Biomass pyrolysis hydrogen production is a promising hydrogen production technology [
1,
2,
3]. However, the tar generated during biomass pyrolysis not only slows down the hydrogen production rate, but also damages the equipment [
4,
5,
6]. Hydroxy derivatives of benzene are important components of tar, accounting for 37% of the tar mass [
7]; hence, it is important to study the mechanism governing the efficient cracking of phenol-like aromatic compounds for hydrogen production in order to remove tar from biomass.
Catalysts, such as metals, metallic oxides, and molecular sieves, effectively promote tar cracking as they can enhance the yield and selectivity of syngas, resulting in an improved pyrolysis efficiency [
8,
9]. CaO is often used as a tar-cracking catalyst owing to its effective catalytic activity, inexpensiveness, and abundant reserves. Incorporating it in biomass pyrolysis can significantly improve tar-cracking efficiency and lead to the adsorption of CO
2 generated by pyrolysis, thereby resulting in an enhanced calorific value of the gas products [
10]. Xue et al. [
11] conducted a comprehensive analysis of the impact of CaO catalyst on the chemical reaction properties of corn stover pyrolysis. The results of the study indicated that the improvement in the addition of CaO led to a consequential reduction in tar output, coupled with a commensurate rise in solid and gas outputs. Furthermore, it was observed that CO
2 content in the pyrolysis gas exhibited a gradual decline, while the H
2 content exhibited a gradual increase. Ni-based catalysts are also highly efficient tar-cracking catalysts as they are also inexpensive; hence, they are extensively used for biomass pyrolysis. A composite catalyst composed of Ni and CaO can significantly improve the tar removal rate [
12]. For instance, Xu et al. [
13] added 10 wt.% Ni/CaO during the pyrolysis of herbal residues, which significantly reduced the yield of condensable liquids and led to a high CO
2 removal rate (>60%) at 700 °C. Therefore, it demonstrated superior catalytic efficiency when compared to that of a CaO catalyst in its pure form. Zhao et al. [
14] executed an analysis concerning the catalytic pyrolysis of herbal waste to generate a hydrogen-enriched gas. The outcomes revealed that the utilization of a 10 wt.% Ni/CaO catalyst substantially lessened the apparent activation energy required to produce hydrogen-enriched gas through the pyrolysis of herbal waste as opposed to pyrolysis lacking a catalyst and with the addition of CaO. Yue et al. [
15] produced a series of bifunctional Ni/CaO catalysts and conducted an examination of the function of different Ni/Ca ratios on the H
2 production resulting from corn cobs pyrolysis. The study showed that a ratio of 1:7 of Ni/Ca catalyst increased the hydrogen content from 11.82 to 68.62 vol.% when compared to the sample without any catalyst. Nevertheless, these catalysts are subjected to severe deactivation due to sintering, metal oxidation, and coking. It has been shown that a suitable carrier can result in better dispersion of the loaded catalyst, higher resistance to deactivation, and better stability owing to the metal–carrier interactions. Dang et al. [
16] utilized a hollow porous Ni-Ca-Al-O bifunctional catalyst in the process of catalyzing the generation of hydrogen from glycerol reforming. The results of their study indicate that the 10Ni-Ca-Al2.8 catalyst exhibited superior cycling stability and maintained a consistent hydrogen production purity of 99% as compared to the control group that did not incorporate a carrier. Liu et al. [
17] prepared Ni-CaO-Al
2O
3 for ethanol adsorption-strengthened steam reforming hydrogen production experiments using the sol-gel method; one of the Ni/Al/Ca-85.5 samples demonstrated the best CO
2 adsorption function and a high hydrogen output over 20 cycles. Meanwhile, Wang et al. [
18] prepared a Ca
12Al
14O
33 carrier-loaded Ni-CaO catalyst for autothermal reforming of acetic acid using a co-precipitation method; the results showed that strong interactions were formed between Ni, CaO, and Ca
12Al
14O
33, which effectively improved the activity of the catalyst. Due to the introduction of said carrier, the catalyst displayed a notable degree of thermal stability and experienced no significant deactivation over the course of the experiment, which lasted for 9 h.
The catalytic pyrolysis of tar is a complex thermochemical transformation process that is significantly influenced by a catalyst. However, improving catalyst performance is time-consuming and labor-intensive. It is a well-known fact that magnetic fields possess the ability to enhance the performance of catalysts and promote an increased degree of product selectivity [
19], owing to their high efficiency, fast speeds, and non-contact nature. For instance, when Kiatphuengporn et al. [
20] added a magnetic field to the CO
2 catalytic hydrogenation reaction, and the magnetic field changed the reaction path of the catalytic thermal transform and promoted the water gas change reaction by a factor of 1.2–1.6. Donphai et al. [
19] conducted a comprehensive analysis of the efficiency of Cu-ZnO/ZrO
2 in the process of CO
2 hydrogenation under various intensities of magnetic fields (0, 20.80, and 27.70 mT) and two opposing magnetic field orientations, namely (N-S) and (S-N) directions. The results show that the efficiency of CO
2 conversion by Cu-ZnO/ZrO
2 catalysts is higher with the magnetism at different temperatures than when the field is absent. The optimal CO
2 conversion rate was attained at 20.80 mT and with an S-N orientation. The implementation of a magnetic field resulted in a notable augmentation of 1.8–3.0 times when compared to the conversion rate attained in its absence. The exceptional performance can be attributed to the magnetized catalyst’s surface being facilitated in its adsorption of reactant gas molecules of CO
2 via the influence of external magnetism. Consequently, there was a significant improvement in the effectiveness of the catalyst in the course of CO
2 hydrogenation. In a previous study [
21], the inquiry examined the influence of magnetic fields on the catalytic pyrolysis of wood chips, and it was observed that the yield of liquid products comprising tar and water was reduced with the augmentation of the magnetic field’s intensity. The fraction of liquid mass was decreased by 16% in comparison to the observations made without magnetism. Based on these above-mentioned studies, magnetic field-assisted catalysts enhance the degradation rate of organic macromolecules. Nevertheless, previous research has failed to elucidate the underlying mechanism responsible for the conversion of tar facilitated by magnetic fields.
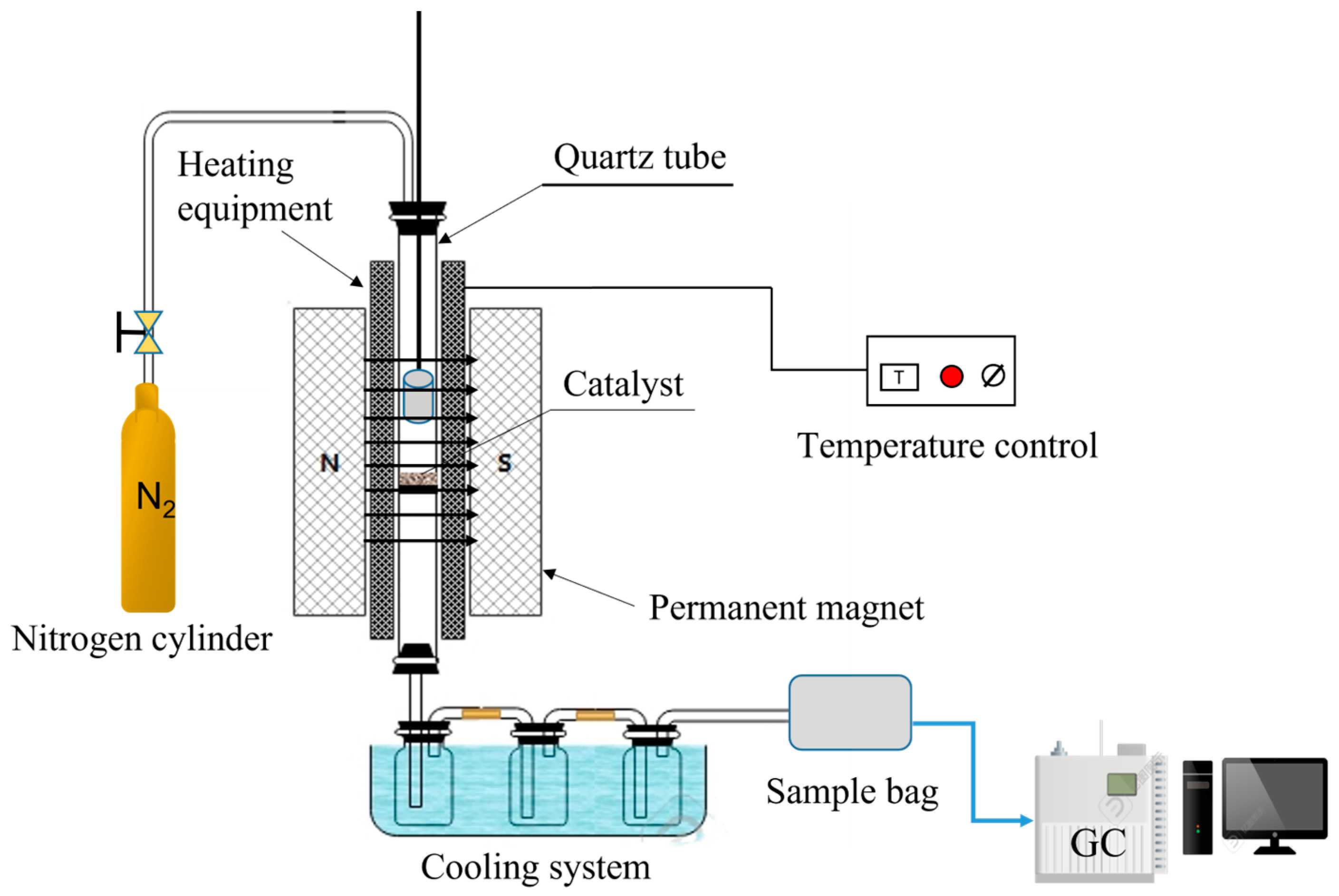
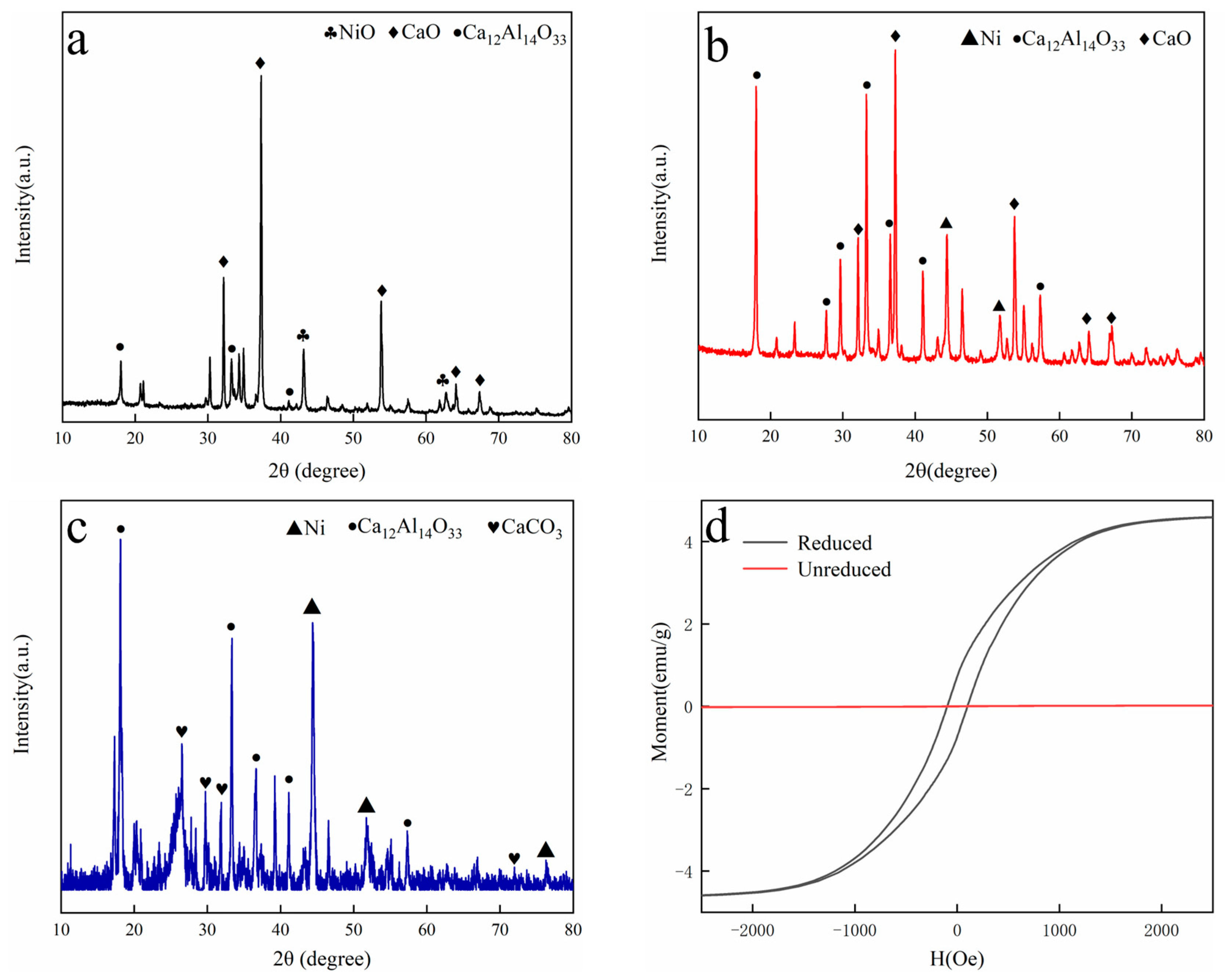
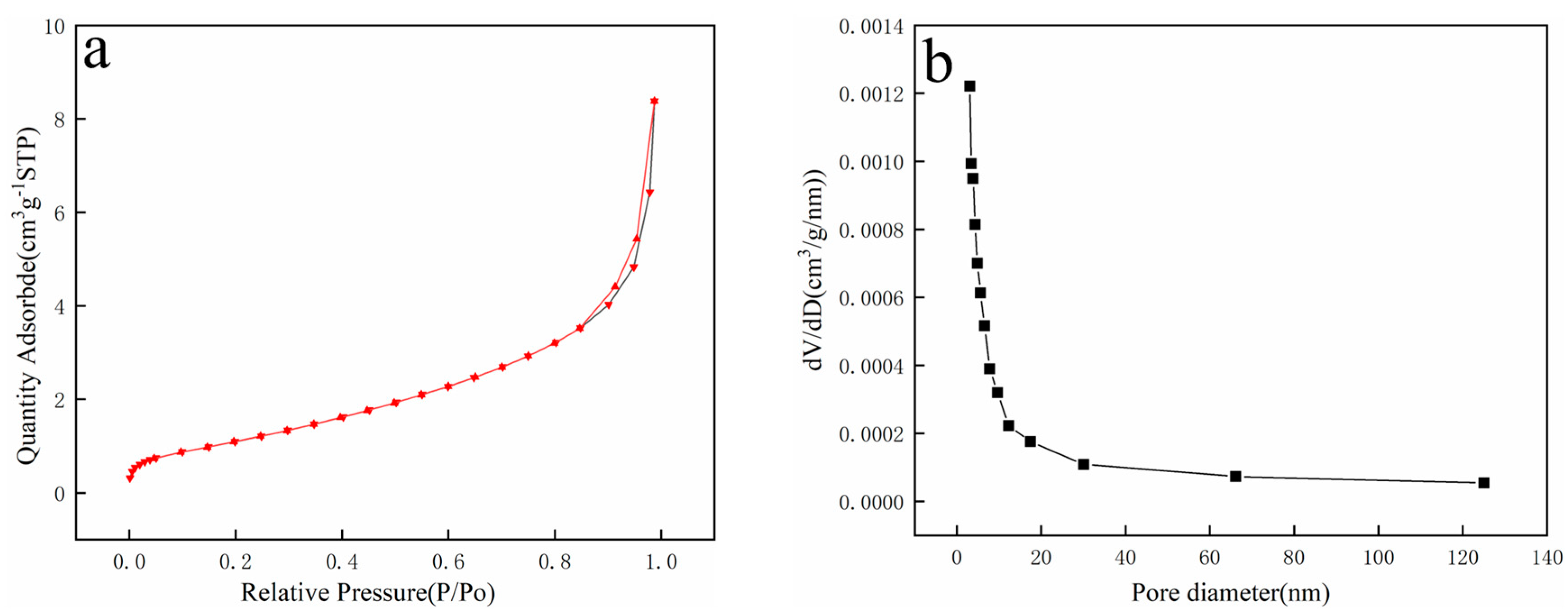
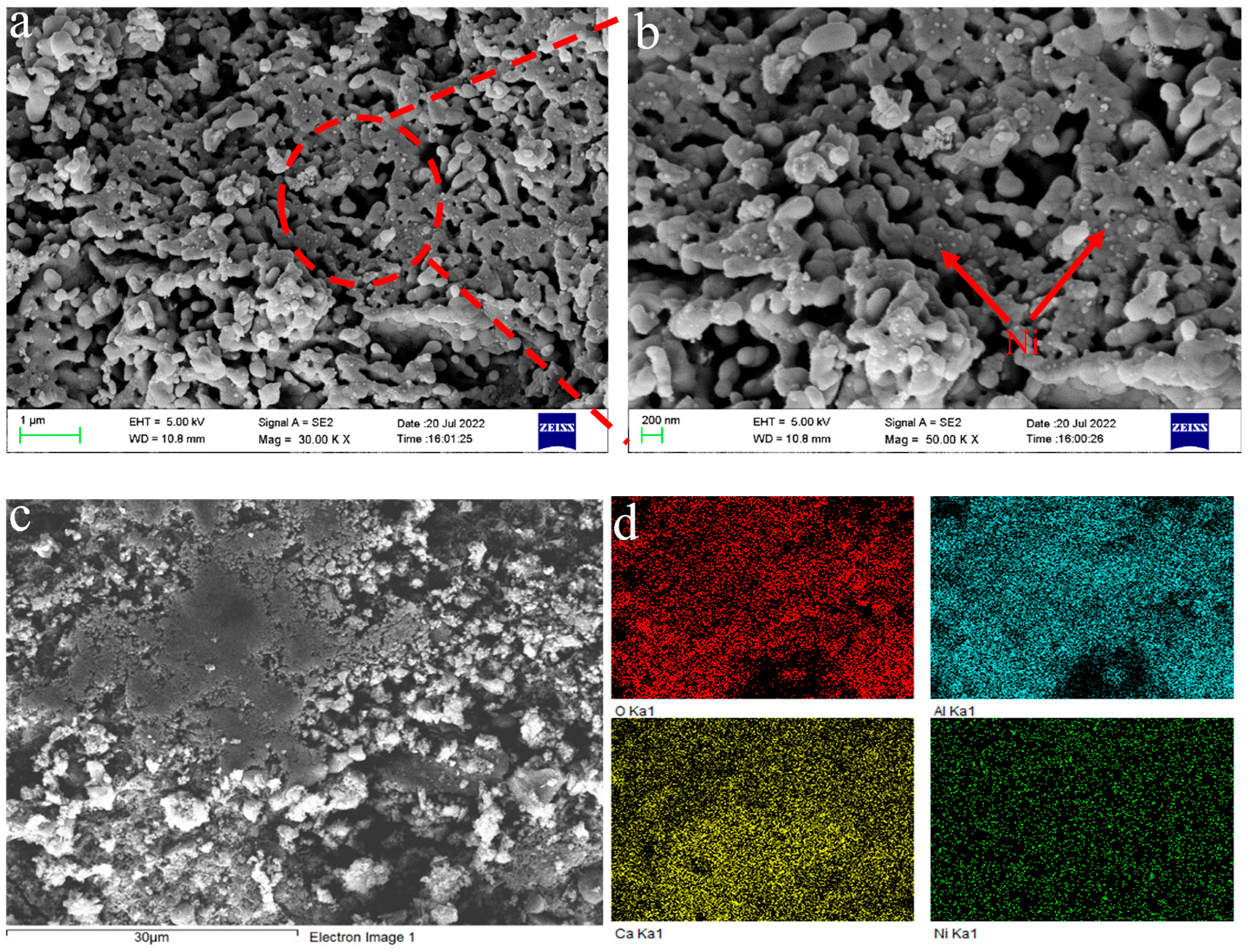
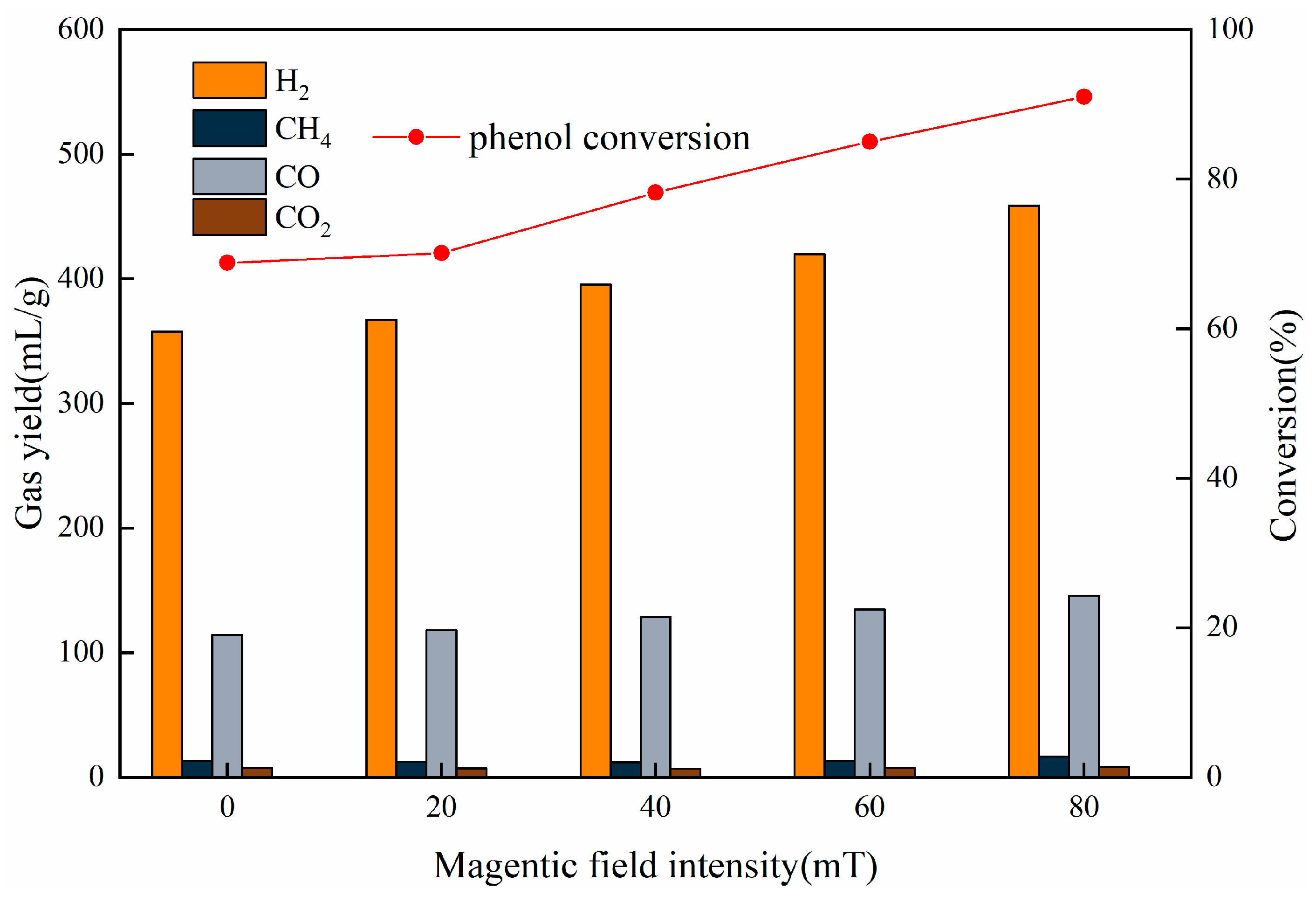
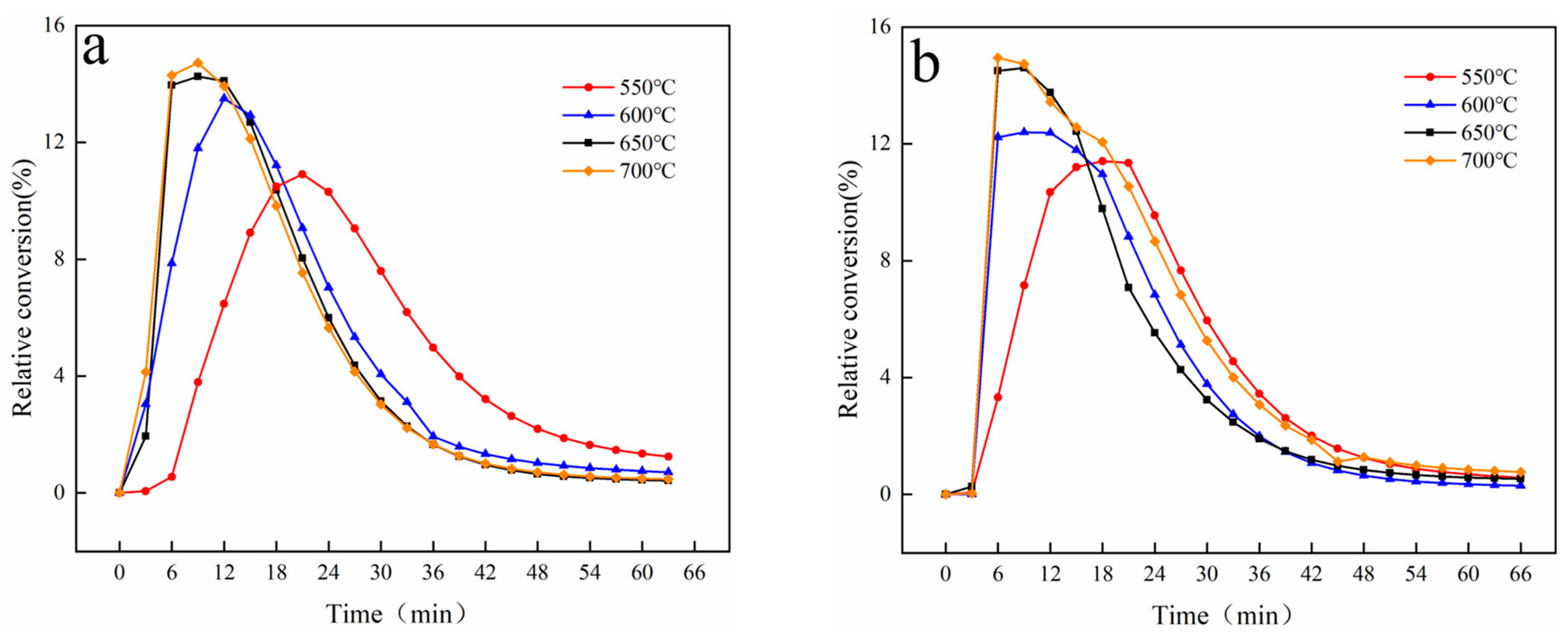
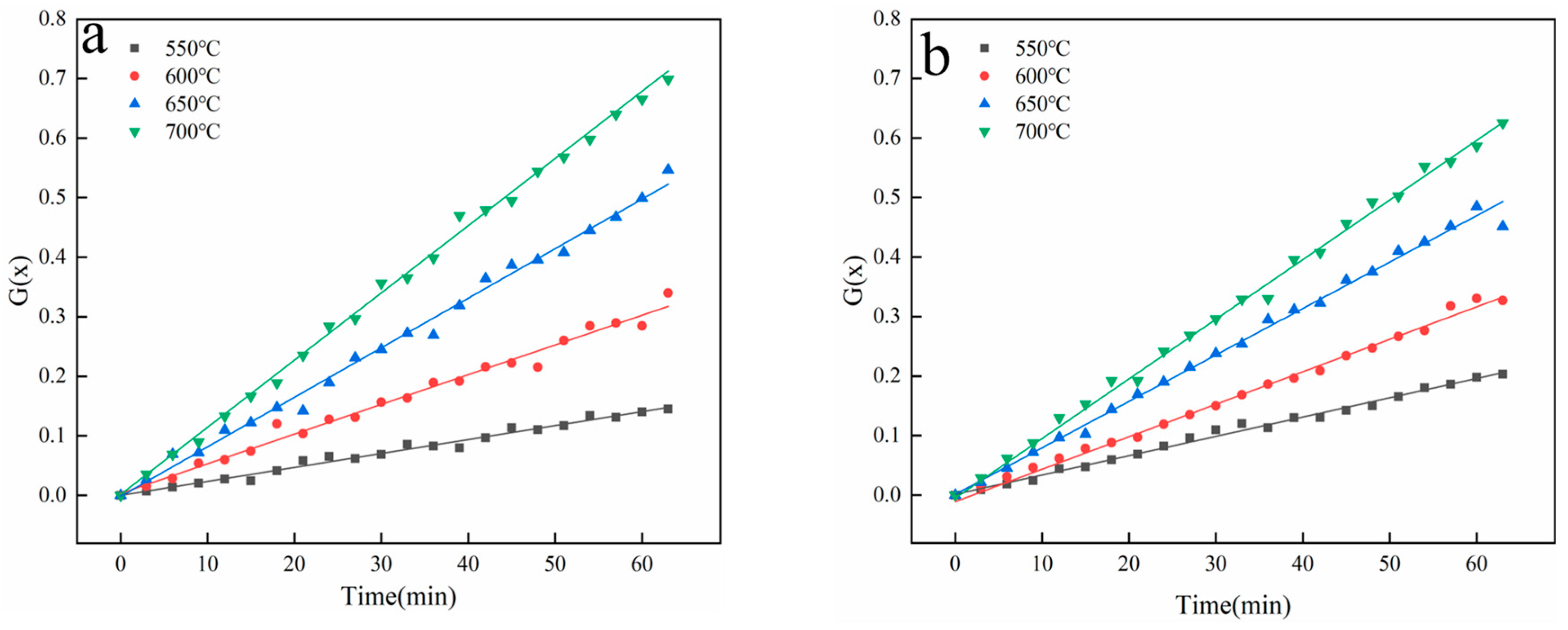
In this work, phenol was chosen as the tar model compound, and a magnetic 10 wt.% Ni/CaO-Ca12Al14O33 was applied. As a result, an investigation into the impact of magnetic fields on the process of converting phenol to hydrogen was discussed using a fixed bed reactor. The outcomes of this examination are projected to offer both theoretical and practical direction for the innovation of advanced biomass hydrogen production technologies.