Microporous Adsorbent-Based Mixed Matrix Membranes for CO2/N2 Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Mixed Matrix Membranes
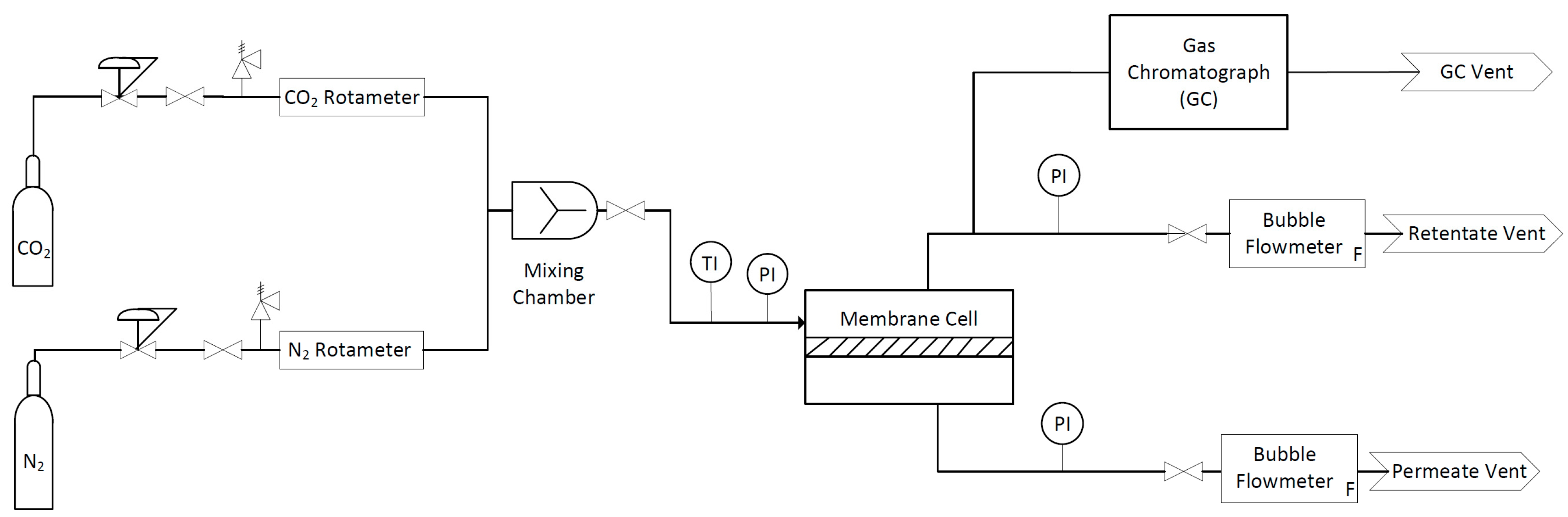
2.2. Single Gas Permeation Characterization
3. Results and Discussion
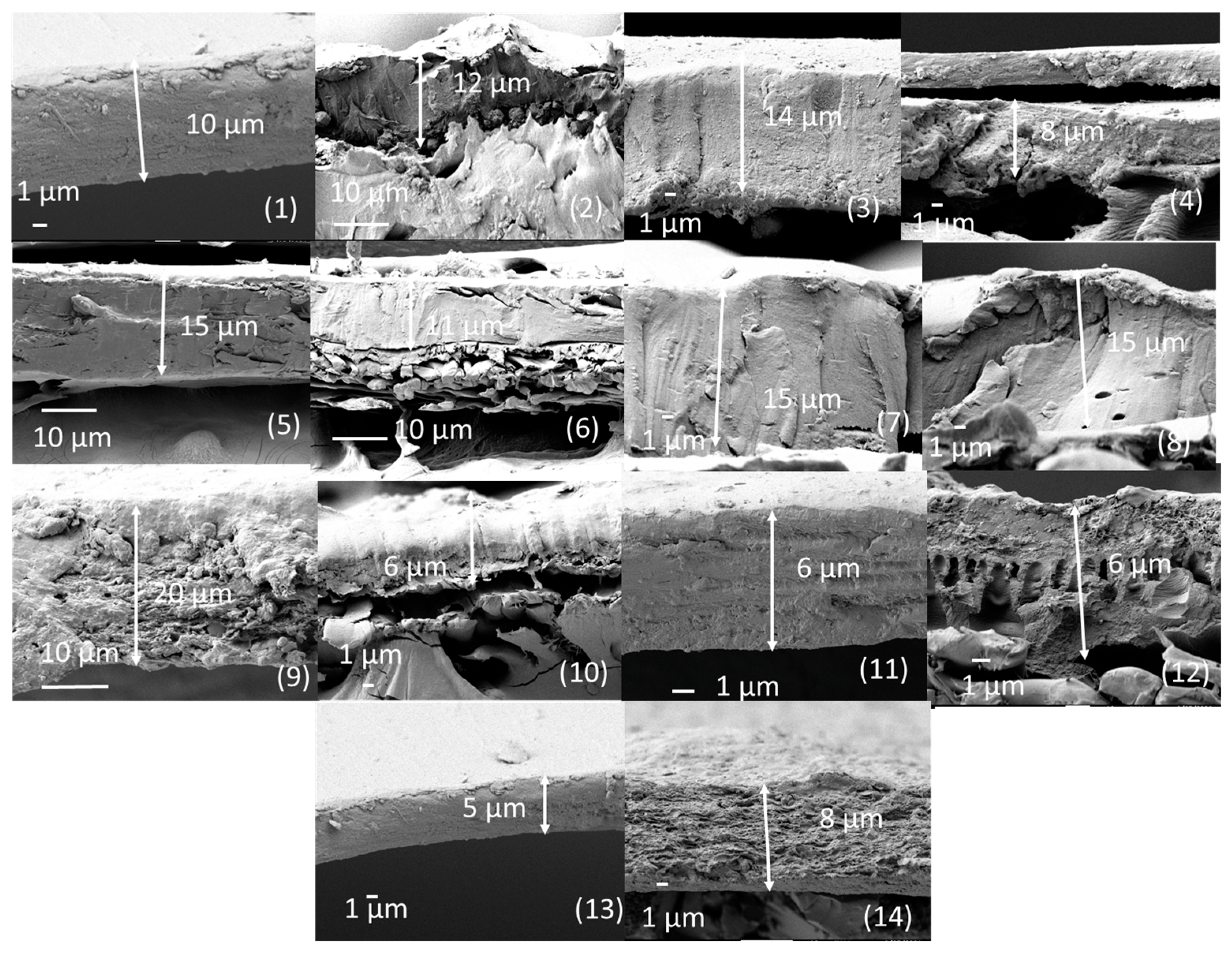
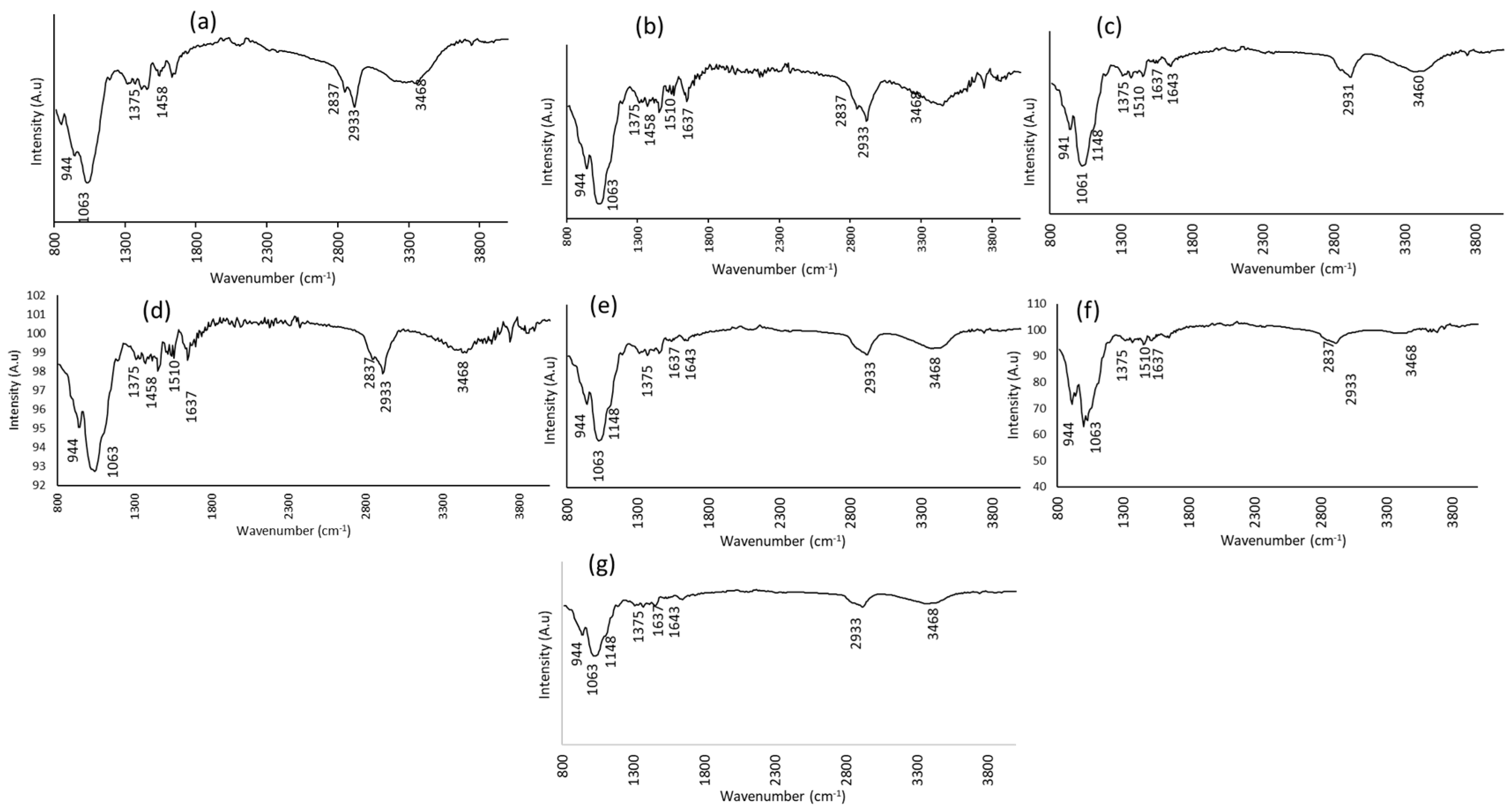
3.1. Structural and Chemical Characterizations
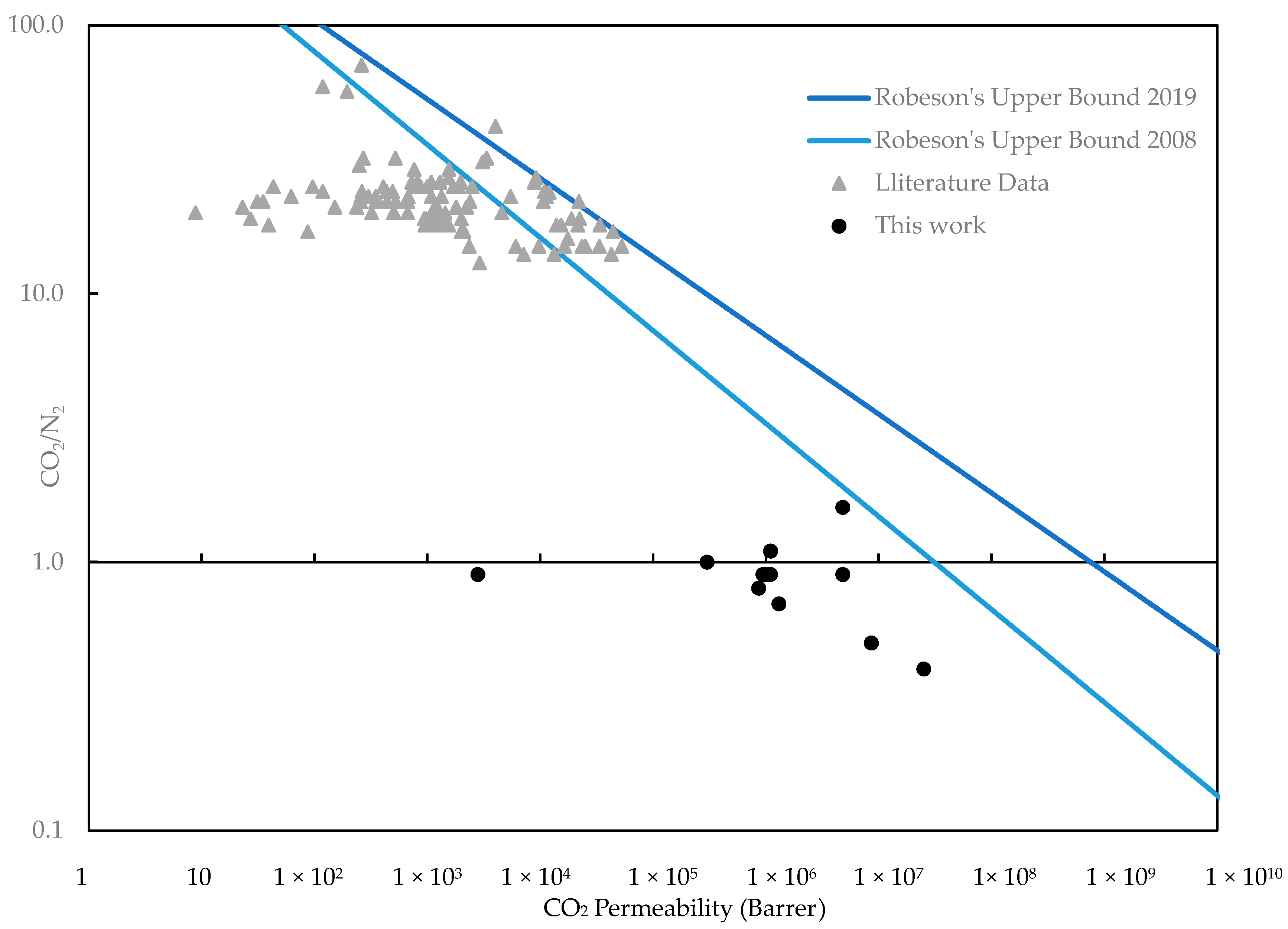
3.2. Gas Permeation Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Membrane area [cm3] |
| F | Volumetric flowrate [cm3/s] |
| L | Membrane thickness [cm] |
| p | Pressure [atm] |
| P | Permeability [Barrer] |
| pSTP | Standard atmospheric pressure [atm] |
| STP | Standard pressure and temperature [K, atm] |
| T | Temperature [K] |
| TSTP | Standard temperature [K] |
| α | Selectivity [dimensionless] |
| Δp | Pressure differential [atm] |
Abbreviations
| CAS | Chemical abstracts service |
| CCUS | Carbon capture, utilization, and storage |
| CO2 | Carbon dioxide |
| COP26 | Conference of the Parties 26 |
| DI | Deionized |
| FTIR | Fourier-transform infrared spectroscopy |
| GHG | Greenhouse gas |
| KOH | Potassium hydroxide |
| MC | Methyl cellulose |
| MMM | Mixed matrix membrane |
| MOF | Metal organic framework |
| N2 | Nitrogen |
| PAA | Poly-N-isopropylallylamine |
| PAH | Polyallylamine hydrochloride |
| PAN | Polyacrylonitrile |
| PVA | Polyvinyl alcohol |
| ZIF-8 | Zeolitic imidazolate framework |
References
- European Association of Remote Sensing Companies. A reporting service for environment and development negotiations. Earth Negot. Bull. 2021, 12. [Google Scholar]
- Khalid, M.; Dharaskar, S.A.; Sillanpa, M.E.T.; Siddiqui, H. Emerging Carbon Capture Technologies: Towards a Sustainable Future; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Chen, G.; Wang, T.; Zhang, G.; Liu, G.; Jin, W. Membrane materials targeting carbon capture and utilization. Adv. Membr. 2022, 2, 100025. [Google Scholar] [CrossRef]
- Criscuoli, A. Membrane Distillation Process. Membranes 2021, 11, 144. [Google Scholar] [CrossRef]
- Jiang, S.; Meng, X.; Chen, B.; Wang, N.; Chen, G. Electrospinning superhydrophobic–superoleophilic PVDF-SiO2 nanofibers membrane for oil–water separation. J. Appl. Polym. Sci. 2020, 137, 49546. [Google Scholar] [CrossRef]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.A.; Nazarenko, S. A review on recent advances in CO2 separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Athanasiou, M.; Manoli, A.; Papagiorgis, P.; Georgiou, K.; Berezovska, Y.; Othonos, A.; Bodnarchuk, M.I.; Kovalenko, M.V.; Itskos, G. Flexible, Free-Standing Polymer Membranes Sensitized by CsPbX3 Nanocrystals as Gain Media for Low Threshold, Multicolor Light Amplification. ACS Photonics 2022, 9, 2385–2397. [Google Scholar] [CrossRef] [PubMed]
- Stogin, B.B.; Gockowski, L.; Feldstein, H.; Claure, H.; Wang, J.; Wong, T.-S. Free-standing liquid membranes as unusual particle separators. Sci. Adv. 2018, 4, eaat3276. [Google Scholar] [CrossRef] [PubMed]
- Chai, S.-H.; Fulvio, P.F.; Hillesheim, P.C.; Qiao, Z.-A.; Mahurin, S.M.; Dai, S. “Brick-and-mortar” synthesis of free-standing mesoporous carbon nanocomposite membranes as supports of room temperature ionic liquids for CO2−N2 separation. J. Memb. Sci. 2014, 468, 73–80. [Google Scholar] [CrossRef]
- Kang, W.R.; Lee, A.S.; Park, S.; Park, S.-H.; Baek, K.-Y.; Lee, K.B.; Lee, S.-H.; Lee, J.-H.; Hwang, S.S.; Lee, J.S. Free-standing, polysilsesquioxane-based inorganic/organic hybrid membranes for gas separations. J. Memb. Sci. 2015, 475, 384–394. [Google Scholar] [CrossRef]
- Zou, J.; Ho, W.S.W. CO2-selective polymeric membranes containing amines in crosslinked poly(vinyl alcohol). J. Memb. [CrossRef]
- Samputu, I. Optimization of Using Polymeric and Mixed Matrix PVA Amine-Based Membranes for CO2/N2 and CO2/CH4 Separation. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2022. [Google Scholar]
- Selyanchyn, O.; Selyanchyn, R.; Fujikawa, S. Critical Role of the Molecular Interface in Double-Layered Pebax-1657/PDMS Nanomembranes for Highly Efficient CO2/N2 Gas Separation. ACS Appl. Mater. Interfaces 2020, 12, 33196–33209. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Memb. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Wang, Z.; Li, M.; Zhang, X.-F.; Zhou, Y.; Yao, J. Integration of natural clay into cellulose membrane for efficient CO2/N2 separation. Cellulose 2022, 29, 1873–1881. [Google Scholar] [CrossRef]
- Tezel, F.H.; Shervani, S.; Strong, C. Composite Material for Thermochemical Energy Storage and Method of Making Same. U.S. Patent US20230332033A1, 19 October 2023. [Google Scholar]
- Meshkat, S.; Kaliaguine, S.; Rodrigue, D. Comparison between ZIF-67 and ZIF-8 in Pebax® MH-1657 mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2020, 235, 116150. [Google Scholar] [CrossRef]
- Mashhadikhan, S.; Moghadassi, A.; Amooghin, A.E.; Sanaeepur, H. Interlocking a synthesized polymer and bifunctional filler containing the same polymer’s monomer for conformable hybrid membrane systems. J. Mater. Chem. A Mater. 2020, 8, 3942–3955. [Google Scholar] [CrossRef]
- Alavi, S.A.; Kargari, A.; Sanaeepur, H.; Karimi, M. Preparation and characterization of PDMS/zeolite 4A/PAN mixed matrix thin film composite membrane for CO2/N2 and CO2/CH4 separations. Res. Chem. Intermed. 2017, 43, 2959–2984. [Google Scholar] [CrossRef]
- Asghari, M.; Mosadegh, M.; Harami, H.R. Supported PEBA-zeolite 13X nano-composite membranes for gas separation: Preparation, characterization and molecular dynamics simulation. Chem. Eng. Sci. 2018, 187, 67–78. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Behbahani, R.M.; Mohammadi, T.; Kargari, A. Ionic liquid-modified Pebax® 1657 membrane filled by ZIF-8 particles for separation of CO2 from CH4, N2 and H2. J. Memb. Sci. 2017, 524, 652–662. [Google Scholar] [CrossRef]
- Rose, L. Carbon Dioxide Gas Separation from Syngas to Increase Conversion of Reverse Water Gas Shift Reaction via Polymeric and Mixed Matrix Membranes. Master’s Thesis, University of Ottawa, Ottawa, ON, Canada, 2018. [Google Scholar]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of Methylcellulose on the Properties and Morphology of Polysulfone Membranes Prepared by Phase Inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef]
- Meléndez-Ortiz, H.I.; Puente-Urbina, B.; León, G.C.-D.; Mercado-Silva, J.A.; Saucedo-Salazar, E.; García-Cerda, L.A. Covalent attachment of poly(allylamine hydrochloride) onto ordered silica foams. J. Porous Mater. 2020, 27, 929–937. [Google Scholar] [CrossRef]
- Kharazmi, A.; Faraji, N.; Hussin, R.M.; Saion, E.; Yunus, W.M.M.; Behzad, K. Structural, optical, opto-thermal and thermal properties of ZnS-PVA nanofluids synthesized through a radiolytic approach. Beilstein J. Nanotechnol. 2015, 6, 529–536. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, J.; Guan, J.; Du, X.; Chen, S.; Song, Y.; Huang, Y. Enhancing CO2 adsorption capacity of ZIF-8 by synergetic effect of high pressure and temperature. Sci. Rep. 2023, 13, 17584. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Seong, J.G.; Hu, X.; Lee, Y.M. Recent progress in microporous polymers from thermally rearranged polymers and polymers of intrinsic microporosity for membrane gas separation: Pushing performance limits and revisiting trade-off lines. J. Polym. Sci. 2020, 58, 2450–2466. [Google Scholar] [CrossRef]



| Membrane | CO2/N2 | PCO2 (Barrer) | PN2 (Barrer) | T (°C) | p (atm) | Ref. |
|---|---|---|---|---|---|---|
| [C2mim][Tf2N]/MC-CB-75 | 36 | 180 | 5 | 22 | 1.35 | [9] |
| LPG64 | 30 | 48 | 1.6 | 35 | 1 | [10] |
| PVA/AIBA/Pam-OH | 6 | 2471 | 416 | 24 | 2.5 | [12] |
| PDMS5/O20.7/Pebax0.1 | 12 | 1417 | 118 | 25 | 1.97 | [13] |
| PEBAX + ZIF-8 | 59 | 118 | 2.0 | 35 | 10.9 | [17] |
| 15wt%Cu(6L)@13X | 117 | 1034 | 8.8 | 25 | 2 | [18] |
| PDMS/4A/PAN | 24 | 12,000 | 508 | 25 | 3 | [19] |
| PEBA-13X | 57 | 194 | 3 | 25 | 13.8 | [20] |
| PEBAX 1657/ZIF-8 | 71 | 261 | 4 | 30 | 2 | [21] |
| No. | Membrane | CO2/N2 | N2/CO2 | PCO2 (Barrer) | PN2 (Barrer) |
|---|---|---|---|---|---|
| 1 | MC | - | - | - | - |
| 2 | MC/PAN | 0.9 | 1.1 | 2.8 × 103 | 3.0 × 103 |
| 3 | MC/PAH/ZIF-8 | 0.9 | 1.1 | 4.8 × 106 | 5.2 × 106 |
| 4 | MC/PAH/ZIF-8/PAN | 0.5 | 1.9 | 8.6 × 105 | 1.6 × 106 |
| 5 | MC/PAH/ZIF-8/PVA | 1.6 | 0.6 | 4.8 × 106 | 2.9 × 106 |
| 6 | MC/PAH/ZIF-8/PVA/PAN | 0.8 | 1.3 | 8.6 × 105 | 1.1 × 106 |
| 7 | MC/PAH/13X | 1.0 | 1.0 | 3.0 × 105 | 3.0 × 105 |
| 8 | MC/PAH/13X/PAN | 0.9 | 1.2 | 9.4 × 105 | 1.1 × 106 |
| 9 | MC/PAH/13X/PVA | 0.4 | 2.8 | 2.5 × 107 | 6.9 × 107 |
| 10 | MC/PAH/13X/PVA/PAN | 0.9 | 1.1 | 1.1 × 106 | 1.2 × 106 |
| 11 | MC/PAH/kaolin | 1.1 | 0.9 | 1.1 × 106 | 9.6 × 105 |
| 12 | MC/PAH/kaolin/PAN | 0.9 | 1.1 | 1.1 × 106 | 1.2 × 106 |
| 13 | MC/PAH/kaolin/PVA | 0.7 | 1.5 | 1.3 × 106 | 2.1 × 106 |
| 14 | MC/PAH/kaolin/PVA/PAN | 0.9 | 1.1 | 1.0 × 106 | 1.1 × 106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shervani, S.; Tansug, L.P.; Tezel, F.H. Microporous Adsorbent-Based Mixed Matrix Membranes for CO2/N2 Separation. Energies 2024, 17, 1927. https://doi.org/10.3390/en17081927
Shervani S, Tansug LP, Tezel FH. Microporous Adsorbent-Based Mixed Matrix Membranes for CO2/N2 Separation. Energies. 2024; 17(8):1927. https://doi.org/10.3390/en17081927
Chicago/Turabian StyleShervani, Suboohi, Lara P. Tansug, and F. Handan Tezel. 2024. "Microporous Adsorbent-Based Mixed Matrix Membranes for CO2/N2 Separation" Energies 17, no. 8: 1927. https://doi.org/10.3390/en17081927






