [Google Scholar] [CrossRef]
- Uddin, M.; Joardder, M.U.H.; Islam, M.N. Design and Construction of Fixed Bed Pyrolysis System and Plum Seed Pyrolysis for Bio-Oil Production. Int. J. Adv. Renew. Energy Res. 2012, 1, 405–409. [Google Scholar]
- Bamido, A. Design of A Fluidized Bed Reactor for Biomass Pyrolysis. Master’s Thesis, University of Cincinnati, Cincinnati, OH, USA, 2019. [Google Scholar]
- Roegiers, J. Heat and Mass Transfer Modelling of Auger Reactors. Master’s Thesis, Universiteit Gent, Ghent, Belgium, 2016. [Google Scholar]
- Garcia-Perez, M.; Adams, T.T.; Goodrum, J.W.; Geller, D.; Das, K.C. Production and Fuel Properties of Pine Chip Bio-Oil/biodiesel Blends. Energy Fuels 2007, 21, 2363–2372. [Google Scholar] [CrossRef]
- Verma, M.; Godbout, S.; Brar, S.K.; Solomatnikova, O.; Lemay, S.P.; Larouche, J.P. Biofuels Production from Biomass by Thermochemical Conversion Technologies. Int. J. Chem. Eng. 2012, 2012, 542426. [Google Scholar] [CrossRef]
- Lappas, A.; Heracleous, E. Production of Biofuels via Fischer-Tropsch Synthesis: Biomass-to-Liquids; Woodhead Publishing Limited: Cambridge, UK, 2011; ISBN 9780857090492. [Google Scholar]
- Morin, M.; Pécate, S.; Hémati, M.; Kara, Y. Pyrolysis of Biomass in a Batch Fluidized Bed Reactor: Effect of the Pyrolysis Conditions and the Nature of the Biomass on the Physicochemical Properties and the Reactivity of Char. J. Anal. Appl. Pyrolysis 2016, 122, 511–523. [Google Scholar] [CrossRef]
- Fernández, Y.; Menéndez, J.A. Influence of Feed Characteristics on the Microwave-Assisted Pyrolysis Used to Produce Syngas from Biomass Wastes. J. Anal. Appl. Pyrolysis 2011, 91, 316–322. [Google Scholar] [CrossRef]
- Lam, S.S.; Russell, A.D.; Chase, H.A. Microwave Pyrolysis, a Novel Process for Recycling Waste Automotive Engine Oil. Energy 2010, 35, 2985–2991. [Google Scholar] [CrossRef]
- Aho, A.; Tokarev, A.; Backman, P.; Kumar, N.; Eränen, K.; Hupa, M.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Catalytic Pyrolysis of Pine Biomass over H-Beta Zeolite in a Dual-Fluidized Bed Reactor: Effect of Space Velocity on the Yield and Composition of Pyrolysis Products. Top. Catal. 2011, 54, 941–948. [Google Scholar] [CrossRef]
- Atutxa, A.; Aguado, R.; Gayubo, A.G.; Olazar, M.; Bilbao, J. Kinetic Description of the Catalytic Pyrolysis of Biomass in a Conical Spouted Bed Reactor. Energy Fuels 2005, 19, 765–774. [Google Scholar] [CrossRef]
- Lappas, A.A.; Samolada, M.C.; Iatridis, D.K.; Voutetakis, S.S.; Vasalos, I.A. Biomass Pyrolysis in a Circulating Fluid Bed Reactor for the Production of Fuels and Chemicals. Fuel 2002, 81, 2087–2095. [Google Scholar] [CrossRef]
- Wang, J.X.; Cao, J.P.; Zhao, X.Y.; Liu, S.N.; Ren, X.Y.; Zhao, M.; Cui, X.; Chen, Q.; Wei, X.Y. Enhancement of Light Aromatics from Catalytic Fast Pyrolysis of Cellulose over Bifunctional Hierarchical HZSM-5 Modified by Hydrogen Fluoride and Nickel/hydrogen Fluoride. Bioresour. Technol. 2019, 278, 116–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Yi, W.; Fu, P.; Li, Z.; Wang, N.; Tian, C. Numerical Simulation and Experiment on Catalytic Upgrading of Biomass Pyrolysis Vapors in V-Shaped Downer Reactors. Bioresour. Technol. 2019, 274, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.R.; Tariq, R.; Shahbaz, M.; Naqvi, M.; Aslam, M.; Khan, Z.; Mackey, H.; Mckay, G.; Al-Ansari, T. Recent Developments on Sewage Sludge Pyrolysis and Its Kinetics: Resources Recovery, Thermogravimetric Platforms, and Innovative Prospects. Comput. Chem. Eng. 2021, 150, 107325. [Google Scholar] [CrossRef]
- Xiu, S.; Shahbazi, A. Bio-Oil Production and Upgrading Research: A Review. Renew. Sustain. Energy Rev. 2012, 16, 4406–4414. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar Physicochemical Properties: Pyrolysis Temperature and Feedstock Kind Effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Pal, S.; Kumar, A.; Sharma, A.K.; Ghodke, P.K.; Pandey, S.; Patel, A. Recent Advances in Catalytic Pyrolysis of Municipal Plastic Waste for the Production of Hydrocarbon Fuels. Processes 2022, 10, 1497. [Google Scholar] [CrossRef]
- Elliott, D.C.; Hart, T.R.; Neuenschwander, G.G.; Rotness, L.J.; Olarte, M.V.; Zacher, A.H.; Solantausta, Y. Catalytic Hydroprocessing of Fast Pyrolysis Bio-Oil from Pine Sawdust. Energy Fuels 2012, 26, 3891–3896. [Google Scholar] [CrossRef]
- Czernik, S.; Bridgwater, A.V. Overview of Applications of Biomass Fast Pyrolysis Oil. Energy Fuels 2004, 18, 590–598. [Google Scholar] [CrossRef]
- Treedet, W.; Suntivarakorn, R. Design and Operation of a Low Cost Bio-Oil Fast Pyrolysis from Sugarcane Bagasse on Circulating Fluidized Bed Reactor in a Pilot Plant. Fuel Process. Technol. 2018, 179, 17–31. [Google Scholar] [CrossRef]
- Ly, H.V.; Kim, S.S.; Choi, J.H.; Woo, H.C.; Kim, J. Fast Pyrolysis of Saccharina Japonica Alga in a Fixed-Bed Reactor for Bio-Oil Production. Energy Convers. Manag. 2016, 122, 526–534. [Google Scholar] [CrossRef]
- Santos, J.; Ouadi, M.; Jahangiri, H.; Hornung, A. Integrated Intermediate Catalytic Pyrolysis of Wheat Husk. Food Bioprod. Process. 2019, 114, 23–30. [Google Scholar] [CrossRef]
- Jouhara, H.; Ahmad, D.; van den Boogaert, I.; Katsou, E.; Simons, S.; Spencer, N. Pyrolysis of Domestic Based Feedstock at Temperatures up to 300 °C. Therm. Sci. Eng. Prog. 2018, 5, 117–143. [Google Scholar] [CrossRef]
- Gong, X.; Kung, C.C.; Zhang, L. An Economic Evaluation on Welfare Distribution and Carbon Sequestration under Competitive Pyrolysis Technologies. Energy Explor. Exploit. 2021, 39, 553–570. [Google Scholar] [CrossRef]
- Lee, X.J.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Mahlia, T.M.I. State of Art Review on Conventional and Advanced Pyrolysis of Macroalgae and Microalgae for Biochar, Bio-Oil and Bio-Syngas Production. Energy Convers. Manag. 2020, 210, 112707. [Google Scholar] [CrossRef]
- Singh, N.; Singhania, R.R.; Nigam, P.S.; di Dong, C.; Patel, A.K.; Puri, M. Global Status of Lignocellulosic Biorefinery: Challenges and Perspectives. Bioresour. Technol. 2022, 344, 126415. [Google Scholar] [CrossRef]
- Lee, X.J.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Ng, H.K. Biochar Potential Evaluation of Palm Oil Wastes through Slow Pyrolysis: Thermochemical Characterization and Pyrolytic Kinetic Studies. Bioresour. Technol. 2017, 236, 155–163. [Google Scholar] [CrossRef]
- Brebu, M.; Vasile, C. Thermal Degradation of Lignin-A Review. Cellul. Chem. Technol. 2010, 44, 353–363. [Google Scholar]
- Quan, C.; Gao, N.; Song, Q. Pyrolysis of Biomass Components in a TGA and a Fixed-Bed Reactor: Thermochemical Behaviors, Kinetics, and Product Characterization. J. Anal. Appl. Pyrolysis 2016, 121, 84–92. [Google Scholar] [CrossRef]
- Luo, D.; Wang, L.; Nan, H.; Cao, Y.; Wang, H.; Kumar, T.V.; Wang, C. Phosphorus Adsorption by Functionalized Biochar: A Review. Environ. Chem. Lett. 2022, 21, 497–524. [Google Scholar] [CrossRef]
- Kang, Z.; Jia, X.; Zhang, Y.; Kang, X.; Ge, M.; Liu, D.; Wang, C.; He, Z. A Review on Application of Biochar in the Removal of Pharmaceutical Pollutants through Adsorption and Persulfate-Based AOPs. Sustainability 2022, 14, 10128. [Google Scholar] [CrossRef]
- Reza, M.S.; Azad, A.K.; Bakar, M.S.A.; Karim, M.R.; Sharifpur, M.; Taweekun, J. Evaluation of Thermochemical Characteristics and Pyrolysis of Fish Processing Waste for Renewable Energy Feedstock. Sustainability 2022, 14, 1203. [Google Scholar] [CrossRef]
- Xu, Y.; Hu, X.; Li, W.; Shi, Y. Preparation and Characterization of Bio-Oil from Biomass. In Progress in Biomass and Bioenergy Production; Shaukat, S., Ed.; InTech: Shanghai, China, 2011; pp. 197–222. ISBN 978-953-307-491-7. [Google Scholar]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast Co-Pyrolysis of Sewage Sludge and Lignocellulosic Biomass in a Conical Spouted Bed Reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Saber, M.; Nakhshiniev, B.; Yoshikawa, K. A Review of Production and Upgrading of Algal Bio-Oil. Renew. Sustain. Energy Rev. 2016, 58, 918–930. [Google Scholar] [CrossRef]
- Lin, B.-J.; Chen, W.-H. Sugarcane Bagasse Pyrolysis in a Carbon Dioxide Atmosphere with Conventional and Microwave-Assisted Heating. Front. Energy Res. 2015, 3, 4. [Google Scholar] [CrossRef]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Demirbas, A. Biofuels Sources, Biofuel Policy, Biofuel Economy and Global Biofuel Projections. Energy Convers. Manag. 2008, 49, 2106–2116. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T. A Review on the Microwave-Assisted Pyrolysis Technique. Renew. Sustain. Energy Rev. 2013, 28, 317–330. [Google Scholar] [CrossRef]
- Yang, C.; Li, R.; Cui, C.; Liu, S.; Qiu, Q.; Ding, Y.; Wu, Y.; Zhang, B. Catalytic Hydroprocessing of Microalgae-Derived Biofuels: A Review. Green Chem. 2016, 18, 3684–3699. [Google Scholar] [CrossRef]
- Campanella, A.; Harold, M.P. Fast Pyrolysis of Microalgae in a Falling Solids Reactor: Effects of Process Variables and Zeolite Catalysts. Biomass Bioenergy 2012, 46, 218–232. [Google Scholar] [CrossRef]
- Aydin, Ö.; Nakajima, H.; Kitahara, T. Current and Temperature Distributions in-Situ Acquired by Electrode-Segmentation along a Microtubular Solid Oxide Fuel Cell Operating with Syngas. J. Power Sources 2015, 293, 1053–1061. [Google Scholar] [CrossRef]
- Samolada, M.C.; Stoicos, T.; Vasalos, I.A. An Investigation of the Factors Controlling the Pyrolysis Product Yield of Greek Wood Biomass in a Fluidized Bed. J. Anal. Appl. Pyrolysis 1990, 18, 127–141. [Google Scholar] [CrossRef]
- Rahman, A.A.; Sulaiman, F.; Abdullah, N. Effect of Temperature on Pyrolysis Product of Empty Fruit Bunches. In Proceedings of the AIP Conference Proceedings 1657, Tahara, Japan, 22–23 October 2015; p. 40011. [Google Scholar]
- Demirbas, A. Effect of Temperature on Pyrolysis Products from Biomass. Energy Sources Part A Recover. Util. Environ. Eff. 2007, 29, 329–336. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Taweekun, J.; Rahman, M.M.; Rasul, M.G.; Mahlia, T.M.I.; Ashrafur, S.M. An Overview of Recent Developments in Biomass Pyrolysis Technologies. Energies 2018, 11, 3115. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic Biomass Pyrolysis: A Review of Product Properties and Effects of Pyrolysis Parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Solís, S. Biochar Production by Biomass Pyrolysis for Various Applications. Master’s Thesis, Luleå University of Technology, Luleå, Sweden, 2016. [Google Scholar]
- Safdari, M.-S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating Rate and Temperature Effects on Pyrolysis Products from Live Wildland Fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Alsaleh, A.; Sattler, M.L. Waste Tire Pyrolysis: Influential Parameters and Product Properties. Curr. Sustain. Energy Rep. 2014, 1, 129–135. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-Oil from Sawdust: Pyrolysis of Sawdust in a Fixed-Bed System. Energy Fuels 2009, 23, 3767–3772. [Google Scholar] [CrossRef]
- Ozbay, N.; Pütün, A.E.; Pütün, E. Bio-Oil Production from Rapid Pyrolysis of Cottonseed Cake: Product Yields and Compositions. Int. J. Energy Res. 2006, 30, 501–510. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9780128129920. [Google Scholar]
- Mastral, F.J.; Esperanza, E.; García, P.; Juste, M.; Garciía, P.; Juste, M. Pyrolysis of High-Density Polyethylene in a Fluidised Bed Reactor. Influence of the Temperature and Residence Time. J. Anal. Appl. Pyrolysis 2002, 63, 1–15. [Google Scholar] [CrossRef]
- Boscagli, C.; Tomasi Morgano, M.; Raffelt, K.; Leibold, H.; Grunwaldt, J.D. Influence of Feedstock, Catalyst, Pyrolysis and Hydrotreatment Temperature on the Composition of Upgraded Oils from Intermediate Pyrolysis. Biomass Bioenergy 2018, 116, 236–248. [Google Scholar] [CrossRef]
- Azeta, O.; Ayeni, A.O.; Agboola, O.; Elehinafe, F.B. A Review on the Sustainable Energy Generation from the Pyrolysis of Coconut Biomass. Sci. African 2021, 13, e00909. [Google Scholar] [CrossRef]
- Solar, J.; de Marco, I.; Caballero, B.M.; Lopez-Urionabarrenechea, A.; Rodriguez, N.; Agirre, I.; Adrados, A. Influence of Temperature and Residence Time in the Pyrolysis of Woody Biomass Waste in a Continuous Screw Reactor. Biomass Bioenergy 2016, 95, 416–423. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S. Effect of Particle Size on Pyrolysis of Single-Component Municipal Solid Waste in Fixed Bed Reactor. Int. J. Hydrogen Energy 2010, 35, 93–97. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste Tyre Pyrolysis—A Review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Aguilar, G.; Muley, P.D.; Henkel, C.; Boldor, D. Effects of Biomass Particle Size on Yield and Composition of Pyrolysis Bio-Oil Derived from Chinese Tallow Tree (Triadica sebifera L.) and Energy Cane (Saccharum complex) in an Inductively Heated Reactor. AIMS Energy 2015, 3, 838–850. [Google Scholar] [CrossRef]
- Bridgwater, A. Fast Pyrolysis of Biomass for the Production of Liquids. In Biomass Combustion Science, Technology and Engineering; Woodhead Publishing: Sawston, UK, 2013; pp. 130–171. ISBN 9780857091314. [Google Scholar]
- Ibrahim, H.A.-H. Introductory Chapter: Pyrolysis. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2020; ISBN 978-1-78984-064-3. [Google Scholar]
- Qiu, B.; Tao, X.; Wang, J.; Liu, Y.; Li, S.; Chu, H. Research Progress in the Preparation of High-Quality Liquid Fuels and Chemicals by Catalytic Pyrolysis of Biomass: A Review. Energy Convers. Manag. 2022, 261, 115647. [Google Scholar] [CrossRef]
- Kakaei, K.; Esrafili, M.D.; Ehsani, A. Introduction to Catalysis. Interface Sci. Technol. 2019, 27, 1–21. [Google Scholar] [CrossRef]
- Fermoso, J.; Coronado, J.M.; Serrano, D.P.; Pizarro, P. Pyrolysis of Microalgae for Fuel Production. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Elsevier Inc.: Duxford, UK, 2017; pp. 259–281. ISBN 9780081010273. [Google Scholar]
- Panda, A.K.; Singh, R.K.; Mishra, D.K. Thermolysis of Waste Plastics to Liquid fuelA Suitable Method for Plastic Waste Management and Manufacture of Value Added products—A World Prospective. Renew. Sustain. Energy Rev. 2010, 14, 233–248. [Google Scholar] [CrossRef]
- Yadav, K.; Jagadevan, S. Influence of Process Parameters on Synthesis of Biochar by Pyrolysis of Biomass: An Alternative Source of Energy. In Recent Advances in Pyrolysis; IntechOpen: London, UK, 2020; pp. 1–14. [Google Scholar] [CrossRef]
- Torri, C.; Reinikainen, M.; Lindfors, C.; Fabbri, D.; Oasmaa, A.; Kuoppala, E. Investigation on Catalytic Pyrolysis of Pine Sawdust: Catalyst Screening by Py-GC-MIP-AED. J. Anal. Appl. Pyrolysis 2010, 88, 7–13. [Google Scholar] [CrossRef]
- Abu Bakar, M.S.; Titiloye, J.O. Catalytic Pyrolysis of Rice Husk for Bio-Oil Production. J. Anal. Appl. Pyrolysis 2013, 103, 362–368. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic Pyrolysis of Biomass for Biofuels Production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Gupta, R.B.; Tu, M.; Taylor, S. Production of Hydrocarbon Fuels from Biomass Using Catalytic Pyrolysis under Helium and Hydrogen Environments. Bioresour. Technol. 2011, 102, 6742–6749. [Google Scholar] [CrossRef]
- Güngör, A.; Önenç, S.; Uçar, S.; Yanik, J. Comparison between the “one-Step” and “two-Step” Catalytic Pyrolysis of Pine Bark. J. Anal. Appl. Pyrolysis 2012, 97, 39–48. [Google Scholar] [CrossRef]
- Li, J.; Li, X.; Zhou, G.; Wang, W.; Wang, C.; Komarneni, S.; Wang, Y. Catalytic Fast Pyrolysis of Biomass with Mesoporous ZSM-5 Zeolites Prepared by Desilication with NaOH Solutions. Appl. Catal. A Gen. 2014, 470, 115–122. [Google Scholar] [CrossRef]
- Stefanidis, S.D.D.; Kalogiannis, K.G.G.; Iliopoulou, E.F.F.; Lappas, A.A.A.; Pilavachi, P.A.A. In-Situ Upgrading of Biomass Pyrolysis Vapors: Catalyst Screening on a Fixed Bed Reactor. Bioresour. Technol. 2011, 102, 8261–8267. [Google Scholar] [CrossRef]
- Pan, P.; Hu, C.; Yang, W.; Li, Y.; Dong, L.; Zhu, L.; Tong, D.; Qing, R.; Fan, Y. The Direct Pyrolysis and Catalytic Pyrolysis of Nannochloropsis Sp. Residue for Renewable Bio-Oils. Bioresour. Technol. 2010, 101, 4593–4599. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Huang, H.; Xiao, G. Comparison of Non-Catalytic and Catalytic Fast Pyrolysis of Corncob in a Fluidized Bed Reactor. Bioresour. Technol. 2009, 100, 1428–1434. [Google Scholar] [CrossRef]
- Compton, D.L.; Jackson, M.A.; Mihalcik, D.J.; Mullen, C.A.; Boateng, A.A. Catalytic Pyrolysis of Oak via Pyroprobe and Bench Scale, Packed Bed Pyrolysis Reactors. J. Anal. Appl. Pyrolysis 2011, 90, 174–181. [Google Scholar] [CrossRef]
- Demiral, İ.; Şensöz, S. The Effects of Different Catalysts on the Pyrolysis of Industrial Wastes (Olive and Hazelnut Bagasse). Bioresour. Technol. 2008, 99, 8002–8007. [Google Scholar] [CrossRef]
- Park, H.J.; Heo, H.S.; Park, Y.-K.; Yim, J.-H.; Jeon, J.-K.; Park, J.; Ryu, C.; Kim, S.-S. Clean Bio-Oil Production from Fast Pyrolysis of Sewage Sludge: Effects of Reaction Conditions and Metal Oxide Catalysts. Bioresour. Technol. 2010, 101, S83–S85. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic Pyrolysis of Rice Husk by Mixing with Zinc Oxide: Characterization of Bio-Oil and Its Rheological Behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Babich, I.V.; van der Hulst, M.; Lefferts, L.; Moulijn, J.A.; O’Connor, P.; Seshan, K. Catalytic Pyrolysis of Microalgae to High-Quality Liquid Bio-Fuels. Biomass Bioenergy 2011, 35, 3199–3207. [Google Scholar] [CrossRef]
- Rutkowski, P. Pyrolysis of Cellulose, Xylan and Lignin with the K2CO3 and ZnCl2 Addition for Bio-Oil Production. Fuel Process. Technol. 2011, 92, 517–522. [Google Scholar] [CrossRef]
- Barbooti, M.M.; Matlub, F.K.; Hadi, H.M. Catalytic Pyrolysis of Phragmites (Reed): Investigation of Its Potential as a Biomass Feedstock. J. Anal. Appl. Pyrolysis 2012, 98, 1–6. [Google Scholar] [CrossRef]
- Wolff, A. Management of Waste from the Health-Care Sector, 1st ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 11, ISBN 9780444638571. [Google Scholar]
- Jerzak, W.; Gao, N.; Kalemba-Rec, I.; Magdziarz, A. Catalytic Intermediate Pyrolysis of Post-Extraction Rapeseed Meal by Reusing ZSM-5 and Zeolite Y Catalysts. Catal. Today 2022, 404, 63–77. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent Insights into Lignocellulosic Biomass Pyrolysis: A Critical Review on Pretreatment, Characterization, and Products Upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Luo, G.; Resende, F.L.P. In-Situ and Ex-Situ Upgrading of Pyrolysis Vapors from Beetle-Killed Trees. Fuel 2016, 166, 367–375. [Google Scholar] [CrossRef]
- Muneer, B.; Zeeshan, M.; Qaisar, S.; Razzaq, M.; Iftikhar, H. Influence of in-Situ and Ex-Situ HZSM-5 Catalyst on Co-Pyrolysis of Corn Stalk and Polystyrene with a Focus on Liquid Yield and Quality. J. Clean. Prod. 2019, 237, 117762. [Google Scholar] [CrossRef]
- Kabakcı, S.B.; Hacıbektaşoğlu, Ş. Catalytic Pyrolysis of Biomass Literature Review. In Pyrolysis; Samer, M., Ed.; IntechOpen Limited: London, UK, 2017; pp. 167–196. [Google Scholar]
- Wan, S.; Wang, Y. A Review on Ex Situ Catalytic Fast Pyrolysis of Biomass. Front. Chem. Sci. Eng. 2014, 8, 280–294. [Google Scholar] [CrossRef]
- Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Overview of Catalytic Upgrading of Biomass Pyrolysis Vapors toward the Production of Fuels and High-Value Chemicals. Wiley Interdiscip. Rev. Energy Environ. 2019, 8, e322. [Google Scholar] [CrossRef]
- Wang, C.; Lei, H.; Zou, R.; Qian, M.; Mateo, W.; Lin, X.; Ruan, R. Biochar-Driven Simplification of the Compositions of Cellulose-Pyrolysis-Derived Biocrude Oil Coupled with the Promotion of Hydrogen Generation. Bioresour. Technol. 2021, 334, 125251. [Google Scholar] [CrossRef]
- Abu Bakar, M. Catalytic Intermediate Pyrolysis of Brunei Rice Husk for Bio-Oil Production. Ph.D. Thesis, Aston University, Birmingham, UK, 2013. [Google Scholar]
- Yang, H.; Coolman, R.; Karanjkar, P.; Wang, H.; Dornath, P.; Chen, H.; Fan, W.; Conner, W.C.; Mountziaris, T.J.; Huber, G. The Effects of Contact Time and Coking on the Catalytic Fast Pyrolysis of Cellulose. Green Chem. 2017, 19, 286–297. [Google Scholar] [CrossRef]
- Li, X.; Su, L.; Wang, Y.; Yu, Y.; Wang, C.; Li, X.; Wang, Z. Catalytic Fast Pyrolysis of Kraft Lignin with HZSM-5 Zeolite for Producing Aromatic Hydrocarbons. Front. Environ. Sci. Eng. China 2012, 6, 295–303. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Carlson, T.R.; Tompsett, G.A.; Conner, W.C.; Huber, G.W. Aromatic Production from Catalytic Fast Pyrolysis of Biomass-Derived Feedstocks. Top. Catal. 2009, 52, 241–252. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. CO2 Methanation: Principles and Challenges. In Studies in Surface Science and Catalysis; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 178, pp. 85–103. [Google Scholar]
- Hervy, M.; Weiss-Hortala, E.; Pham Minh, D.; Dib, H.; Villot, A.; Gérente, C.; Berhanu, S.; Chesnaud, A.; Thorel, A.; Le Coq, L.; et al. Reactivity and Deactivation Mechanisms of Pyrolysis Chars from Bio-Waste during Catalytic Cracking of Tar. Appl. Energy 2019, 237, 487–499. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Zhu, L.; Qian, M.; Chan, J.C.; Zhu, X.; Liu, Y.; Yadavalli, G.; Yan, D.; Wang, L.; et al. Development of a Catalytically Green Route from Diverse Lignocellulosic Biomasses to High-Density Cycloalkanes for Jet Fuels. Catal. Sci. Technol. 2016, 6, 4210–4220. [Google Scholar] [CrossRef]
- Onwudili, J.A.; Muhammad, C.; Williams, P.T. Influence of Catalyst Bed Temperature and Properties of Zeolite Catalysts on Pyrolysis-Catalysis of a Simulated Mixed Plastics Sample for the Production of Upgraded Fuels and Chemicals. J. Energy Inst. 2019, 92, 1337–1347. [Google Scholar] [CrossRef]
- Zhou, G.; Jensen, P.A.; Le, D.M.; Knudsen, N.O.; Jensen, A.D. Direct Upgrading of Fast Pyrolysis Lignin Vapor over the HZSM-5 Catalyst. Green Chem. 2016, 18, 1965–1975. [Google Scholar] [CrossRef]
- Williams, P.T.; Nugranad, N. Comparison of Products from the Pyrolysis and Catalytic Pyrolysis of Rice Husks. Energy 2000, 25, 493–513. [Google Scholar] [CrossRef]
- Al-Salem, S.M.; Antelava, A.; Constantinou, A.; Manos, G.; Dutta, A. A Review on Thermal and Catalytic Pyrolysis of Plastic Solid Waste (PSW). J. Environ. Manag. 2017, 197, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Liu, R.; Cai, J. Catalytic Fast Pyrolysis of Biomass over Zeolites for High Quality Bio-Oil—A Review. Fuel Process. Technol. 2018, 180, 32–46. [Google Scholar] [CrossRef]
- Martin, A. Zeolite Catalysis. Appl. Catal. 1990, 59, 118. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The Role of Shape Selectivity in Catalytic Fast Pyrolysis of Lignin with Zeolite Catalysts. Appl. Catal. A Gen. 2012, 447–448, 115–123. [Google Scholar] [CrossRef]
- Gao, L.; Sun, J.; Xu, W.; Xiao, G. Catalytic Pyrolysis of Natural Algae over Mg-Al Layered Double oxides/ZSM-5 (MgAl-LDO/ZSM-5) for Producing Bio-Oil with Low Nitrogen Content. Bioresour. Technol. 2017, 225, 293–298. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. In Situ Fast Pyrolysis of Biomass with Zeolite Catalysts for Bioaromatics/gasoline Production: A Review. Energy Convers. Manag. 2015, 105, 338–354. [Google Scholar] [CrossRef]
- Shirazi, L.; Jamshidi, E.; Ghasemi, M.R. The Effect of Si/Al Ratio of ZSM-5 Zeolite on Its Morphology, Acidity and Crystal Size. Cryst. Res. Technol. 2008, 43, 1300–1306. [Google Scholar] [CrossRef]
- Speight, J.G. Upgrading by Hydrocracking; Elsevier: Amsterdam, The Netherlands, 2019; pp. 467–528. [Google Scholar]
- Rehan, M.; Miandad, R.; Barakat, M.A.; Ismail, I.M.I.; Almeelbi, T.; Gardy, J.; Hassanpour, A.; Khan, M.Z.; Demirbas, A.; Nizami, A.S. Effect of Zeolite Catalysts on Pyrolysis Liquid Oil. Int. Biodeterior. Biodegrad. 2017, 119, 162–175. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; López, J.M.; Callén, M.S.; Murillo, R.; García, T. Production of Upgraded Bio-Oils by Biomass Catalytic Pyrolysis in an Auger Reactor Using Low Cost Materials. Fuel 2015, 141, 17–22. [Google Scholar] [CrossRef]
- Ly, H.V.; Lim, D.-H.; Sim, J.W.; Kim, S.-S.; Kim, J. Catalytic Pyrolysis of Tulip Tree (Liriodendron) in Bubbling Fluidized-Bed Reactor for Upgrading Bio-Oil Using Dolomite Catalyst. Energy 2018, 162, 564–575. [Google Scholar] [CrossRef]
- Mehmood, M. Dolomite and Dolomitization Model—A Short Review. Int. J. Hydrol. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Maisano, S.; Urbani, F.; Mondello, N.; Chiodo, V. Catalytic Pyrolysis of Mediterranean Sea Plant for Bio-Oil Production. Int. J. Hydrog. Energy 2017, 42, 28082–28092. [Google Scholar] [CrossRef]
- Valle, B.; Aramburu, B.; Santiviago, C.; Bilbao, J.; Gayubo, A.G. Upgrading of Bio-Oil in a Continuous Process with Dolomite Catalyst. Energy Fuels 2014, 28, 6419–6428. [Google Scholar] [CrossRef]
- Tursunov, O. A Comparison of Catalysts Zeolite and Calcined Dolomite for Gas Production from Pyrolysis of Municipal Solid Waste (MSW). Ecol. Eng. 2014, 69, 237–243. [Google Scholar] [CrossRef]
- Gerçel, H.F. Bio-Oil Production from Onopordum Acanthium L. by Slow Pyrolysis. J. Anal. Appl. Pyrolysis 2011, 92, 233–238. [Google Scholar] [CrossRef]
- Sulman, M.; Kosivtsov, Y.; Sulman, E.; Alfyorov, V.; Lugovoy, Y.; Molchanov, V.; Tyamina, I.; Misnikov, O.; Afanasjev, A.; Kumar, N.; et al. Influence of Aluminosilicate Materials on the Peat Low-Temperature Pyrolysis and Gas Formation. Chem. Eng. J. 2009, 154, 355–360. [Google Scholar] [CrossRef]
- Suprianto, T.; Winarto; Wijayanti, W.; Wardana, I. Effect of Activated Carbon Catalyst on the Cracking of Biomass Molecules into Light Hydrocarbons in Biomass Pyrolysis. In IOP Conference Series: Materials Science and Engineering, Proceedings of the 2nd International Conference on Mechanical Engineering Research and Application (iCOMERA 2020), Malang, Indonesia, 7–9 October 2020; IOPScience: Bristol, UK, 2021; Volume 1034, p. 12079. [Google Scholar]
- Zhang, Y.; Lei, H.; Yang, Z.; Duan, D.; Villota, E.; Ruan, R. From Glucose-Based Carbohydrates to Phenol-Rich Bio-Oils Integrated with Syngas Production via Catalytic Pyrolysis over an Activated Carbon Catalyst. Green Chem. 2018, 20, 3346–3358. [Google Scholar] [CrossRef]
- Duan, D.; Zhang, Y.; Lei, H.; Villota, E.; Ruan, R. Renewable Jet-Fuel Range Hydrocarbons Production from Co-Pyrolysis of Lignin and Soapstock with the Activated Carbon Catalyst. Waste Manag. 2019, 88, 1–9. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Liu, Y.; Liang, J.; Tang, J. Renewable Phenols Production by Catalytic Microwave Pyrolysis of Douglas Fir Sawdust Pellets with Activated Carbon Catalysts. Bioresour. Technol. 2013, 142, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lei, H.; Wang, L.; Yadavalli, G.; Wei, Y.; Zhang, X.; Zhu, L.; Liu, Y. Biofuel Production from Catalytic Microwave Pyrolysis of Douglas Fir Pellets over Ferrum-Modified Activated Carbon Catalyst. J. Anal. Appl. Pyrolysis 2015, 112, 74–79. [Google Scholar] [CrossRef]
- Oxide—Wikipedia. Available online: https://en.wikipedia.org/wiki/Oxide (accessed on 4 May 2021).
- Shi, K.; Shao, S.; Huang, Q.; Liang, X.; Lan, J.; Ya, L. Review of Catalytic Pyrolysis of Biomass for Bio-Oil. In Proceedings of the ICMREE2011—Proceedings 2011 International Conference on Materials for Renewable Energy and Environment, Shanghai, China, 20–22 May 2011; Volume 1, pp. 317–321. [Google Scholar]
- Liu, C.; Wang, H.; Karim, A.M.; Sun, J.; Wang, Y. Catalytic Fast Pyrolysis of Lignocellulosic Biomass. Chem. Soc. Rev. 2014, 43, 7594–7623. [Google Scholar] [CrossRef]
- Oxide|Chemical Compound|Britannica. Available online: https://www.britannica.com/science/oxide (accessed on 12 April 2023).
- Zhang, C.; Hu, X.; Guo, H.; Wei, T.; Dong, D.; Hu, G.; Hu, S.; Xiang, J.; Liu, Q.; Wang, Y. Pyrolysis of Poplar, Cellulose and Lignin: Effects of Acidity and Alkalinity of the Metal Oxide Catalysts. J. Anal. Appl. Pyrolysis 2018, 134, 590–605. [Google Scholar] [CrossRef]
- Che, Q.; Yang, M.; Wang, X.; Chen, X.; Chen, W.; Yang, Q.; Yang, H.; Chen, H. Aromatics Production with Metal Oxides and ZSM-5 as Catalysts in Catalytic Pyrolysis of Wood Sawdust. Fuel Process. Technol. 2019, 188, 146–152. [Google Scholar] [CrossRef]
- Li, D.; Yao, F.; Guo, Q.X. Upgraded Acidic Components of Bio-Oil through Catalytic Ketonic Condensation. Energy Fuels 2009, 23, 564–568. [Google Scholar] [CrossRef]
- Aysu, T. Pyrolysis of Isochrysis Microalgae with Metal Oxide Catalysts for Bio-Oil Production. J. Turk. Chem. Soc. Sect. A Chem. 2016, 4, 395. [Google Scholar] [CrossRef]
- Chong, Y.Y.; Thangalazhy-Gopakumar, S.; Ng, H.K.; Lee, L.Y.; Gan, S. Effect of Oxide Catalysts on the Properties of Bio-Oil from in-Situ Catalytic Pyrolysis of Palm Empty Fruit Bunch Fiber. J. Environ. Manag. 2019, 247, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, J.; Wang, D.; Zheng, Z. Advanced Catalytic Upgrading of Biomass Pyrolysis Vapor to Bio-Aromatics Hydrocarbon: A Review. Appl. Energy Combust. Sci. 2022, 10, 100061. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, J.; Liu, C.; Lu, Y.; Lin, X.; Li, W.; Zheng, Z. Efficient and Stable Ni-Cu Catalysts for Ex Situ Catalytic Pyrolysis Vapor Upgrading of Oleic Acid into Hydrocarbon: Effect of Catalyst Support, Process Parameters and Ni-to-Cu Mixed Ratio. Renew. Energy 2020, 154, 797–812. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Dai, L.; Yu, Z.; Yang, Q.; Yang, S.; Jiang, D.; Ma, Z.; Wu, Q.; Zhang, B.; et al. Co-Pyrolysis of Biomass and Soapstock in a Downdraft Reactor Using a Novel ZSM-5/SiC Composite Catalyst. Bioresour. Technol. 2019, 279, 202–208. [Google Scholar] [CrossRef]
- Kaewpengkrow, P.; Atong, D.; Sricharoenchaikul, V. Selective Catalytic Fast Pyrolysis of Jatropha Curcas Residue with Metal Oxide Impregnated Activated Carbon for Upgrading Bio-Oil. Int. J. Hydrogen Energy 2017, 42, 18397–18409. [Google Scholar] [CrossRef]
- Nikkhah, H.; Tavasoli, A.; Jafarian, S. Investigating the Influence of Acid Washing Pretreatment and Zn/activated Biochar Catalyst on Thermal Conversion of Cladophora Glomerata to Value-Added Bio-Products. Energy Convers. Manag. 2020, 225, 113392. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Kim, C.S.; Ellis, N.; Bi, X. Microwave-Assisted Catalytic Pyrolysis of Switchgrass for Improving Bio-Oil and Biochar Properties. Bioresour. Technol. 2016, 201, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Vispute, T. Pyrolysis Oils: Characterization, Stability Analysis, and Catalytic Upgrading to Fuels and Chemicals. Ph.D. Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2011. [Google Scholar]
- Thangalazhy-Gopakumar, S.; Adhikari, S.; Ravindran, H.; Gupta, R.B.; Fasina, O.; Tu, M.; Fernando, S.D. Physiochemical Properties of Bio-Oil Produced at Various Temperatures from Pine Wood Using an Auger Reactor. Bioresour. Technol. 2010, 101, 8389–8395. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Yang, H.; Wang, X.; Che, Q.; Chen, W.; Chen, H. Catalytic Fast Pyrolysis of Biomass: Selective Deoxygenation to Balance the Quality and Yield of Bio-Oil. Bioresour. Technol. 2019, 273, 153–158. [Google Scholar] [CrossRef]
- Veses, A.; Aznar, M.; Martínez, I.; Martínez, J.D.; López, J.M.; Navarro, M.V.; Callén, M.S.; Murillo, R.; García, T. Catalytic Pyrolysis of Wood Biomass in an Auger Reactor Using Calcium-Based Catalysts. Bioresour. Technol. 2014, 162, 250–258. [Google Scholar] [CrossRef]
- Thangalazhy-Gopakumar, S.; Wei Lee, C.; Gan, S.; Kiat Ng, H.; Yee Lee, L. Comparison of Bio-Oil Properties from Non-Catalytic and In-Situ Catalytic Fast Pyrolysis of Palm Empty Fruit Bunch. Mater. Today Proc. 2018, 5, 23456–23465. [Google Scholar] [CrossRef]
- Muley, P.D.; Henkel, C.; Abdollahi, K.K.; Boldor, D. Pyrolysis and Catalytic Upgrading of Pinewood Sawdust Using an Induction Heating Reactor. Energy Fuels 2015, 29, 7375–7385. [Google Scholar] [CrossRef]
- Sheng, C.; Azevedo, J.L.T. Estimating the Higher Heating Value of Biomass Fuels from Basic Analysis Data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Pulidori, E.; Gonzalez-Rivera, J.; Pelosi, C.; Ferrari, C.; Bernazzani, L.; Bramanti, E.; Tiné, M.R.; Duce, C.; Pulidori, E.; Gonzalez-Rivera, J.; et al. Thermochemical Evaluation of Different Waste Biomasses (Citrus Peels, Aromatic Herbs, and Poultry Feathers) towards Their Use for Energy Production. Thermo 2023, 3, 66–75. [Google Scholar] [CrossRef]
- Ben, H.; Wu, F.; Wu, Z.; Han, G.; Jiang, W.; Ragauskas, A.J. A Comprehensive Characterization of Pyrolysis Oil from Softwood Barks. Polymers 2019, 11, 1387. [Google Scholar] [CrossRef] [PubMed]
- Aysu, T.; Durak, H.; Güner, S.; Bengü, A.Ş.; Esim, N. Bio-Oil Production via Catalytic Pyrolysis of Anchusa Azurea: Effects of Operating Conditions on Product Yields and Chromatographic Characterization. Bioresour. Technol. 2016, 205, 7–14. [Google Scholar] [CrossRef]
- Zabeti, M.; Nguyen, T.S.; Lefferts, L.; Heeres, H.J.; Seshan, K. In Situ Catalytic Pyrolysis of Lignocellulose Using Alkali-Modified Amorphous Silica Alumina. Bioresour. Technol. 2012, 118, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Gao, L.; Wang, W.; Zhang, G.; Ji, J. Bio-Fuel Production from the Catalytic Pyrolysis of Soybean Oil over Me-Al-MCM-41 (Me = La, Ni or Fe) Mesoporous Materials. J. Anal. Appl. Pyrolysis 2013, 104, 325–329. [Google Scholar] [CrossRef]
- Nokkosmäki, M.I.; Kuoppala, E.T.; Leppämäki, E.A.; Krause, A.O.I. Catalytic Conversion of Biomass Pyrolysis Vapours with Zinc Oxide. J. Anal. Appl. Pyrolysis 2000, 55, 119–131. [Google Scholar] [CrossRef]
- Mante, O.D.; Agblevor, F.A. Catalytic Conversion of Biomass to Bio-Syncrude Oil. Biomass Convers. Biorefinery 2011, 1, 203–215. [Google Scholar] [CrossRef]
- Dong, Z.; Bai, X.; Xu, D.; Li, W. Machine Learning Prediction of Pyrolytic Products of Lignocellulosic Biomass Based on Physicochemical Characteristics and Pyrolysis Conditions. Bioresour. Technol. 2023, 367, 128182. [Google Scholar] [CrossRef]
- Bal, M.; Amasyali, M.F.; Sever, H.; Kose, G.; Demirhan, A. Performance Evaluation of the Machine Learning Algorithms Used in Inference Mechanism of a Medical Decision Support System. Sci. World J. 2014, 2014, 137896. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, X.; Xia, A.; Shah, A.A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. The Role of Machine Learning to Boost the Bioenergy and Biofuels Conversion. Bioresour. Technol. 2022, 343, 126099. [Google Scholar] [CrossRef] [PubMed]
- Ascher, S.; Watson, I.; You, S. Machine Learning Methods for Modelling the Gasification and Pyrolysis of Biomass and Waste. Renew. Sustain. Energy Rev. 2022, 155, 111902. [Google Scholar] [CrossRef]
- Pan, R.; Martins, M.F.; Debenest, G. Optimization of Oil Production through Ex-Situ Catalytic Pyrolysis of Waste Polyethylene with Activated Carbon. Energy 2022, 248, 123514. [Google Scholar] [CrossRef]
- Yang, K.; Wu, K.; Zhang, H. Machine Learning Prediction of the Yield and Oxygen Content of Bio-Oil via Biomass Characteristics and Pyrolysis Conditions. Energy 2022, 254, 124320. [Google Scholar] [CrossRef]
- Balsora, H.K.; Kartik, A.; Dua, V.; Joshi, J.B.; Kataria, G.; Sharma, A.; Chakinala, A.G. Machine Learning Approach for the Prediction of Biomass Pyrolysis Kinetics from Preliminary Analysis. J. Environ. Chem. Eng. 2022, 10, 108025. [Google Scholar] [CrossRef]
- Sharma, A.; Suryawanshi, B.; Mohanty, B.; Sawarkar, A.N. Comparison of Artificial Neural Network and Response Surface Methodology for Evaluation of the Predictive Capability of Bio-Oil Yield from Pyrolysis of Mangifera Indica Wood Sawdust. Fuel 2023, 338, 127251. [Google Scholar] [CrossRef]
- Anex, R.P.; Aden, A.; Kazi, F.K.; Fortman, J.; Swanson, R.M.; Wright, M.M.; Satrio, J.A.; Brown, R.C.; Daugaard, D.E.; Platon, A.; et al. Techno-Economic Comparison of Biomass-to-Transportation Fuels via Pyrolysis, Gasification, and Biochemical Pathways. Fuel 2010, 89, S29–S35. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, K.; Bhaskar, T. Hydochar and Biochar: Production, Physicochemical Properties and Techno-Economic Analysis. Bioresour. Technol. 2020, 310, 123442. [Google Scholar] [CrossRef] [PubMed]
- López-González, D.; Puig-Gamero, M.; Acién, F.G.; García-Cuadra, F.; Valverde, J.L.; Sanchez-Silva, L. Energetic, Economic and Environmental Assessment of the Pyrolysis and Combustion of Microalgae and Their Oils. Renew. Sustain. Energy Rev. 2015, 51, 1752–1770. [Google Scholar] [CrossRef]
- Hoang, A.T.; Ong, H.C.; Fattah, I.M.R.; Chong, C.T.; Cheng, C.K.; Sakthivel, R.; Ok, Y.S. Progress on the Lignocellulosic Biomass Pyrolysis for Biofuel Production toward Environmental Sustainability. Fuel Process. Technol. 2021, 223, 106997. [Google Scholar] [CrossRef]
- Haeldermans, T.; Campion, L.; Kuppens, T.; Vanreppelen, K.; Cuypers, A.; Schreurs, S. A Comparative Techno-Economic Assessment of Biochar Production from Different Residue Streams Using Conventional and Microwave Pyrolysis. Bioresour. Technol. 2020, 318, 124083. [Google Scholar] [CrossRef]
- Zein, S.H.; Antony, A. Techno-Economic Analysis and Feasibility of Industrial-Scale Activated Carbon Production from Agricultural Pea Waste Using Microwave-Assisted Pyrolysis: A Circular Economy Approach. Processes 2022, 10, 1702. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Ruziwa, W.R.; Musademba, D. A Systematic Review of the Techno-Economic Assessment and Biomass Supply Chain Uncertainties of Biofuels Production from Fast Pyrolysis of Lignocellulosic Biomass. Fuel Commun. 2023, 14, 100086. [Google Scholar] [CrossRef]
- Nematian, M.; Keske, C.; Ng’ombe, J.N. A Techno-Economic Analysis of Biochar Production and the Bioeconomy for Orchard Biomass. Waste Manag. 2021, 135, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.C.; Zhang, N. Renewable Energy from Pyrolysis Using Crops and Agricultural Residuals: An Economic and Environmental Evaluation. Energy 2015, 90, 1532–1544. [Google Scholar] [CrossRef]
- Thilakaratne, R.; Brown, T.; Li, Y.; Hu, G.; Brown, R. Mild Catalytic Pyrolysis of Biomass for Production of Transportation Fuels: A Techno-Economic Analysis. Green Chem. 2014, 16, 627–636. [Google Scholar] [CrossRef]
- Shoaib Ahmed Khan, M.; Grioui, N.; Halouani, K.; Benelmir, R. Techno-Economic Analysis of Production of Bio-Oil from Catalytic Pyrolysis of Olive Mill Wastewater Sludge with Two Different Cooling Mechanisms. Energy Convers. Manag. X 2022, 13, 100170. [Google Scholar] [CrossRef]
- Wang, Y.; Akbarzadeh, A.; Chong, L.; Du, J.; Tahir, N.; Awasthi, M.K. Catalytic Pyrolysis of Lignocellulosic Biomass for Bio-Oil Production: A Review. Chemosphere 2022, 297, 134181. [Google Scholar] [CrossRef] [PubMed]
- Vasalos, I.A.; Lappas, A.A.; Kopalidou, E.P.; Kalogiannis, K.G. Biomass Catalytic Pyrolysis: Process Design and Economic Analysis. Wiley Interdiscip. Rev. Energy Environ. 2016, 5, 370–383. [Google Scholar] [CrossRef]
- Rajesh Banu, J.; Preethi; Kavitha, S.; Gunasekaran, M.; Kumar, G. Microalgae Based Biorefinery Promoting Circular Bioeconomy-Techno Economic and Life-Cycle Analysis. Bioresour. Technol. 2020, 302, 122822. [Google Scholar] [CrossRef]




| Feedstocks | Catalysts | Pyrolyzer Type | Ref. |
|---|---|---|---|
| Pine sawdust | ZSM-5 | Fluidized bed | [157] |
| Rice husk | ZSM-5 | Fixed bed | [158] |
| Aspen wood | ZSM-5 | Tubular bed | [159] |
| Pinewood chip | ZSM-5 | Fixed bed | [160] |
| Pine barks | ZSM-5 | Fixed bed | [161] |
| Sawdust | ZSM-5 | Fluidized bed | [162] |
| Beechwood | ZSM-5 | Fixed bed | [163] |
| Nannochloropsis sp. | ZSM-5 | Fixed bed | [164] |
| Corncob | ZSM-5 | Fluidized bed | [165] |
| Pine sawdust oak | Mordenite | Packed bed | [166] |
| Beechwood | FeO, Al2O3 | Fixed bed | [163] |
| Hazelnut bagasse | FeO, Al2O3 | Fixed bed | [167] |
| Sewage sludge | La2O3 | Fluidized bed | [168] |
| Rice husk | ZnO | Fixed bed | [169] |
| Rice husk | Al-MSU-F, Al-MCM-41, Rice husk ash | Fixed bed | [158] |
| Microalgae | Na2CO3 | Fixed bed | [170] |
| Cellulose | K2CO3 | Fixed bed | [171] |
| Reed | K2CO3 | Fixed bed | [172] |
| Sample | Catalyst | Products Yield/wt% | Major Components in Bio-Oil | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochar | Syngas | Bio-Oil | Organic Acids | Aromatic Hydrocarbon | Ketones | Furans | Heavy Phenols | Light Phenols | ANHYDROSUGARS | ||
| Rice husk | -- | 41.92 | 18.47 | 39.61 | 16.06 | 1.51 | 14.75 | 8.99 | 13.57 | 12.77 | 3.65 |
| ZSM-5 | 42.27 | 19.45 | 38.29 | 15.49 | 2.24 | 12.96 | 9.63 | 13.48 | 14.44 | 2.04 | |
| Al-MSU-F | 43.31 | 19.18 | 39.59 | 15.51 | 1.57 | 13.82 | 9.57 | 14.24 | 12.56 | 2.34 | |
| Al-MCM-41 | 43.15 | 18.80 | 39.98 | 14.17 | 1.42 | 13.65 | 9.57 | 14.99 | 12.60 | 2.88 | |
| BRHA | 42.27 | 21.62 | 38.29 | 16.16 | 1.53 | 14.76 | 9.48 | 12.84 | 12.66 | 2.75 | |
| Sample | Catalyst | Temperature, °C | Products, wt% | Reference | ||
|---|---|---|---|---|---|---|
| Biochar | Bio-Oil | Syngas | ||||
| Tulip tree | Sand | 450 | 17.95 | 49.03 | 21.84 | [203] |
| Dolomite | 450 | 26.04 | 34.26 | 39.70 | ||
| Sample | Catalyst | Products, wt% | Major Components, wt% | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Bio-oil | Syngas | Biochar | Phenols | Guaiacols | Furans | Ketones/Aldehydes | |||
| Douglas fir sawdust pellets | No catalyst | 45.20 | 11.80 | 43.00 | 2.540 | 48.78 | 3.90 | 10.36 | [213] |
| GAC 830 PLUS (Bituminous coal) | 31.00 | 44.60 | 24.40 | 25.74 | 15.65 | 14.24 | 16.85 | ||
| DARCO MRX (Wood) | 26.50 | 52.67 | 20.83 | 71.87 | 1.33 | - | 3.03 | ||
| DARCO 830 (Lignite coal) | 28.97 | 47.53 | 23.5 | 74.77 | - | - | 2.02 | ||
| Catalyst/Biomass | Phenol | Levoglucosenone | 2-Furancarboxaldehyde, 5-methyl- | ||||||
| Glucose | 0 | 63.3 | 18.8 | 17.6 | - | 29.9 | 6.8 | [211] | |
| 0.28 | 52.8 | 18.7 | 24.0 | 4.7 | 18.8 | 8.8 | |||
| 0.4 | 49.5 | 18.9 | 25.6 | 10.9 | 20.5 | 10.8 | |||
| 0.7 | 45.8 | 18.8 | 29.1 | 34.5 | - | 24.4 | |||
| 1.13 | 44.6 | 18.7 | 29.6 | 88.4 | - | - | |||
| Sample | Catalyst | Products, wt% | Major Components, wt% | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Bio-oil | Biochar | Syngas | Acetic Acid | Anhydrosugar | Furan | Phenol | |||
| Palm empty fruit bunches | No catalyst | 42.3 | 27.2 | 30.5 | 1.95 | 0.22 | 0.38 | 1.54 | [223] |
| CaO, 5% | 39.9 | 29.4 | 30.7 | 1.53 | 0.07 | 0.39 | 1.17 | ||
| CaO, 10% | 40.4 | 30.7 | 28.9 | 0.9 | 0.1 | 0.38 | 0.64 | ||
| MgO, 5% | 39.3 | 29 | 31.6 | 2.32 | 0.06 | 0.49 | 1.11 | ||
| MgO, 10% | 42.3 | 25.7 | 32 | 2.08 | 0.04 | 0.46 | 0.85 | ||
| ZnO, 5% | 44.7 | 27 | 28.2 | 2.29 | 0.07 | 0.41 | 1.2 | ||
| ZnO, 10% | 42.2 | 26.8 | 31 | 2.23 | 0.1 | 0.52 | 1.2 | ||
| Isochrysis Microalgae | No catalyst | 16.82 | 37.86 | 45.32 | [222] | ||||
| 20.13 | 33.96 | 45.91 | |||||||
| 18.07 | 30.76 | 51.17 | |||||||
| CeO2 | 20.82 | 34.83 | 44.35 | ||||||
| 22.97 | 31.08 | 45.95 | |||||||
| 19.91 | 28.48 | 51.61 | |||||||
| TiO2 | 22.41 | 33.18 | 44.41 | ||||||
| 24.30 | 29.53 | 46.17 | |||||||
| 21.10 | 26.39 | 52.51 | |||||||
| Al2O3 | 19.36 | 36.47 | 44.17 | ||||||
| 22.10 | 34.34 | 43.56 | |||||||
| 20.03 | 31.80 | 48.17 | |||||||
| Sample | Catalyst | Products Yield (wt%) | ||
|---|---|---|---|---|
| Biochar | Bio-Oil | Syngas | ||
| Acid-washed Cladophora Glomerata (ACG) | No catalyst | 34 | 46 | 20 |
| Zn/Activated Carbon | 37 | 35 | 28 | |
| Sample | Catalyst | Temperature (°C) | pH | Reference |
|---|---|---|---|---|
| Cotton stalk | No catalyst | 600 | 2.8 (±0.1) | [232] |
| CaO | 4.8 (±0.1) | |||
| MgO | 3.9 (±0.2) | |||
| Al2O3 | 4.1 (±0.1) | |||
| ZSM-5 | 3.9 (±0.2) | |||
| MCM-41 | 2.8 (±0.1) | |||
| CuO | 4.1 (±0.2) | |||
| Fe2O3 | 3.5 (±0.1) | |||
| NiO | 3.9 (±0.1) | |||
| ZnO | 4.3 (±0.1) | |||
| ZrO2 | 4.0 (±0.2) | |||
| TiO2 | 4.0 (±0.1) | |||
| Brunei rice husk | No catalyst | 450 | 3 | [158] |
| ZSM-5 | 2.74 | |||
| Al-MCM-41 | 2.83 | |||
| Al-MSU-F | 2.69 | |||
| BRHA | 2.79 | |||
| Pinus halepensis | Sand | 450 | 2.9 | [233] |
| Sand-CaO | 4.3 | |||
| Sand-CaO.MgO | 4.6 | |||
| Empty fruit bunch (EFB) | No catalyst | 550 | 3.8 ± 0.2 | [234] |
| 1% CaO | 3.9 ± 0.2 | |||
| 5% CaO | 3.92 ± 0.2 | |||
| 10% CaO | 4.16 ± 0.3 |
| Sample | Catalyst | Temperature (°C) | Water (wt%) | Reference |
|---|---|---|---|---|
| Pinewood chips | No catalyst | 425 | 20.8 ± 3.9 | [231] |
| 450 | 21.0 ± 4.6 | |||
| 475 | 20.3 ± 3.9 | |||
| 500 | 20.6 ± 3.9 | |||
| Brunei rice husk | No catalyst | 450 (°C) | 52.60 | [158]. |
| ZSM-5 | 55.56 | |||
| Al-MCM-41 | 54.66 | |||
| Al-MSU-F | 54.64 | |||
| BRHA | 55.43 | |||
| Pinus halepensis | Sand | 450 | 3.5 | [233]. |
| Sand-CaO | 5.9 | |||
| Sand-CaO·MgO | 6.3 |
| Sample | Catalyst | Primary Reactor Temperature, °C | HHV-Wet Basis, MJ/kg | HHV-Dry Basis, MJ/kg |
|---|---|---|---|---|
| Pinewood chips | No catalyst | 425 | 18.6 ± 0.8 | |
| 450 | 19.1 ± 1.3 | |||
| 475 | 18.4 ± 0.5 | |||
| 500 | 19.7 ± 1.2 | |||
| Brunei Rice husk | No catalyst | 450 | 13.61 | 28.71 |
| ZSM-5 | 13.33 | 30.01 | ||
| Al-MCM-41 | 14.41 | 31.79 | ||
| Al-MSU-F | 14.98 | 33.02 | ||
| BRHA | 17.17 | 38.53 | ||
| Empty Fruit Bunch (EFB) | No catalyst | 550 | 13.46 ± 1.98 | |
| 1% CaO | 14.95 ± 0.58 | |||
| 5% CaO | 15.80 ± 0.21 | |||
| 10% CaO | 17.61 ± 1.94 |
| Temperature, °C | Components | No catalyst | Na2CO3 | ZnCl2 | Al2O3 | Ca(OH)2 |
|---|---|---|---|---|---|---|
| 350 | C, wt% | 45.68 | 56.00 | 46.52 | 49.82 | 50.76 |
| H, wt% | 7.15 | 7.48 | 8.19 | 6.96 | 7.16 | |
| N, wt% | 1.57 | 3.37 | 1.74 | 2.28 | 2.64 | |
| O, wt% | 45.60 | 33.15 | 43.55 | 40.94 | 39.44 | |
| H/C molar ratio | 1.87 | 1.60 | 2.11 | 1.67 | 1.69 | |
| O/C molar ratio | 0.74 | 0.44 | 0.70 | 0.61 | 0.58 | |
| 550 | C, wt% | 45.59 | 57.06 | 47.41 | 49.90 | 57.01 |
| H, wt% | 7.11 | 7.48 | 7.21 | 7.43 | 7.05 | |
| N, wt% | 1.58 | 2.71 | 2.14 | 1.44 | 2.89 | |
| O, wt% | 45.72 | 32.75 | 43.24 | 41.23 | 33.05 | |
| H/C molar ratio | 1.87 | 1.57 | 1.82 | 1.78 | 1.48 | |
| O/C molar ratio | 0.75 | 0.43 | 0.68 | 0.62 | 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reza, M.S.; Iskakova, Z.B.; Afroze, S.; Kuterbekov, K.; Kabyshev, A.; Bekmyrza, K.Z.; Kubenova, M.M.; Bakar, M.S.A.; Azad, A.K.; Roy, H.; et al. Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review. Energies 2023, 16, 5547. https://doi.org/10.3390/en16145547
Reza MS, Iskakova ZB, Afroze S, Kuterbekov K, Kabyshev A, Bekmyrza KZ, Kubenova MM, Bakar MSA, Azad AK, Roy H, et al. Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review. Energies. 2023; 16(14):5547. https://doi.org/10.3390/en16145547
Chicago/Turabian StyleReza, Md Sumon, Zhanar Baktybaevna Iskakova, Shammya Afroze, Kairat Kuterbekov, Asset Kabyshev, Kenzhebatyr Zh. Bekmyrza, Marzhan M. Kubenova, Muhammad Saifullah Abu Bakar, Abul K. Azad, Hridoy Roy, and et al. 2023. "Influence of Catalyst on the Yield and Quality of Bio-Oil for the Catalytic Pyrolysis of Biomass: A Comprehensive Review" Energies 16, no. 14: 5547. https://doi.org/10.3390/en16145547







