On the Thermal Stability of a Counter-Current Fixed-Bed Gasifier
Abstract
:1. Introduction
- The equations are formulated in non-dimensional form;
- They are simplified to such an extent that they can be easily solved, in same cases also analytically;
- Non-dimensional parameters which arise in the non-dimensional equations are responsible alone for the thermal structure of the system.
2. The Method of the Investigation
2.1. The Equation of the Model
- Total mass balance (for the gas and the solid system);
- Enthalpy balance written as an equation for the temperature (for both systems);
- Mass balances for each component in each phase.
- Drying and pyrolysis of the fuel.
- Heterogeneous reactions of the fixed char with the gaseous phase (heterogeneous oxidation and gasification reactions);
- Homogeneous reactions in the gas phase (homogeneous oxidation and water gas shift reaction).
2.2. Assumptions and Simplifications
- Ideal gas with constant specific heat. The specific enthalpy can then be calculated as follows:
- Ideal solid with constant specific heat. From this assumption, it follows that:
- Constant porosity .
- Negligible thermal conductivity of the gas ().
- Negligible mass diffusivity for both phases ().
- 6.
- Heterogeneous oxidation and pyrolysis are the only two processes considered here.
- 7.
- Pyrolysis is a single reaction in which the fuel is converted into gas and char. The rate is chemically controlled and is written using the classical Arrhenius rate:in which is the mass fraction of the fuel in the solid.
- 8.
- The pyrolysis energy is negative, and the associated source term is present in the gas–energy equation only. This assumption is justified when the enthalpy of the gas alone determines the pyrolysis process. The gas phase has a higher temperature than the solid, and heat is transferred from the gas into the particle. A thin film on the particle surface is heated, and chemical bonds will be promptly disrupted before the heat can be transferred into the particle’s internal region. Only the gas takes part energetically in the pyrolysis process.
- 9.
- In the modeling of the oxidation rate, both kinetics and diffusion of oxygen in the bulk are considered. The reaction order for the oxygen is assumed equal to one, and the mathematical expression of the reaction rate takes the classical form:where and are the kinetic and diffusion reaction velocity per unit area, respectively. The term represents the area of contact between the phases per unit volume, and is the mass fraction of the char in the solid phase. The kinetics velocity is expressed in an Arrhenius form, while the diffusion velocity is defined as a power of the temperature T.
- 10.
- The heat of combustion is positive, and the associated source term is split between the gas and the solid using a factor f, expressing the amount of heat absorbed directly by the solid phase.
2.3. The Equations in Non-Dimensional Form
- The length L of the reactor is used to introduce the variable
- The non-dimensional temperature is defined as:where is the inlet temperature of the gas (usually room temperature) and a normalizing temperature taken . The ignition temperature is not used since its value changes depending on the reactor input conditions, and it is not a property of the fuel alone. As it has been chosen here, a fixed temperature allows for a quick comparison among the temperature profiles for different configurations.
- The length of the reactor and the initial velocity of the solid are used to normalize the time:
- The pressure is normalized using the atmospheric pressure:
- The densities of both phases ( and ) are normalized to and using the initial density of the respective phase, namely at for the gas and at for the solid.
- The fluxes are also normalized using the initial values of the respective streams as:
- The mass sources are normalized using the mass flow rate of the solid:
- The heat of reactions ( and ) are both normalized using the temperature difference and the specific heat of the solid phase:
2.4. The Mathematical Solution
- The shooting method in which the differential equations are written as a system of first-order ordinary differential equations. Boundary conditions at one interval endpoint must be guessed or solved to meet the conditions at the other endpoint. The advantage of this method is that the solution of the ODE system can be easily calculated using highly accurate methods (such as Runge–Kutta methods), where the accuracy can be controlled by grid refining.
- The method proposed by Schwabauer in [30] in which the gas phase and the solid phase are solved separately and iteratively until convergence is achieved.
- The solution of a time-dependent problem (or pseudo-time dependent) for a long enough time interval until steady-state is reached.
3. Solution Using the Flame Sheet Approximation
- The solutions of the equations for the total mass, Equations (50), are straightforward.
- Since the details of the flame front is not resolved in this approximation, the equations for the temperatures are decoupled from the Equations (51) for the fluxes and the concentrations.
- The equations for the temperatures can be analytically solved (see later).
- The position and are determined by the temperature profile as a function of the parameters of the problem.
3.1. The Temperature Profile
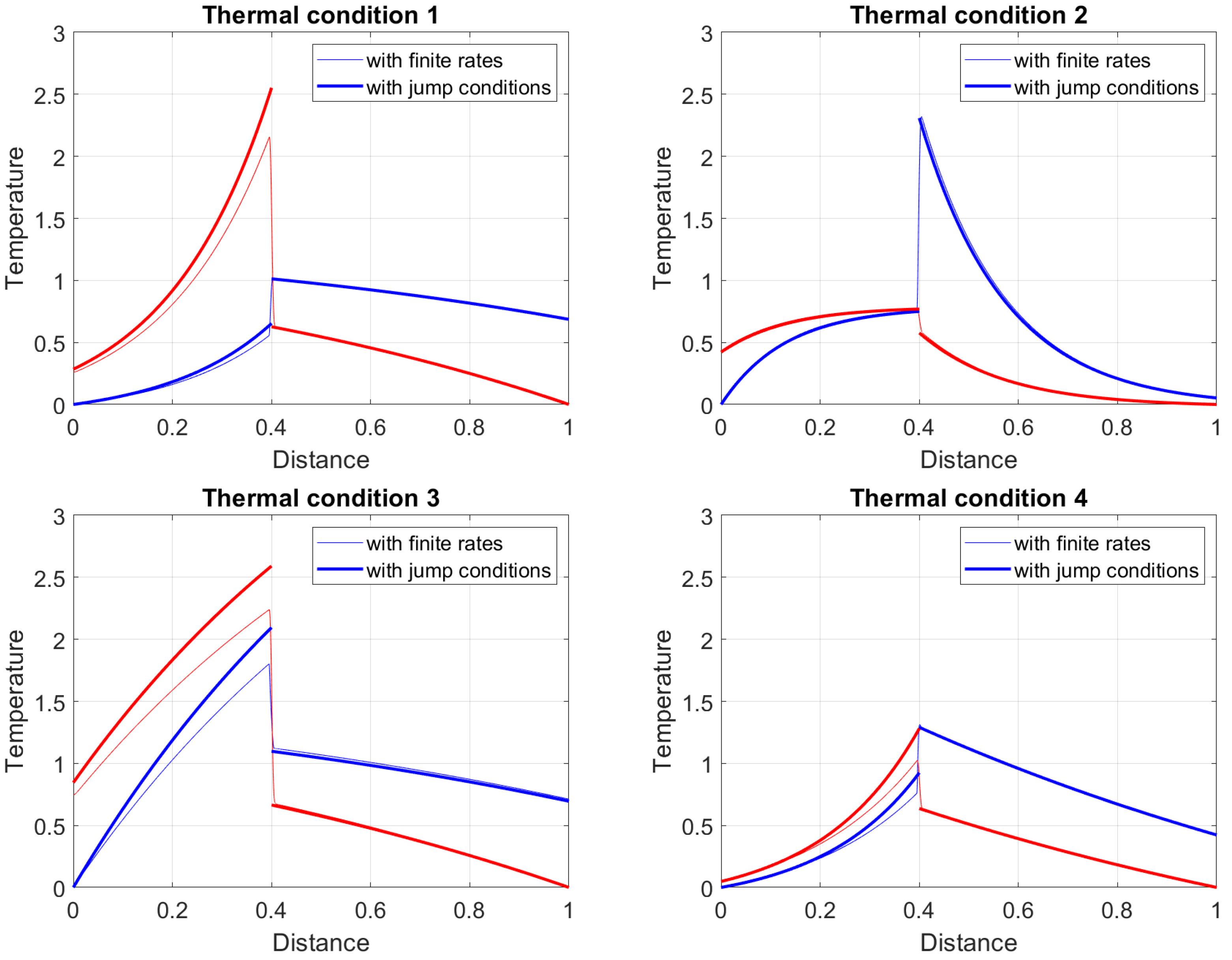
- Thermal state TC1, in which and , at the top left part of Figure 1.
- Thermal state TC2, in which and , at the top right part of Figure 1.
- Thermal state TC3, in which and , at bottom left part of Figure 1.
- Thermal state TC4, in which and , at the bottom right part of Figure 1.
3.1.1. Conditions for the Existence of Each Thermal Condition
3.1.2. Relaxation of Various Assumptions
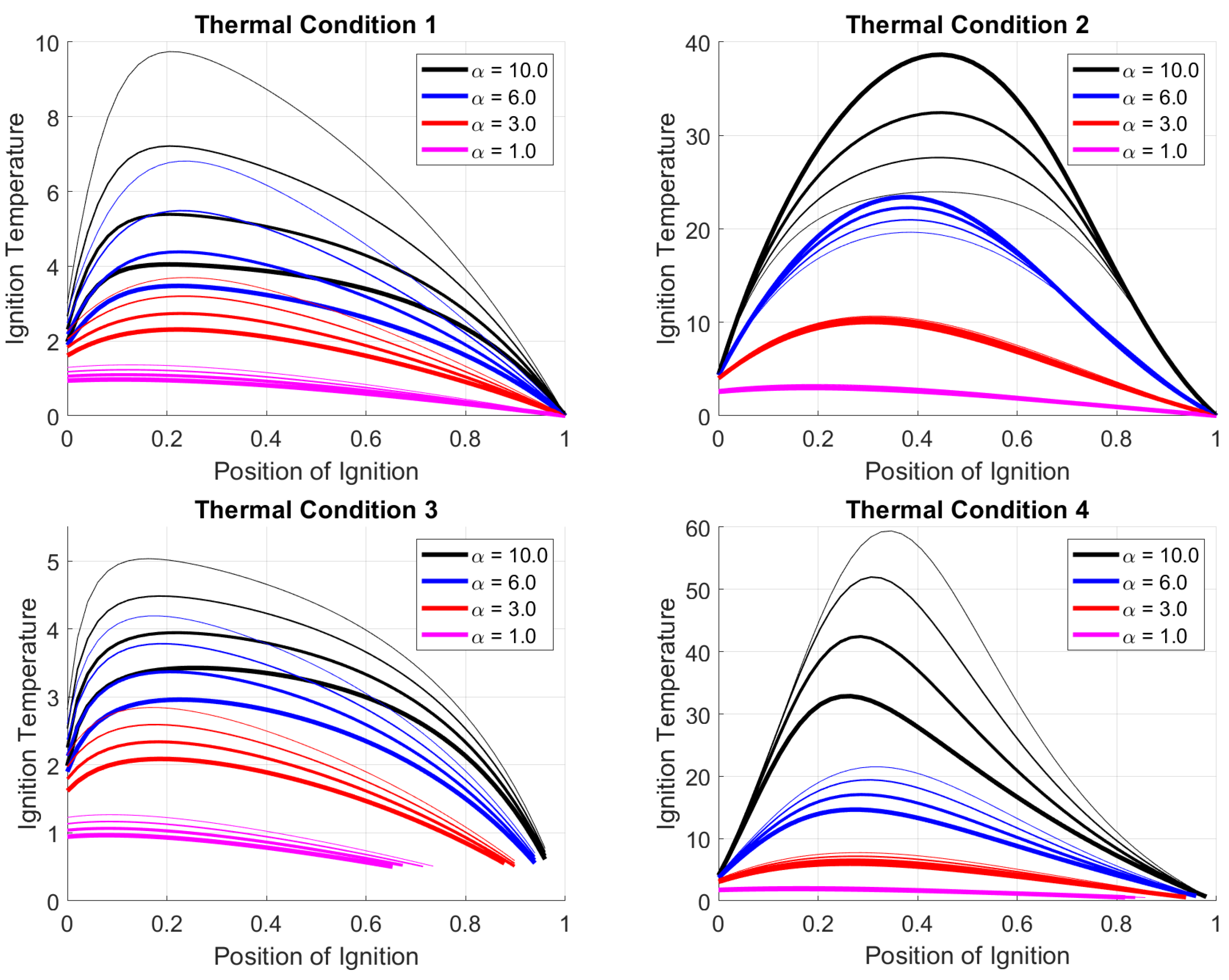
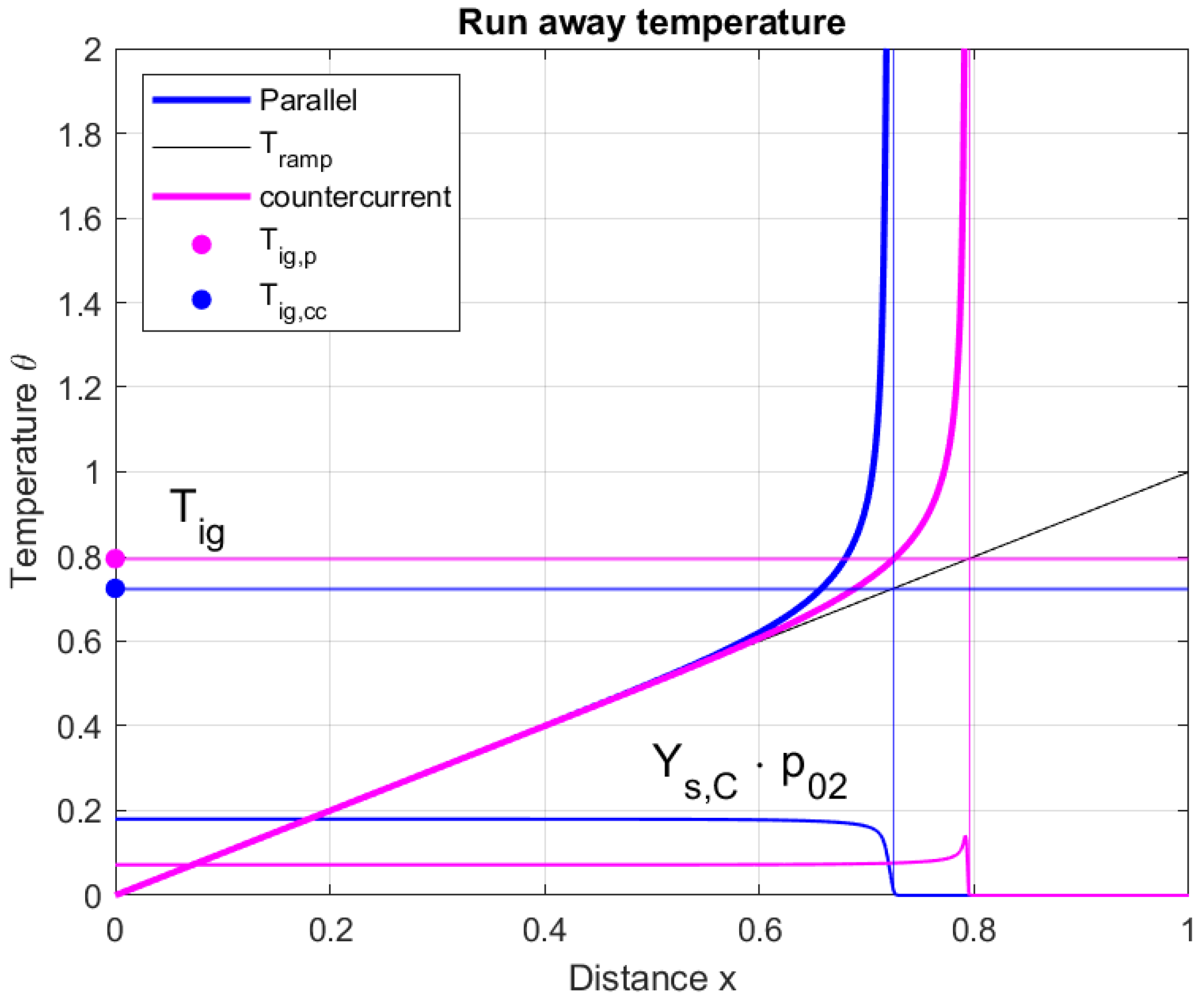
3.2. The Position of the Runaway
- Only materials with a finite range of ignition temperatures can reach a stable position in the reactor.
- As a consequence of the assumption that the ignition temperature is equal to a specific solid temperature, an oxidation front is present even for small , and it is located at a position near , the upper part of the reactor.
- If a stable oxidation front is stabilized in the upper part of the reactor, an increase of causes a decrease of , and the reaction front moves deeper into the reactor.
- The ignition temperatures that cause the reaction front to be at the bottom part of the reactor , are higher than the ones which stabilize the front in the upper part.
- With an increase of , the maximum clearly increases, and this means that, for materials with a fixed ignition temperature, the oxidation front shifts toward the two ends of the reactor as thermal couplings is increased.
- In most of the conditions, two possible positions exist for a given . Only for small thermal couplings one position is present in the upper part of the reactor.
- A critical parameter exists for which there is no stabilization when . This condition describes the situation in which the exothermic reactions and the thermal coupling cannot heat up the solid to the ignition temperature.
- If the number of solutions depend on a particular value :
- (a)
- If , only one runaway position is possible for the allowed range of ignition temperatures. For values of q slightly higher than , the oxidation front stabilizes in the vicinity of .
- (b)
- If , the system respond to a variation of q in a more complex way. It exists a value for which, when , two positions of the front are allowed, while if , only one position is allowed at the reactor top. When q is slightly higher than , the front stabilizes in a position near the center of the reactor.
4. Improvements of the Flame Sheet Solution
4.1. The Role of the Finite Reaction Rate
- If , the reactor is in TC1 for all the values of ;
- If and we have the sequence TC2 → TC3 → TC1 for a decreasing ;
- If and we have the sequence TC2 → TC4 → TC1 for a decreasing .
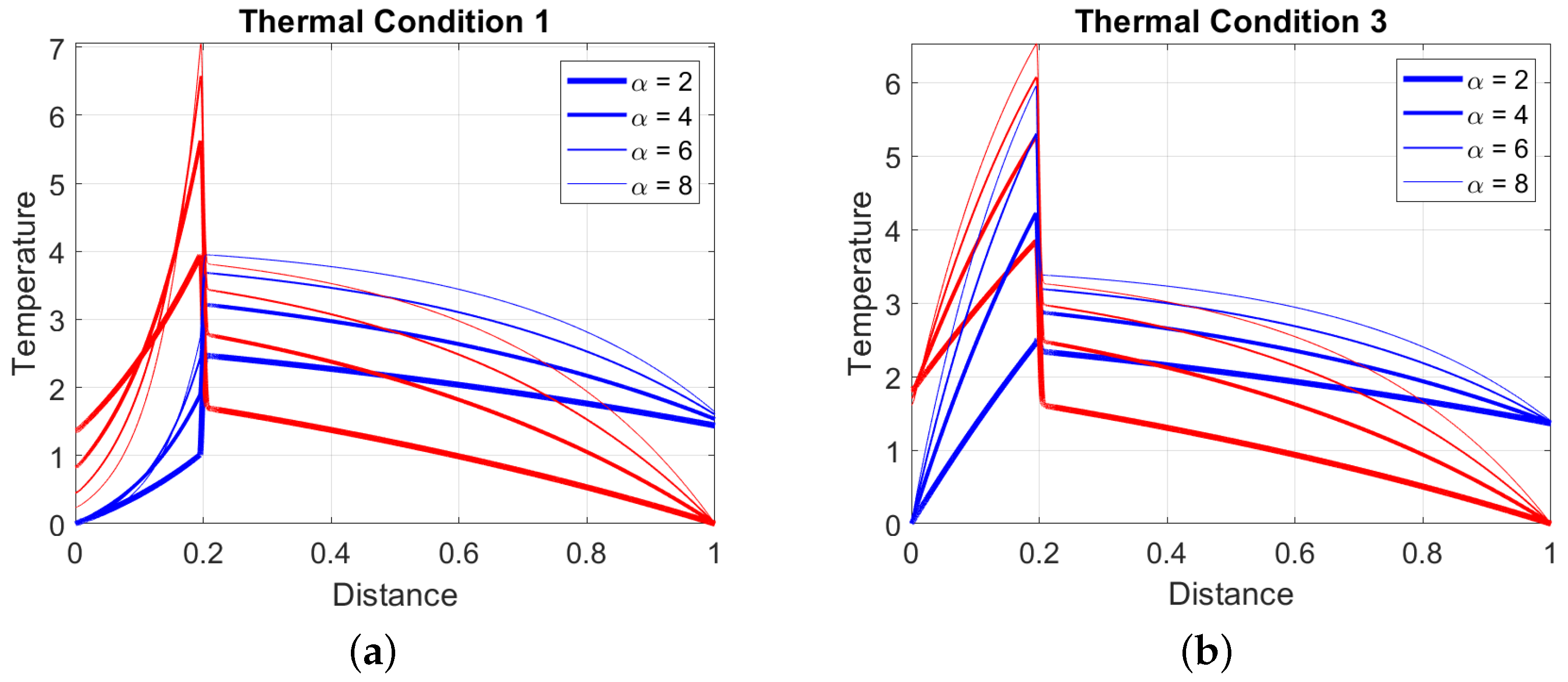
4.2. The Role of the Effective Solid Conduction
- The heat is removed from the highest temperature region and stored in the central part of the reactor;
- For sufficiently large , the reactor freezes, and the oxidation zone is quenched;
- The ignition temperature increases with the critical parameters and q;
- The oxidation region widens, and if partial oxidation occurs (as in the case shown in the figure), the burnout increases;
- The steady state position of the oxidation front moves towards the upper part of the reactor.
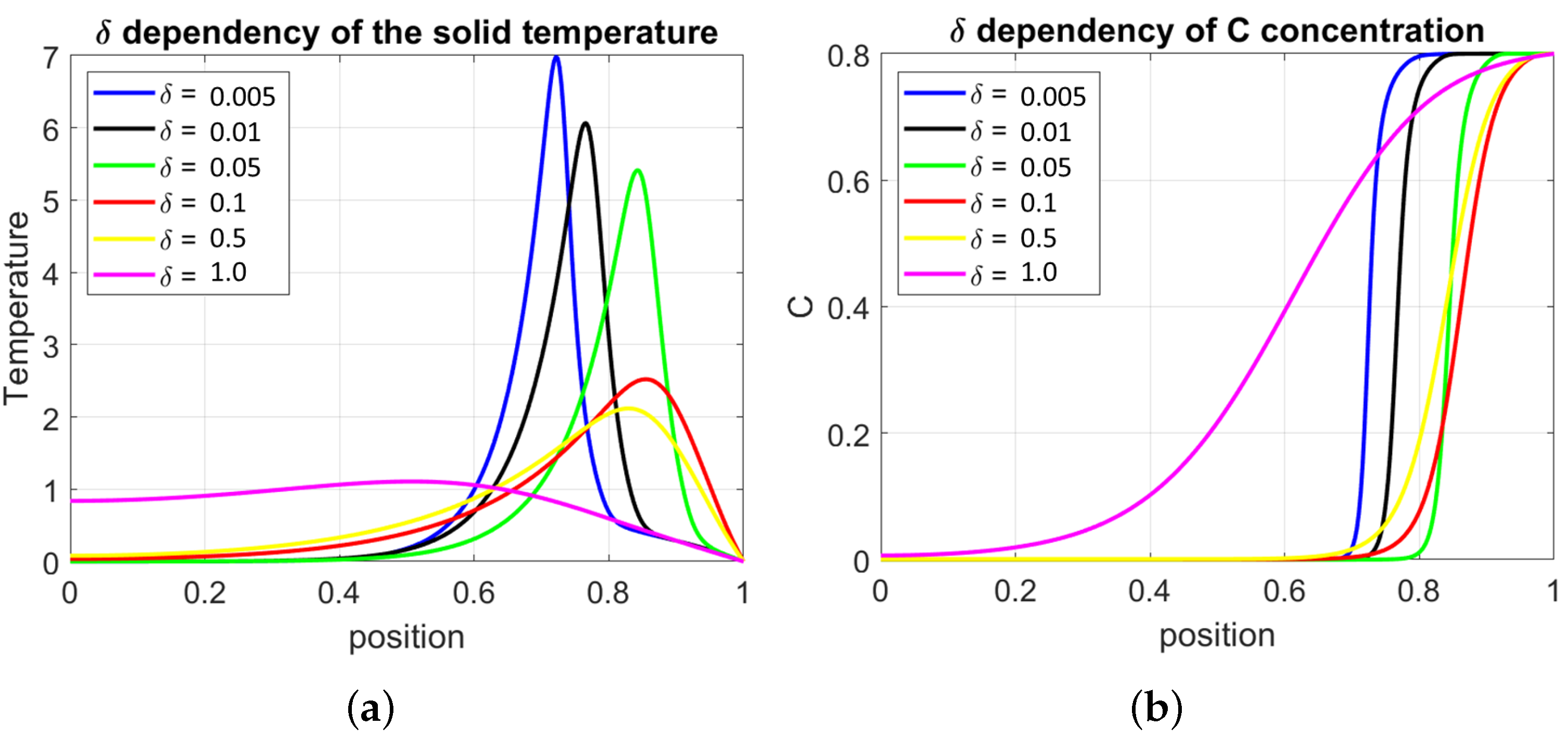
4.3. The Role of Pyrolysis
- Both mass fluxes are changed. The gas-phase flux increases after the region where pyrolysis is active, while the solid-phase flux decreases. The reactor is divided into three regions instead of the two regions as considered in the previous sections.
- The total physical energy available in the system, previously released by oxidation only, will decrease due to endothermic pyrolysis reactions. Consequently, the temperatures decrease.
5. Discussion of the Results
- The oscillations before reactor quenching;
- The effects of mathematical description of the pyrolysis reactions;
- The difference between ignition temperature in a co-flow and counter-current flow configuration.
5.1. The Stability of the Steady Solutions
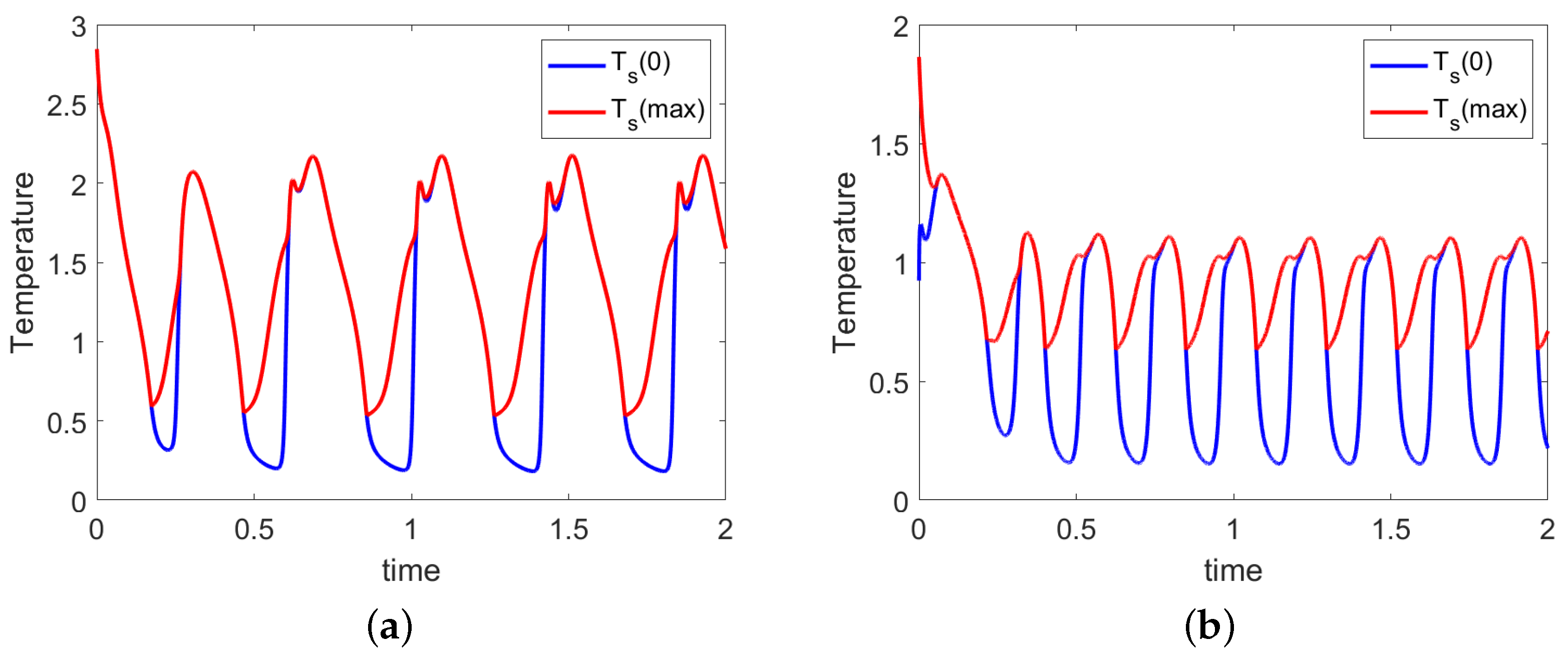
5.2. Oscillations before Flame Quenching
5.3. On the Modeling of Pyrolysis
5.4. The Ignition Temperature
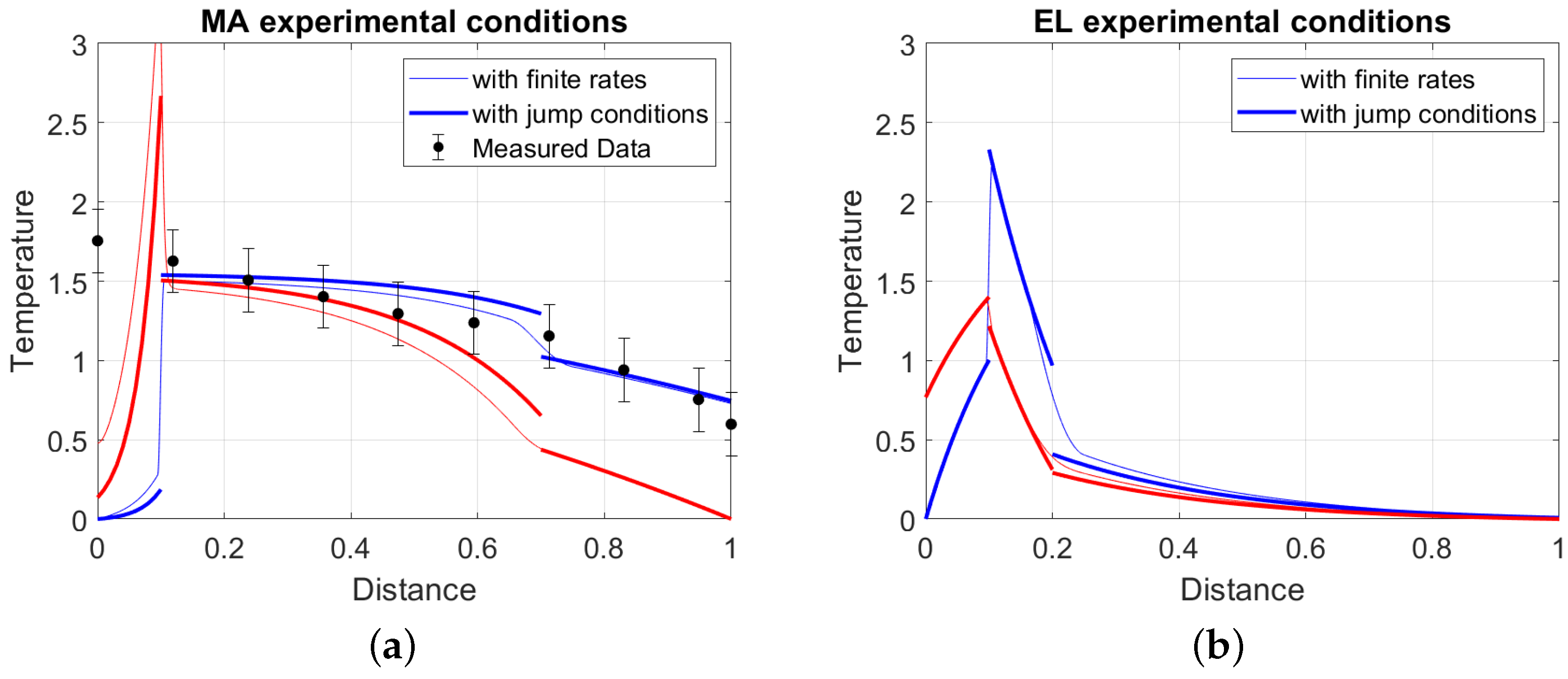
5.5. Application to the Test Cases
6. Conclusions
- There are four possible thermal structures in a counter-current reactor;
- The constraints on the thermal structure allow, for most of the parameters, only two steady-state positions of the oxidation front, one in the upper part of the reactor and one in the lower part of the reactor;
- An increase in the heating value of the solid material or an increase in thermal coupling results in forcing both steady state positions to drift towards the extremities of the reactor;
- The position of the oxidation zone in the upper part of the reactor is stable in fuel-lean conditions (combustion mode), while the position in the bottom part is stable in fuel-reach conditions (gasification mode);
- A critical minimum heat value of the feedstock exists under which the reactor cannot be stabilized by an oxidation front; this value is primarily a function of the thermal coupling between the phases;
- Thermal oscillations can exist for heating values smaller than the critical heating value if enough char is available in the oxidation zone and if the thermal coupling is not too strong.
- Interaction between the combustion zone and the pyrolysis zone. In the previous sections, it has been demonstrated that there is an interaction between the two regions. As a unique result, it has been shown that the thermal structure of the reactor is primarily responsible for this interaction, but a more detailed analysis taking into account more precise reaction rates needs to be performed.
- The existence of possible oscillations was discovered in [48] and in this work a more accurate theoretical explanation has been provided. Although probably not relevant for industrial applications, the study of oscillations in an analysis of reactor dynamic phenomena is of interest. In particular, the amplitude and frequency of the oscillations depend on the operating conditions of the reactor, the property of the feedstock, and the detail of the pyrolysis reactions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Thermo-physical quantities: | ||
| Porosity | ||
| Density | ||
| U | Velocity | |
| Mass sources | ||
| Specific heat | ||
| T | Temperature | |
| Heat source | ||
| h | Convective coefficient | |
| k | Thermal conductivity | |
| Volumetric contact surface | ||
| Mass flux | ||
| Heat capacity | ||
| f | Energy splitting factor | - |
| Mass fraction | ||
| D | Mass diffusion coefficient | |
| p | Pressure | |
| Stoichiometric coefficients | ||
| Air-fuel equivalence ratio | - | |
| Enthalpy of products | ||
| Specific heat of products | ||
| A | Pre-exponential factor | |
| E | Activation energy | |
| Q | Heat of reaction | |
| Geometrical quantities: | ||
| z | Variable for the reactor height | |
| t | Variable for the time | |
| L | Length of the reactor | |
| Indexes: | ||
| g | Gas | |
| s | Solid | |
| c | Contact gas–solid | |
| o | Oxidation | |
| p | Pyrolysis | |
| Reduced variables: | ||
| x | Height | |
| Time | ||
| Temperature | ||
| Fluxes | ||
| Mass fraction | ||
| Pressure | ||
| Mass sources | ||
| Thermal coupling | ||
| Conduction | ||
| q | Heat of Reaction | |
| c | Specific heats ratio | |
| g | Mass fluxes ratio | |
| w | Heat capacities ratio | |
| Product specific heat ratio | ||
References
- Papa, A.A.; Di Carlo, A.; Bocci, E.; Taglieri, L.; Del Zotto, L.; Gallifuoco, A. Energy Analysis of an Integrated Plant: Fluidized Bed Steam Gasification of Hydrothermally Treated Biomass Coupled to Solid Oxide Fuel Cells. Energies 2021, 14, 7331. [Google Scholar] [CrossRef]
- Barisano, D.; Canneto, G.; Nanna, F.; Villone, A.; Fanelli, E.; Freda, C.; Grieco, M.; Lotierzo, A.; Cornacchia, G.; Braccio, G.; et al. Investigation of an Intensified Thermo-Chemical Experimental Set-Up for Hydrogen Production from Biomass: Gasification Process Integrated to a Portable Purification System;Part II. Energies 2022, 15, 4580. [Google Scholar] [CrossRef]
- Papa, A.A.; Savuto, E.; Di Carlo, A.; Tacconi, A.; Rapagnà, S. Synergic Effects of Bed Materials and Catalytic Filter Candle for the Conversion of Tar during Biomass Steam Gasification. Energies 2023, 16, 595. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y. Research on Greenhouse Gas Emissions and Economic Assessment of Biomass Gasification Power Generation Technology in China Based on LCA Method. Sustainability 2022, 14, 729. [Google Scholar] [CrossRef]
- Kolb, T.; Aigner, M.; Kneer, R.; Müller, M.; Weber, R.; Djordjevic, N. Tackling the challenges in modelling entrained-flow gasification of low-grade feedstock. J. Energy Inst. 2016, 89, 485–503. [Google Scholar] [CrossRef]
- Brown, R.C. The Role of Pyrolysis and Gasification in a Carbon Negative Economy. Processes 2021, 9, 882. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Özgür Çolpan, C.; Ayol, A. Biomass gasification for sustainable energy production: A review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- Sobolewski, A.; Chmielniak, T.; Bigda, J.; Billig, T.; Fryza, R.; Popowicz, J. Closing of Carbon Cycle by Waste Gasification for Circular Economy Implementation in Poland. Energies 2022, 15, 4983. [Google Scholar] [CrossRef]
- Xu, H.; Shi, B. Design and System Evaluation of Mixed Waste Plastic Gasification Process Based on Integrated Gasification Combined Cycle System. Processes 2022, 10, 499. [Google Scholar] [CrossRef]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2023, 1, 140–165. [Google Scholar] [CrossRef]
- Gŏmez-Barea, A.; Leckner, B. Modeling of biomass gasification in fluidized bed. Prog. Energy Combust. Sci. 2010, 36, 444–509. [Google Scholar] [CrossRef]
- Higman, C. Gasification process technology. In Advances in Clean Hydrocarbon Fuel Processing; Khan, M.R., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2011; pp. 155–185. [Google Scholar] [CrossRef]
- Chopra, S.; Jain, A.K. A Review of Fixed Bed Gasification Systems for Biomass. Agric. Eng. Int. CIGR J. 2007, 9, 1–23. [Google Scholar]
- Breault, R.W. Gasification Processes Old and New: A Basic Review of the Major Technologies. Energies 2010, 3, 216–240. [Google Scholar] [CrossRef]
- Eberhard, M.; Santo, U.; Michelfelder, B.; Günther, A.; Weigand, P.; Matthes, J.; Waibel, P.; Hagenmeyer, V.; Kolb, T. The bioliq® Entrained-Flow Gasifier—A Model for the German Energiewende. ChemBioEng Rev. 2020, 7, 106–118. [Google Scholar] [CrossRef]
- Mularski, J.; Modliński, N. Entrained-Flow Coal Gasification Process Simulation with the Emphasis on Empirical Char Conversion Models Optimization Procedure. Energies 2021, 14, 1729. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, Y.; Zhang, X.; Yusuf, A.; Famiyeh, L.; Murindababisha, D.; Jin, H.; Liu, Y.; He, J.; Wang, Y.; et al. Hydrogen Production by Fluidized Bed Reactors: A Quantitative Perspective Using the Supervised Machine Learning Approach. J 2021, 4, 266–287. [Google Scholar] [CrossRef]
- Di Renzo, A.; Scala, F.; Heinrich, S. Recent Advances in Fluidized Bed Hydrodynamics and Transport Phenomena—Progress and Understanding. Processes 2021, 9, 639. [Google Scholar] [CrossRef]
- Grohn, P.; Lawall, M.; Oesau, T.; Heinrich, S.; Antonyuk, S. CFD-DEM Simulation of a Coating Process in a Fluidized Bed Rotor Granulator. Processes 2020, 8, 1090. [Google Scholar] [CrossRef]
- Shim, S.; Won, S.; Reza, A.; Kim, S.; Ahmed, N.; Ra, C. Design and Optimization of Fluidized Bed Reactor Operating Conditions for Struvite Recovery Process from Swine Wastewater. Processes 2020, 8, 422. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Zhang, M.; Han, Z.; Jia, X.; Bai, D.; Duo, W.; Bi, X.; Abudula, A.; Guan, G.; et al. A two-stage circulated fluidized bed process to minimize tar generation of biomass gasification for fuel gas production. Appl. Energy 2022, 323, 119639. [Google Scholar] [CrossRef]
- Costa, M.; La Villetta, M.; Piazzullo, D.; Cirillo, D. A Phenomenological Model of a Downdraft Biomass Gasifier Flexible to the Feedstock Composition and the Reactor Design. Energies 2021, 14, 4226. [Google Scholar] [CrossRef]
- Havilah, P.R.; Sharma, A.K.; Govindasamy, G.; Matsakas, L.; Patel, A. Biomass Gasification in Downdraft Gasifiers: A Technical Review on Production, Up-Gradation and Application of Synthesis Gas. Energies 2022, 15, 3938. [Google Scholar] [CrossRef]
- Paiva, M.; Vieira, A.; Gomes, H.T.; Brito, P. Simulation of a Downdraft Gasifier for Production of Syngas from Different Biomass Feedstocks. ChemEngineering 2021, 5, 20. [Google Scholar] [CrossRef]
- Huang, Y.; Wan, Y.; Liu, S.; Zhang, Y.; Ma, H.; Zhang, S.; Zhou, J. A Downdraft Fixed-Bed Biomass Gasification System with Integrated Products of Electricity, Heat, and Biochar: The Key Features and Initial Commercial Performance. Energies 2019, 12, 2979. [Google Scholar] [CrossRef]
- Ghorbani, S.; Atashkari, K.; Borji, M. Three-stage model-based evaluation of a downdraft biomass gasifier. Renew. Energy 2022, 194, 734–745. [Google Scholar] [CrossRef]
- Sharma, P.; Gupta, B.; Pandey, M.; Singh Bisen, K.; Baredar, P. Downdraft biomass gasification: A review on concepts, designs analysis, modelling and recent advances. Mater. Today Proc. 2021, 46, 5333–5341. [Google Scholar] [CrossRef]
- Fazil, A.; Kumar, S.; Mahajani, S.M. Downdraft co-gasification of high ash biomass and plastics. Energy 2022, 243, 123055. [Google Scholar] [CrossRef]
- Calì, G.; Deiana, P.; Bassano, C.; Meloni, S.; Maggio, E.; Mascia, M.; Pettinau, A. Syngas Production, Clean-Up and Wastewater Management in a Demo-Scale Fixed-Bed Updraft Biomass Gasification Unit. Energies 2020, 13, 2594. [Google Scholar] [CrossRef]
- Schwabauer, A.; Mancini, M.; Poyraz, Y.; Weber, R. On the Mathematical Modelling of a Moving-Bed Counter-Current Gasifier Fuelled with Wood-Pellets. Energies 2021, 14, 5840. [Google Scholar] [CrossRef]
- Zhu, F.; Shen, L.; Xu, P.; Yuan, H.; Hu, M.; Qi, J.; Chen, Y. Numerical Simulation of an Improved Updraft Biomass Gasifier Based on Aspen Plus. Int. J. Environ. Res. Public Health 2022, 19, 17089. [Google Scholar] [CrossRef]
- Preisner, N.C.; Linder, M. A Moving Bed Reactor for Thermochemical Energy Storage Based on Metal Oxides. Energies 2020, 13, 1232. [Google Scholar] [CrossRef]
- Song, H.; Yang, G.; Xue, P.; Li, Y.; Zou, J.; Wang, S.; Yang, H.; Chen, H. Recent development of biomass gasification for H2 rich gas production. Appl. Energy Combust. Sci. 2022, 10, 100059. [Google Scholar] [CrossRef]
- Nunes, L.J. Biomass gasification as an industrial process with effective proof-of-concept: A comprehensive review on technologies, processes and future developments. Results Eng. 2022, 14, 100408. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on biomass gasification: Gasifiers, gasifying mediums, and operational parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Patuzzi, F.; Basso, D.; Vakalis, S.; Antolini, D.; Piazzi, S.; Benedetti, V.; Cordioli, E.; Baratieri, M. State-of-the-art of small-scale biomass gasification systems: An extensive and unique monitoring review. Energy 2021, 223, 120039. [Google Scholar] [CrossRef]
- Safronov, D.; Förster, T.; Schwitalla, D.; Nikrityuk, P.; Guhl, S.; Richter, A.; Meyer, B. Numerical study on entrained-flow gasification performance using combined slag model and experimental characterization of slag properties. Fuel Process. Technol. 2017, 161, 62–75. [Google Scholar] [CrossRef]
- Ecoloop. Recycle to Gas. Available online: https://www.ecoloop.eu/ (accessed on 19 January 2023).
- Hüser, T. Synthesegas aus Kunststoffabfällen. Available online: https://www.process.vogel.de/synthesegas-aus-kunststoffabfaellen-a-533433/ (accessed on 19 January 2023).
- Möller, R. Synthesegasherstellung aus Kunststoffabfällen—Erfahrungen und Kennzahlen aus der Inbetriebnahme einer 32 MW-Pilotanlage. In Alternativen zur Abfallverbrennung; Neuruppin: Brandenburg, Germany, 2014; Volume 11, pp. 797–888. [Google Scholar]
- Stefan Brinker, A.L.; Mancini, M.; Muster, M.; Schwabauer, A.; Weber, R. Energieeffiziente Nutzung von pyrolysierbarem Material als Energieträger in Kalkwerken (Projekt Ecoloop); Teilvorhaben: Mechanische und verfahrenstechnische Optimierung des Prozesses; Final Report; Technical University of Clausthal: Clausthal-Zellerfeld, Germany, 2017. [Google Scholar]
- Hobbs, M.L.; Radulovic, P.T.; Smoot, L.D. Modeling fixed-bed coal gasifiers. AIChE J. 1992, 38, 681–702. [Google Scholar] [CrossRef]
- Hobbs, M.; Radulovic, P.; Smoot, L. Combustion and gasification of coals in fixed-beds. Prog. Energy Combust. Sci. 1993, 19, 505–586. [Google Scholar] [CrossRef]
- Thunman, H.; Leckner, B. Co-current and counter-current fixed bed combustion of biofuel: A comparison. Fuel 2003, 82, 275–283. [Google Scholar] [CrossRef]
- Thunman, H.; Leckner, B. Modeling of the combustion front in a countercurrent fuel converter. Proc. Combust. Inst. 2002, 29, 511–518. [Google Scholar] [CrossRef]
- Yang, W.; Ryu, C.; Choi, S. Unsteady one-dimensional model for a bed combustion of solid fuels. Proc. Inst. Mech. Eng. Part A J. Power Energy 2004, 218, 589–598. [Google Scholar] [CrossRef]
- Radulovic, P.T.; Ghani, M.; Smoot, L. An improved model for fixed bed coal combustion and gasification. Fuel 1995, 74, 582–594. [Google Scholar] [CrossRef]
- Di Blasi, C. Modeling wood gasification in a countercurrent fixed-bed reactor. AIChE J. 2004, 50, 2306–2319. [Google Scholar] [CrossRef]
- Kayal, T.; Chakravarty, M.; Biswas, G. Mathematical modelling of continuous updraft gasification of bundled jute stick—A low ash content woody biomass. Bioresour. Technol. 1994, 49, 61–73. [Google Scholar] [CrossRef]
- Girgis, E.; Hallett, W.L.H. Wood Combustion in an Overfeed Packed Bed, Including Detailed Measurements within the Bed. Energy Fuels 2010, 24, 1584–1591. [Google Scholar] [CrossRef]
- Mandl, C.; Obernberger, I.; Biedermann, F. Modelling of an updraft fixed-bed gasifier operated with softwood pellets. Fuel 2010, 89, 3795–3806. [Google Scholar] [CrossRef]
- Howell, J.; Mengüc, M.; Siegel, R. Thermal Radiation Heat Transfer, 7th ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Ergun, S.; Orning, A.A. Fluid Flow through Randomly Packed Columns and Fluidized Beds. Ind. Eng. Chem. 1949, 41, 1179–1184. [Google Scholar] [CrossRef]
- Pereira, F.M.; Oliveira, A.A.; Fachini, F.F. Asymptotic analysis of stationary adiabatic premixed flames in porous inert media. Combust. Flame 2009, 156, 152–165. [Google Scholar] [CrossRef]
- Schiesser, W.E. The Numerical Method of Lines: Integration of Partial Differential Equations; Academic Press: San Diego, CA, USA, 1991. [Google Scholar]
- Schiesser, W.E.; Griffiths, G.W. A Compendium of Partial Differential Equation Models: Method of Lines Analysis with Matlab; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Trefethen, L.N. Spectral Methods in MATLAB; SIAM: Philadelphia, PA, USA, 2000. [Google Scholar]
- Weideman, J.A.; Reddy, S.C. A MATLAB differentiation matrix suite. ACM Trans. Math. Softw. (TOMS) 2000, 26, 465–519. [Google Scholar] [CrossRef]
- Shampine, L.F.; Reichelt, M.W. The MATLAB ODE Suite. SIAM J. Sci. Comput. 1997, 18, 1–22. [Google Scholar] [CrossRef]
- Shampine, L.F.; Reichelt, M.W.; Kierzenka, J.A. Solving Index-1 DAEs in MATLAB and Simulink. SIAM Rev. 1999, 41, 538–552. [Google Scholar] [CrossRef]
- Laurendeau, N.M. Heterogeneous kinetics of coal char gasification and combustion. Prog. Energy Combust. Sci. 1978, 4, 221–270. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]
- Guizani, C.; Jeguirim, M.; Valin, S.; Limousy, L.; Salvador, S. Biomass Chars: The Effects of Pyrolysis Conditions on Their Morphology, Structure, Chemical Properties and Reactivity. Energies 2017, 10, 796. [Google Scholar] [CrossRef]
- Kalina, M.; Sovova, S.; Svec, J.; Trudicova, M.; Hajzler, J.; Kubikova, L.; Enev, V. The Effect of Pyrolysis Temperature and the Source Biomass on the Properties of Biochar Produced for the Agronomical Applications as the Soil Conditioner. Materials 2022, 15, 8855. [Google Scholar] [CrossRef]
- Hanif, M.U.; Zwawi, M.; Capareda, S.C.; Iqbal, H.; Algarni, M.; Felemban, B.F.; Bahadar, A.; Waqas, A. Influence of Pyrolysis Temperature on Product Distribution and Characteristics of Anaerobic Sludge. Energies 2020, 13, 79. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Yeboah, Y.D.; Pisupati, S.V. Influence of Pyrolysis Gas on Volatile Yield and CO2 Reaction Kinetics of the Char Samples Generated in a High-Pressure, High-Temperature Flow Reactor. Energies 2019, 12, 107. [Google Scholar] [CrossRef]
- Kobayashi, H.; Howard, J.; Sarofim, A. Coal devolatilization at high temperatures. Symp. (Int.) Combust. 1977, 16, 411–425. [Google Scholar] [CrossRef]







| Oxidation | Pyrolysis | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g | c | w | q | E | A | q | E | A | ||||
| EL process | ||||||||||||
| 9.0 | 2.2 | 0.9 | 1.98 | 0.06 | 15 | 10.8 | 0.14 | −3 | 6.5 | 400 | ||
| MA process | ||||||||||||
| 10 | 0.7 | 1.5 | 1.05 | 0.25 | 5 | 9.6 | 0.68 | −0.1 | 8.0 | 500 | ||
| Derived parameters | ||||||||||||
| Thermal case | w | |||||||||||
| EL | 0.73 | 0.78 | 0.54 | TC 2 | 1.25 | 1.20 | 1.44 | 1.98 | ||||
| MA | −13.2 | −0.52 | −0.6 | TC 1 | 14.25 | 1.37 | 1.63 | 1.05 | ||||
| TC 1 | TC 2 | TC 3 | TC 4 | |
|---|---|---|---|---|
| m | 0.4 | 0.4 | 0.4 | 0.4 |
| g | 1.6 | 1.6 | 8 | 1.6 |
| q | 4.0 | 4.0 | 4.0 | 4.0 |
| [10, 6, 3, 1] | ||||
| c | 0.8 | 2.3 | 0.6 | 2.3 |
| derived parameters | ||||
| 3.33 | 3.33 | 3.33 | 3.33 | |
| 2.12 | 2.12 | 6.60 | 2.12 | |
| w | 1.28 | 3.68 | 4.00 | 2.40 |
| −2.05 | 0.35 | 0.67 | −0.93 | |
| −0.84 | 1.56 | −2.60 | 0.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mancini, M.; Schwabauer, A. On the Thermal Stability of a Counter-Current Fixed-Bed Gasifier. Energies 2023, 16, 3762. https://doi.org/10.3390/en16093762
Mancini M, Schwabauer A. On the Thermal Stability of a Counter-Current Fixed-Bed Gasifier. Energies. 2023; 16(9):3762. https://doi.org/10.3390/en16093762
Chicago/Turabian StyleMancini, Marco, and Andreas Schwabauer. 2023. "On the Thermal Stability of a Counter-Current Fixed-Bed Gasifier" Energies 16, no. 9: 3762. https://doi.org/10.3390/en16093762





