Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects
Abstract
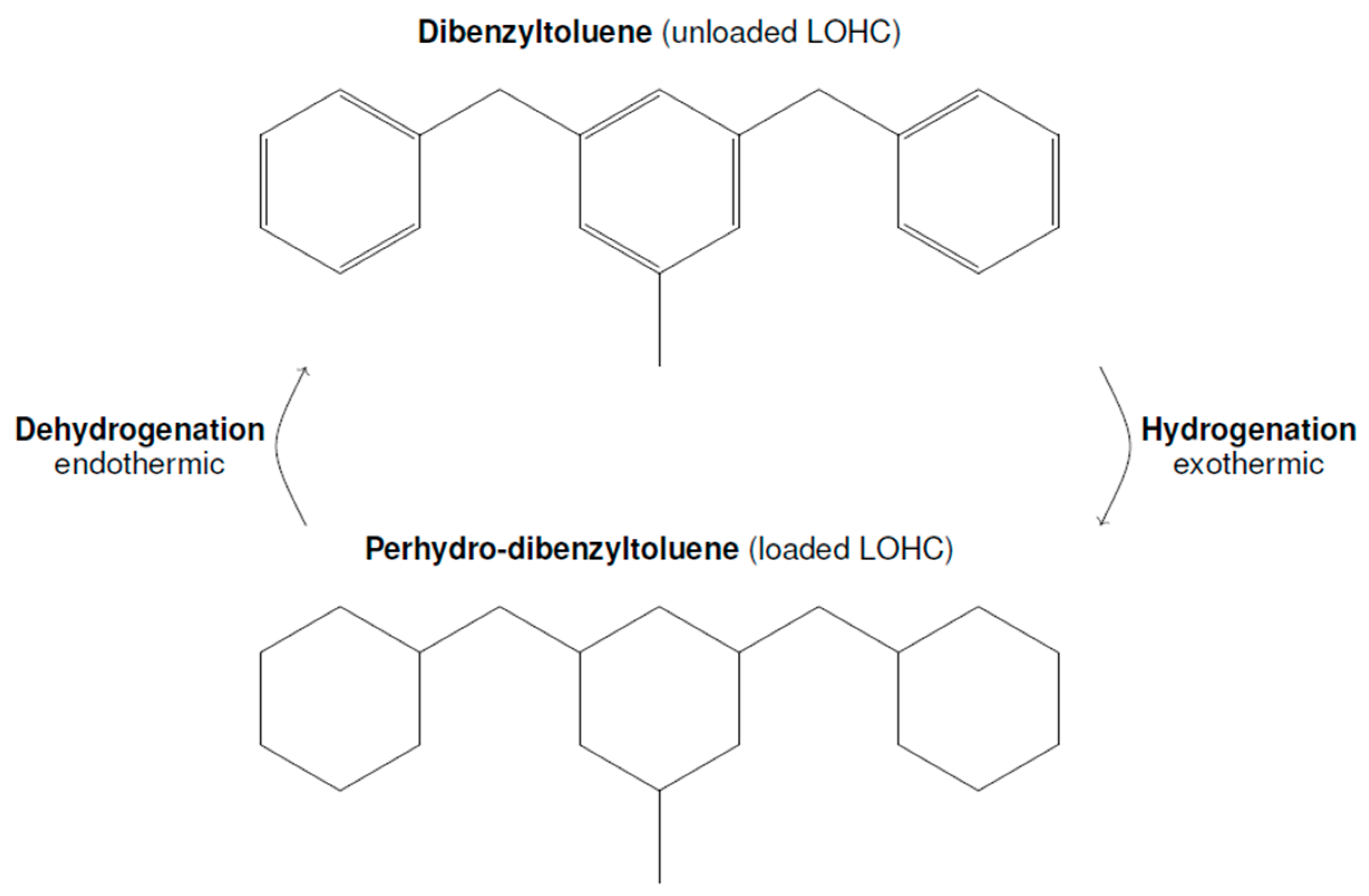
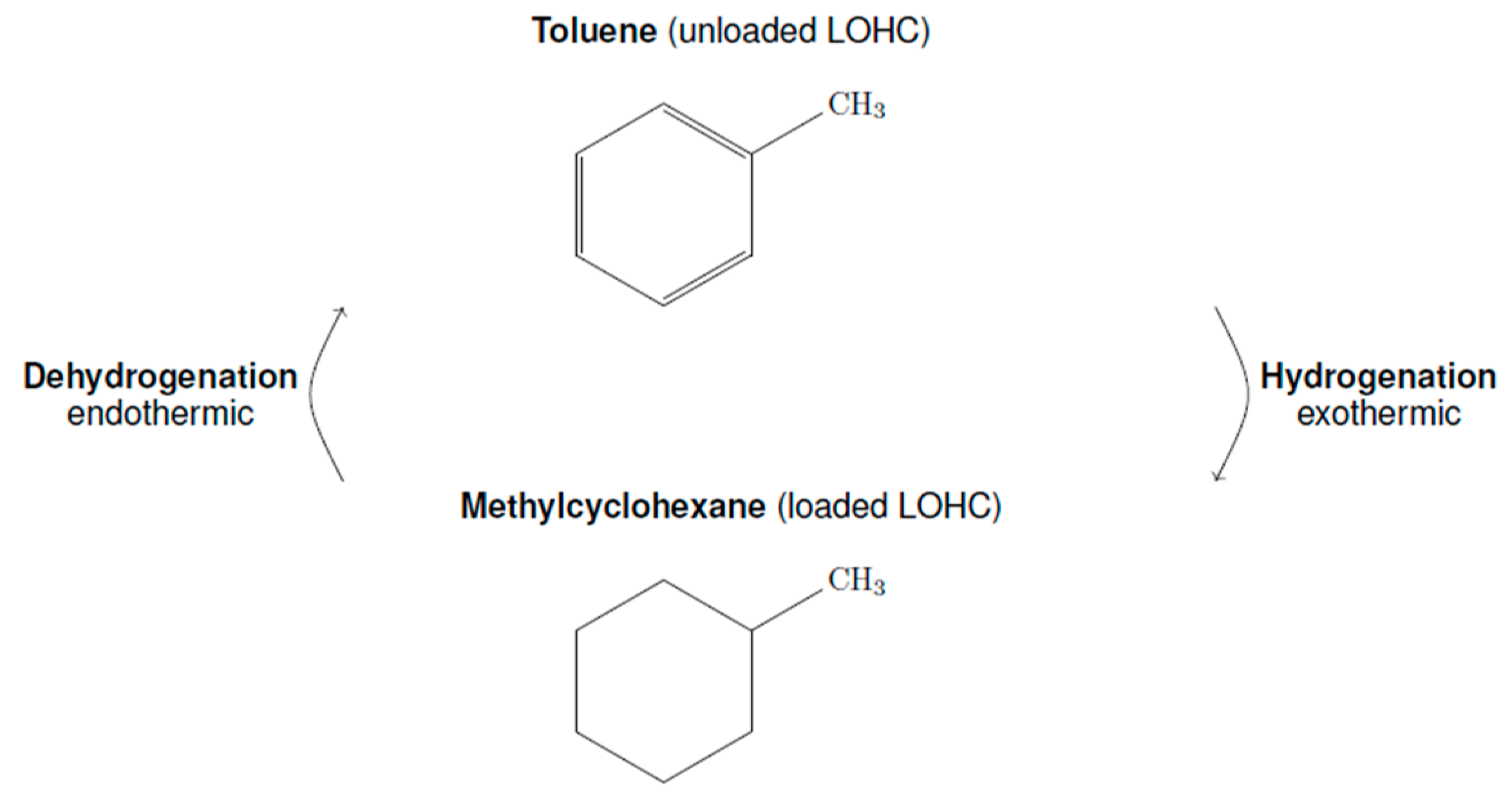
:1. Introduction
2. LOHC Projects
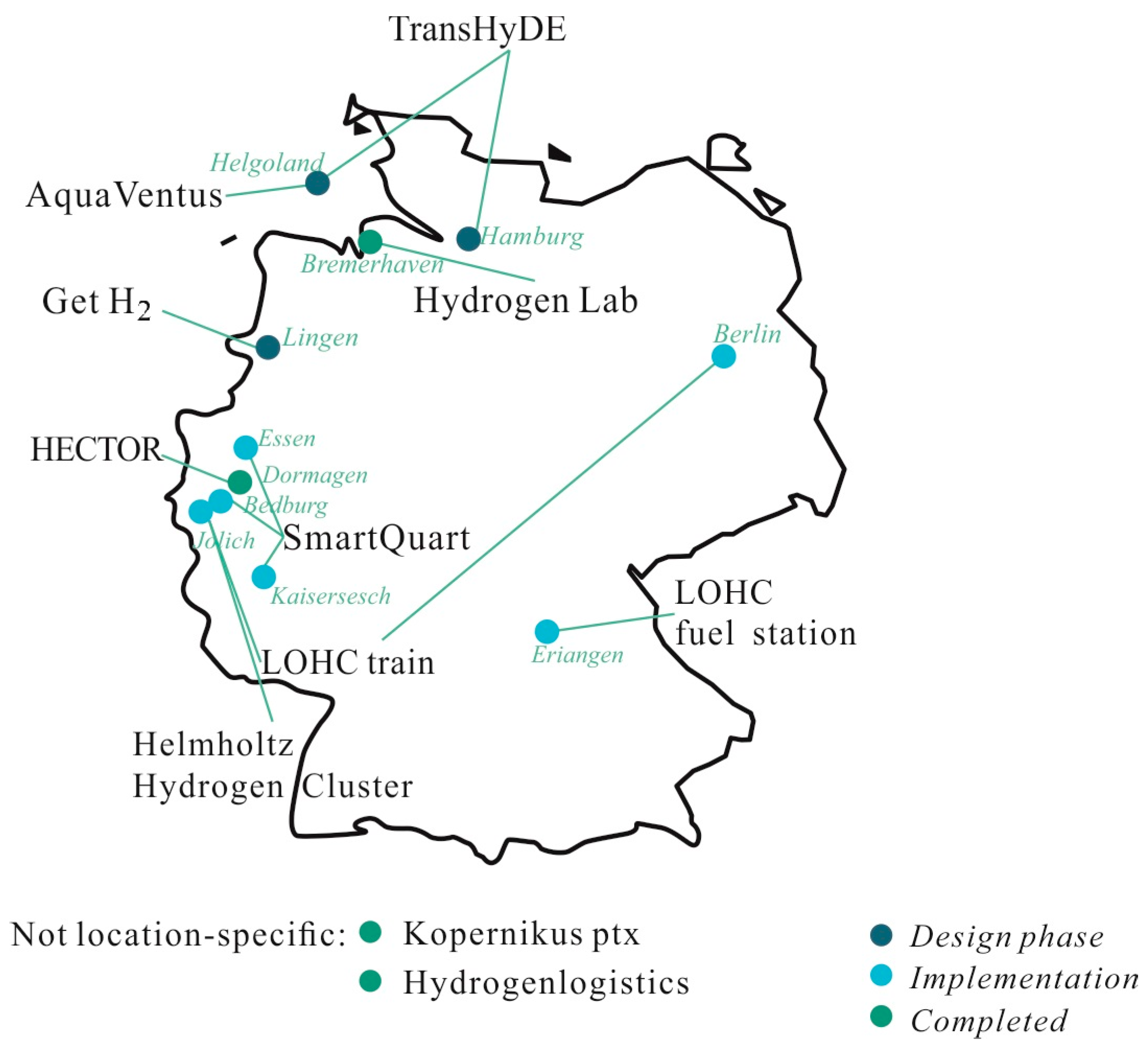
2.1. Projects in Germany
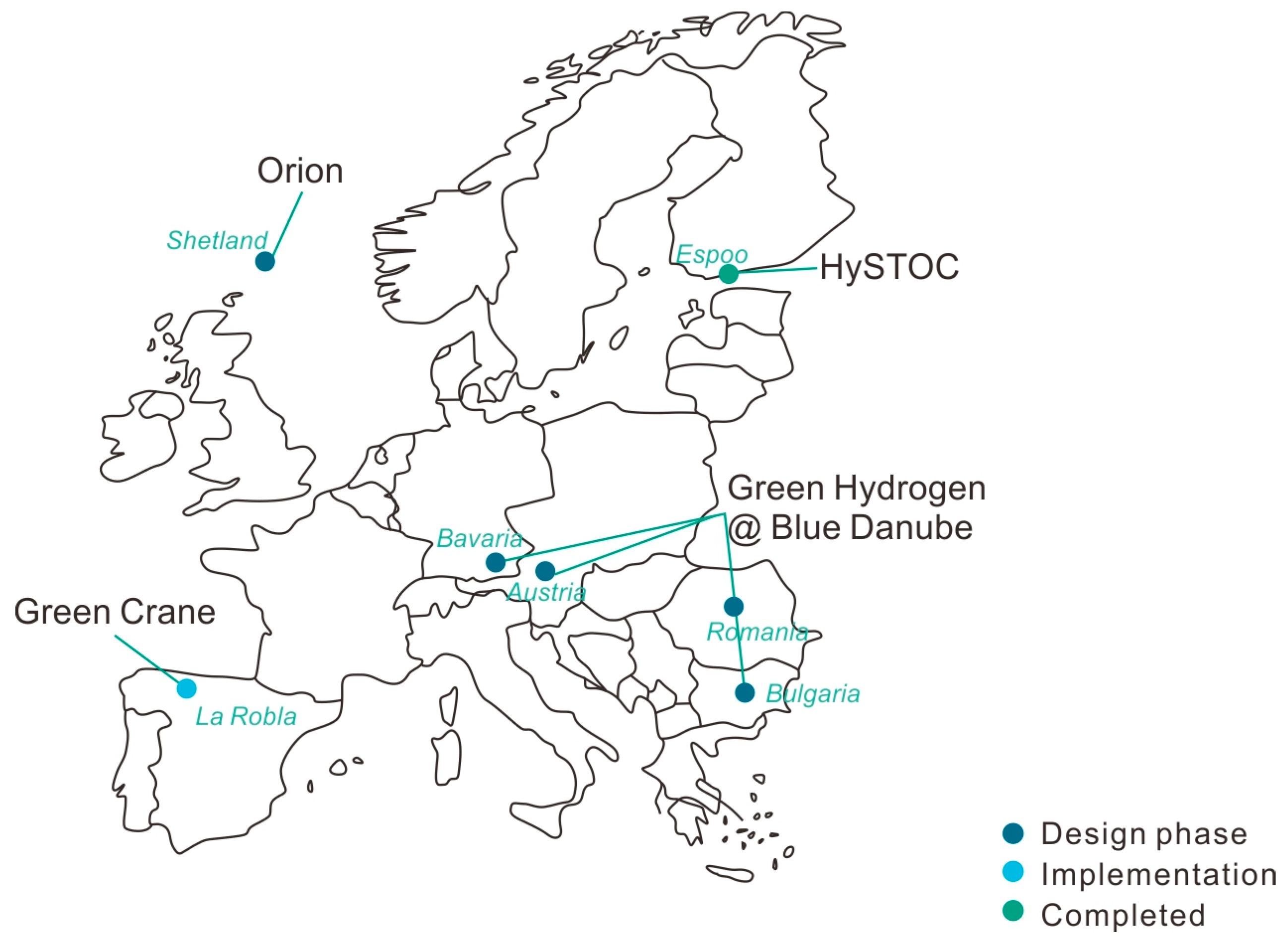
2.2. Projects across Europe
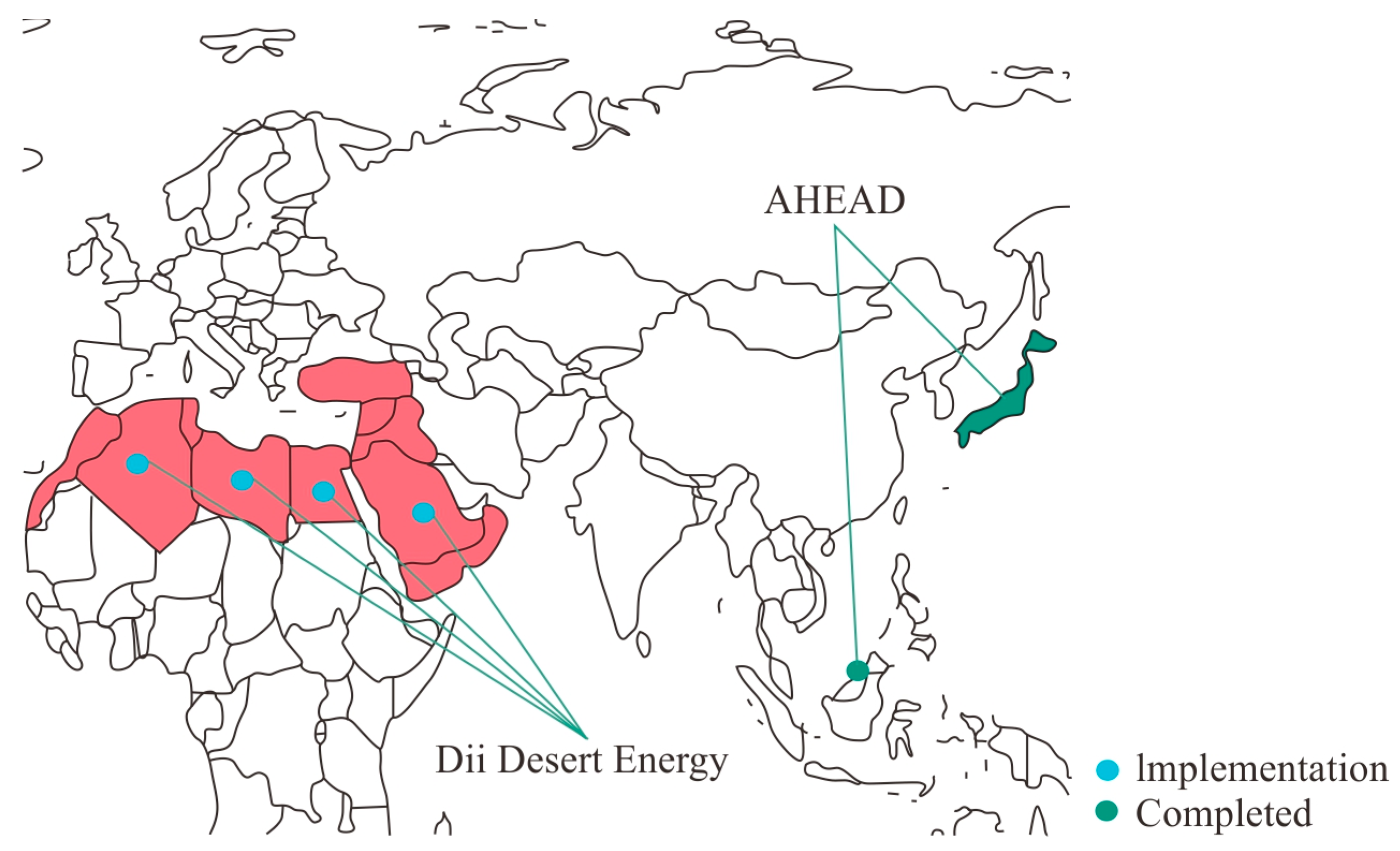
2.3. International Projects
| No. | Project Name | Project Specifications |
|---|---|---|
| 1 | Dii Desert Energy [55] 2009 onwards | Description: Production and export of green hydrogen Location: Middle East and North Africa Target: Build up emission-free and stable energy supply chains with the help of the LOHC technology Consortium: Dii network, H2 Industries Financing: No information available Supply chain: Electrolysis, hydrogenisation, transport LOHC compound: No information available Electrolysis: Wind and solar energy Hydrogenisation: No information available Transport: Water, ship |
| 2 | AHEAD Demonstrator Project [56] 2017–2020 | Description: Production of hydrogen via steam reforming and export from Brunei in the form of LOHCs to Japan Location: Japan/Brunei Target: Showing the possibilities of building up a hydrogen transport chain with LOHCs Consortium: Advanced Hydrogen Energy Chain Association for Technology Development (AHEAD): Chiyoda Corporation, Mitsubishi Corporation, Mitsui & Co., Ltd., Nippon Yusen Kabushiki Kaisha Financing: National Research and Development Agency, New Energy and Industrial Technology Development Organization (NEDO) Supply chain: Hydrogenisation, transport, dehydrogenisation, usage LOHC compound: Toluene Hydrogenisation: No information available Transport: Water, ship Dehydrogenation: No information available Usage: Energy |
3. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef] [PubMed]
- Klerke, A.; Christensen, C.H.; Nørskov, J.K.; Vegge, T. Ammonia for hydrogen storage: Challenges and opportunities. J. Mater. Chem. 2008, 18, 2304–2310. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Toray Advanced Composites. Toray Advanced Composites to Lead Research Consortium for Development of Liquid Hydrogen Composite Tanks for Civil Aviation. 2021. Available online: https://www.toraytac.com/media/news-item/2021/12/14/Toray-Advanced-Composites-to-lead-research-consortium-for-development-of-liquid-hydrogen-composite-tanks-for-civil-aviation (accessed on 20 December 2023).
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: Golden, CO, USA, 2013. [Google Scholar]
- Bick, D.S.; Schmücker, A. H2-Tauglichkeit des Ferngasnetzes der Open Grid Europe. Status, Erforderliche Anpassungen und Fahrplan zur Umsetzung. 2020. Available online: https://h2-news.eu/wp-content/uploads/2020/09/gwf-ge_03_2020_Bick-1.pdf (accessed on 20 December 2023).
- Adam, P.; Engelshove, S.; Heunemann, F.; Thiemann, T.; Bussche, C.V.D. Wasserstoffinfrastruktur-Tragende Säule der Energiewende: Umstellung von Ferngasnetzen auf Wasserstoffbetrieb in der Praxis. 2020. Available online: https://www.gascade.de/fileadmin/downloads/wasserstoff/whitepaper-h2-infrastruktur.pdf (accessed on 20 December 2023).
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOHC) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Schwarz, M.; Bachmann, P.; Silva, T.N.; Mohr, S.; Scheuermeyer, M.; Späth, F.; Bauer, U.; Düll, F.; Steinhauer, J.; Hohner, C.; et al. Model Catalytic Studies of Novel Liquid Organic Hydrogen Carriers: Indole, Indoline and Octahydroindole on Pt(111). Chem. A Eur. J. 2017, 23, 14806–14818. [Google Scholar] [CrossRef]
- Modisha, P.M.; Ouma, C.N.M.; Garidzirai, R.; Wasserscheid, P.; Bessarabov, D. The Prospect of Hydrogen Storage Using Liquid Organic Hydrogen Carriers. Energy Fuels 2019, 33, 2778–2796. [Google Scholar] [CrossRef]
- Bachmann, P.; Steinhauer, J.; Späth, F.; Düll, F.; Bauer, U.; Eschenbacher, R.; Hemauer, F.; Scheuermeyer, M.; Bösmann, A.; Büttner, M.; et al. Dehydrogenation of the liquid organic hydrogen carrier system 2-methylindole/2-methylindoline/2-methyloctahydroindole on Pt(111). J. Chem. Phys. 2019, 151, 144711. [Google Scholar] [CrossRef]
- Huynh, N.-D.; Hur, S.H.; Kang, S.G. Tuning the dehydrogenation performance of dibenzyl toluene as liquid organic hydrogen carriers. Int. J. Hydrogen Energy 2021, 46, 34788–34796. [Google Scholar] [CrossRef]
- Southall, E.; Lukashuk, L. Hydrogen Storage and Transportation Technologies to Enable the Hydrogen Economy: Liquid Organic Hydrogen Carriers. Johns. Matthey Technol. Rev. 2022, 66, 246–258. [Google Scholar] [CrossRef]
- Cao, T.; Lee, W.; Huang, R.; Gorte, R.J.; Vohs, J.M. Liquid-Organic hydrogen carriers as endothermic fuels. Fuel 2022, 313, 123063. [Google Scholar] [CrossRef]
- Biniwale, R.B.; Rayalu, S.; Devotta, S.; Ichikawa, M. Chemical hydrides: A solution to high capacity hydrogen storage and supply. Int. J. Hydrogen Energy 2008, 33, 360–365. [Google Scholar] [CrossRef]
- Bourane, A.; Elanany, M.; Pham, T.V.; Katikaneni, S.P. An overview of organic liquid phase hydrogen carriers. Int. J. Hydrogen Energy 2016, 41, 23075–23091. [Google Scholar] [CrossRef]
- Cho, J.-Y.; Kim, H.; Oh, J.-E.; Park, B.Y. Recent Advances in Homogeneous/Heterogeneous Catalytic Hydrogenation and Dehydrogenation for Potential Liquid Organic Hydrogen Carrier (LOHC) Systems. Catalysts 2021, 11, 1497. [Google Scholar] [CrossRef]
- Kwak, Y.; Moon, S.; Ahn, C.I.; Kim, A.R.; Park, Y.; Kim, Y.; Sohn, H.; Jeong, H.; Nam, S.W.; Yoon, C.W.; et al. Effect of the support properties in dehydrogenation of biphenyl-based eutectic mixture as liquid organic hydrogen carrier (LOHC) over Pt/Al2O3 catalysts. Fuel 2021, 284, 119285. [Google Scholar] [CrossRef]
- Clot, E.; Eisenstein, O.; Crabtree, R.H. Computational structure–activity relationships in H2 storage: How placement of N atoms affects release temperatures in organic liquid storage materials. Chem. Commun. 2007, 22, 2231–2233. [Google Scholar] [CrossRef] [PubMed]
- Pez, G.P.; Scott, A.R.; Cooper, A.C.; Cheng, H.; Wilhelm, F.C.; Abdourazak, A.H. Hydrogen Storage by Reversible Hydrogenation of pi-Conjugated Substrates. U.S. Patent US7351395B1, 1 April 2008. [Google Scholar]
- Cui, Y.; Kwok, S.; Bucholtz, A.; Davis, B.; Whitney, R.A.; Jessop, P.G. The effect of substitution on the utility of piperidines and octahydroindoles for reversible hydrogen storage. New J. Chem. 2008, 32, 1027–1037. [Google Scholar] [CrossRef]
- Niermann, M.; Timmerberg, S.; Drünert, S.; Kaltschmitt, M. Liquid Organic Hydrogen Carriers and alternatives for international transport of renewable hydrogen. Renew. Sustain. Energy Rev. 2021, 135, 110171. [Google Scholar] [CrossRef]
- Reuß, M.; Grube, T.; Robinius, M.; Preuster, P.; Wasserscheid, P.; Stolten, D. Seasonal storage and alternative carriers: A flexible hydrogen supply chain model. Appl. Energy 2017, 200, 290–302. [Google Scholar] [CrossRef]
- Arlt, W.; Obermeier, J. Machbarkeitsstudie: Wasserstoff und Speicherung im Schwerlastverkehr. 2017. Available online: https://www.encn.de/fileadmin/user_upload/Studie_Wasserstoff_und_Schwerlastverkehr_WEB.pdf (accessed on 20 December 2023).
- Sterner, M.; Stadler, I. Energiespeicher-Bedarf, Technologien, Integration; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Jorschick, H.; Geißelbrecht, M.; Eßl, M.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications. Int. J. Hydrogen Energy 2020, 45, 14897–14906. [Google Scholar] [CrossRef]
- Jorschick, H.; Preuster, P.; Bösmann, A.; Wasserscheid, P. Hydrogenation of aromatic and heteroaromatic compounds—A key process for future logistics of green hydrogen using liquid organic hydrogen carrier systems. Sustain. Energy Fuels 2021, 5, 1311–1346. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of industrially applied heat-transfer fluids as liquid organic hydrogen carrier systems. ChemSusChem 2014, 7, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Sekine, Y.; Higo, T. Recent Trends on the Dehydrogenation Catalysis of Liquid Organic Hydrogen Carrier (LOHC): A Review. Top. Catal. 2021, 64, 470–480. [Google Scholar] [CrossRef]
- RWE. Hydrogen Production in the North Sea. Available online: https://www.rwe.com/en/research-and-development/hydrogen-projects/aquaventus/ (accessed on 25 December 2023).
- Bdew. Smart Quart-Energiewende Leben. Available online: https://www.bdew.de/energie/waermewende/waerme-schafft-effizienz/smart-quart-energiewende-leben/ (accessed on 25 December 2023).
- SmartQuart. Climate Neutral Local Digital. 2023. Available online: https://smartquart.energy/ (accessed on 25 December 2023).
- GetH2. Kickstarting the Energy Transition with Hydrogen. Available online: https://www.get-h2.de/en/initiativeandvision/ (accessed on 25 December 2023).
- bp in Deutschland. GET H2 Nukleus: Umfangreiche CO2-Einsparungen Durch Erstes Öffentlich Zugängliches Wasserstoffnetz. 2020. Available online: https://www.bp.com/de_de/germany/home/presse/pressemeldungen/geth2.html (accessed on 25 December 2023).
- Wasserstoff-Leitprojekte. How Partners in the H2Mare Flagship Project Intend to Produce Hydrogen on the High Seas. 2021. Available online: https://www.wasserstoff-leitprojekte.de/projects/h2mare (accessed on 25 December 2023).
- Bundesministerium für Forschung und Bildung. How the Kopernikus Project P2X Converts Renewable Electricity into Plastics and Fuels, Gases and Heat. Available online: https://www.kopernikus-projekte.de/en/projects/p2x (accessed on 25 December 2023).
- Hydrogenious. Worldwide Novelty: Hydrogenious Suppliez Hydrogen Filling Station in Erlangen/Germany via Liquid Organic Hydrogen Carriers; Hydrogenious: Erlangen, Germany, 2022. [Google Scholar]
- Fraunhofer-Institut für Windenergiesysteme. Hydrogen Lab Bremerhaven. Available online: https://www.hydrogen-labs.fraunhofer.de/de/hydrogen-lab-bremerhaven.html (accessed on 25 December 2023).
- Forschungszentrum Jülich GmbH. From the Lab to the Rails. 2018. Available online: https://www.fz-juelich.de/de/aktuelles/news/pressemitteilungen/2018/2018-04-19-lohc-zug?expand=translations,fzjsettings,nearest-institut (accessed on 25 December 2023).
- CORDIS EU Research Results. Enabling the Hydrogen Economy. 2022. Available online: https://cordis.europa.eu/project/id/757082 (accessed on 25 December 2023).
- Helmholtz. SPITZENFORSCHUNG FÜR GROSSE HERAUSFORDERUNGEN: Kompetenzatlas Wasserstoff in der Helmholtz-Gemeinschaft. 2020. Available online: https://www.helmholtz.de/fileadmin/user_upload/01_forschung/01_Energie/Wasserstoffatlas_Mai2020.pdf (accessed on 25 December 2023).
- Hydrogenious LOHC Technologies. Kick-Off for Construction and Operation of the World’s Largest Plant for Storing Green Hydrogen in Liquid Organic Hydrogen Carrier. 2021. Available online: https://hydrogenious.net/kick-off-for-construction-and-operation-of-the-worlds-largest-plant-for-storing-green-hydrogen-in-liquid-organic-hydrogen-carrier/ (accessed on 25 December 2023).
- Verbund. Green Hydrogen Blue Danube. 2020. Available online: https://www.verbund.com/en-at/about-verbund/news-press/press-releases/2020/11/17/greenhydrogenbluedanube (accessed on 4 January 2024).
- CORDIS EU Research Results. Hydrogen Supply and Transportation Using Liquid Organic Hydrogen Carriers. 2022. Available online: https://cordis.europa.eu/project/id/779694/factsheet (accessed on 4 January 2024).
- Orion. Building a World-Leading Clean Energy Island. Available online: https://www.orioncleanenergy.com/ (accessed on 4 January 2024).
- Hydrogen Valleys. Green Crane (Western Route). Available online: https://h2v.eu/hydrogen-valleys/green-crane-western-route (accessed on 4 January 2024).
- Aziz, M.; Oda, T.; Kashiwagi, T. Comparison of liquid hydrogen, methylcyclohexane and ammonia on energy efficiency and economy. Energy Procedia 2019, 158, 4086–4091. [Google Scholar] [CrossRef]
- Ali, A.-G.A.; Ali, L.I.; Aboul-Fotouh, S.M.; Aboul-Gheit, A.K. Hydrogenation of aromatics on modified platinum-alumina catalysts. Appl. Catal. A Gen. 1998, 170, 285–296. [Google Scholar] [CrossRef]
- Nakano, A.; Manabe, S.; Higo, T.; Seki, H.; Nagatake, S.; Yabe, T.; Ogo, S.; Nagatsuka, T.; Sugiura, Y.; Iki, H.; et al. Effects of Mn addition on dehydrogenation of methylcyclohexane over Pt/Al2O3 catalyst. Appl. Catal. A Gen. 2017, 543, 75–81. [Google Scholar] [CrossRef]
- Yan, J.; Wang, W.; Miao, L.; Wu, K.; Chen, G.; Huang, Y.; Yang, Y. Dehydrogenation of methylcyclohexane over PtSn supported on MgAl mixed metal oxides derived from layered double hydroxides. Int. J. Hydrogen Energy 2018, 43, 9343–9352. [Google Scholar] [CrossRef]
- Zhang, X.; He, N.; Lin, L.; Zhu, Q.; Wang, G.; Guo, H. Study of the carbon cycle of a hydrogen supply system over a supported Pt catalyst: Methylcyclohexane–toluene–hydrogen cycle. Catal. Sci. Technol. 2020, 10, 1171–1181. [Google Scholar] [CrossRef]
- Chiyoda Corporation. Spera Hydrogen System. 2021. Available online: https://www.chiyodacorp.com/en/service/spera-hydrogen/innovations/ (accessed on 4 January 2024).
- Dii Desert Energy. World’s First Organization for Energy from the Deserts. Available online: https://dii-desertenergy.org/ (accessed on 4 January 2024).
- AHEAD. World’s First International Transport of Hydrogen: Foreign-Produced Hydrogen Has Arrived in Japan for the First Time from Brunei Darussalam. 2019. Available online: https://www.ahead.or.jp/en/pdf/20191218_ahead_press.pdf (accessed on 4 January 2024).
| No. | Project Name | Project Specifications |
|---|---|---|
| 1 | AquaVentus [32] 2023–2035 | Description: Use electricity from offshore wind farms to produce green hydrogen Location: Helgoland Target: 10 GW green hydrogen (offshore wind) until 2035, transport per pipeline with later storage as LOHCs (2024) Consortium: Agentur für Wirtschaftsförderung Cuxhaven, Aktiengesellschaft EMS, Brunsbüttel Ports GmbH, Buckstay Group GmbH, Deutsche AVIA Mineralöl-GmbH, CHATHAM PARTNERS, Cuxport GmbH, DNV, Deutsche Shell Holding GmbH, Fraunhofer IFAM, EnBW Energie Baden-Württemberg AG, EnTec Industrial Services GmbH & Co. KG, Entwicklungsgesellschaft Brunsbüttel mbH, E.ON SE, GASCADE Gastransport GmbH, Gemeinde Helgoland, German LNG Terminal GmbH, GÖRG Partnerschaft von Rechtsanwälten mbB, H2-Industries SE, HHLA Hamburger Hafen und Logistik Aktiengesellschaft, HanseWerk AG, H. C. Hagemann GmbH & Co. KG, Hydrogenious LOHC Technologies GmbH, HynamicsDeutschland GmbH, ILF Beratende Ingenieure GmbH, Kongstein GmbH, Linde GmbH, Mabanaft GmbH, MHI Vestas Offshore Wind A/S, Northland PowerN.V.,Nederlandse Gasunie, Orsted Wind Power Germany GmbH, OWT Offshore Wind Technologie GmbH, Parkwind nv, Reuther STC, ROSEN Technology and Research Center GmbH, RWE Renewables GmbH, Siemens Gamesa Renewable Energy SA, Siemens Energy AG, Stiftung Offshore Windenergie, Tractebel Overdick GmbH, Vallourec Deutschland GmbH, Vattenfall Innovation GmbH, Versorgungsbetriebe Helgoland, VIRYA ENERGY NV, Weidmüller Interface GmbH, WindMW GmbH Financing: No information available Supply chain: Electrolysis, hydrogenisation, transport LOHC compound: No information available Electrolysis: Offshore wind energy and on-site electrolysis Hydrogenisation: Excess hydrogenation heat to cover residential demand of heating energy Transport: Primary mode of transport: water, tank ship |
| 2 | SmartQuart [33,34] 2020–2024 | Description: Products and solutions for the planning, construction and operation of energy-optimized neighbourhoods in Germany Location: Bedburg, Essen and Kaisersesch Target: Development of a smart concept for a sustainable hydrogen infrastructure including the LOHC technology; different scenarios for industrial and residential applications Consortium: E.ON, gridX, Hydrogenious LOHC Technologies, RWTH Aachen, Viessmann, H2 MOBILITY Germany, RWE Power Financing: Partly funded by BMWi (8.6 million EUR ) Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: (Di-)Benzyltoluene Electrolysis: Use of excess renewable energy (wind, solar); usage of excess electrolyser heat to power a nearby wastewater treatment plant Hydrogenisation: No information available Transport: Planned usage of existing fossil fuel infrastructure Dehydrogenisation: No information available Usage: Heat, electricity, mobility, and industry |
| 3 | Get H2 [35,36] 2024–2030 | Description: Planning of realization of infrastructures for the production, purchase, transport, and storage of green hydrogen (H2) in several projects Location: Lingen Target: Build up a countrywide hydrogen infrastructure Consortium: RWE Generation SE, Siemens, ENERTRAG, public utility Lingen, Hydrogenious LOHC Technologies, Nowega, Research center Jülich, IKEM, Salzgitter Flachstahl, Thyssengas, bp, Evonik, OGE Financing: Applied for IPCEI funding Supply chain: Electrolysis, hydrogenisation, transport, usage LOHC compound: Dibenzyltoluene Electrolysis: Power-to-gas plants, electricity from wind and sun, usage of excess electrolyser heat Hydrogenisation: No information available Transport: Road, tank trucks Usage: Focus on mobility and large-scale industrial consumers |
| 4 | TransHyDE/H2Mare [37] 2021–2025 | Description: The H2Mare-lead project develops ways to produce hydrogen and hydrogen downstream products using off-grid wind turbines directly at sea Location: Helgoland/Hamburg Target: Generate a LOHC supply chain from off-shore wind energy, dehydrogenation at the port of Hamburg Consortium: More than 200 partners, not further disclosed Financing: BMBF funded (total volume of 700 million EUR, fraction for TransHyDE/H2Mare not declared) Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation LOHC compound: No information available Electrolysis: No information available Hydrogenisation: No information available Transport: Road, water; usage of existing fossil fuel tank ship infrastructure Dehydrogenisation: No information available |
| 5 | Kopernikus Power-to-X [38] 2016–2025 | Description: Investigating Power-to-X (ptx) technologies Location: Not location-specific Target: Research on possible technologies to convert green electrical energy into chemical forms of energy (e.g., LOHCs) Consortium: AREVA H2GEN GmbH, AUDI AG, AVL List GmbH, Beiersdorf AG, Bund für Umwelt und Naturschutz Deutschland e.V. (BUND), CAT Catalytic Centre, Clariant Produkte, Deutschland) GmbH, Climeworks Deutschland GmbH, Covestro Deutschland AG, DB Energie, DECHEMA Gesellschaft für Chemische Technik und Biotechnologie e.V., DECHEMA), Deutsches Institut für Wirtschaftsforschung (DIW Berlin), Deutsches Zentrum für Luft und Raumfahrt e.V. (DLR), Evonik Creavis GmbH, Ford-Werke GmbH, Forschungszentrum Jülich GmbH (FZJ), Framatome GmbH, Fraunhofer-Institut für Solare Energiesysteme (ISE), Friedrich-Alexander-Universität Erlangen Nürnberg (FAU), Greenerity GmbH, Helmholtz-Institut Erlangen-Nürnberg, Helmholtz-Zentrum Berlin für Materialien und Energie GmbH, Heraeus GmbH & Co. KG, H-TEC SYSTEMS GmbH, Hydrogenious LOHC Technologies GmbH, International Association for Sustainable Aviation e.V. (IASA), INERATEC GmbH, Institut für Energie- und Umweltforschung Heidelberg GmbH (ifeu), Institut für ZukunftsEnergie und Stoffstromsysteme gGmbH (IZES), Karlsruher Institut für Technologie (KIT), Leibniz-Institut für Interaktive Materialien e.V. (DWI), Linde AG, Ludwig-Maximilians-Universität München (LMU), Max-Planck-Institut für Chemische Energiekonversion (CEC), Öko-Institut e.V., Ostbayerische Technische Hochschule Regensburg (OTH), RWTH Aachen, SCHOTT AG, Siemens AG, sunfire GmbH, Technische Universität München (TUM), Volkswagen AG (VW), Wacker Chemie AG, Wissenschaftszentrum Berlin für Sozialforschung (WZB), WWF Deutschland, Zentrum für Angewandte Energieforschung Bayern e.V. (ZAE Bayern), International Association for Sustainable Aviation e.V. Financing: BMBF 29.7 million EUR Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: Dibenzyltoluene -> Benzyltoluene Electrolysis: Search for ways to reduce the amount of iridium used in electrolysis as much as possible, without making the process less efficient Hydrogenisation: No information available Transport: Road, tank trucks; usage of existing infrastructure (e.g., storage, tank vehicles) possible Dehydrogenisation: Investigation of alternative ways to provide dehydrogenation heat Usage: Mobility, Chemical industry, Industrial furnaces (glass manufacturers) |
| 6 | LOHC fuel station Erlangen [39] 2021 onwards | Description: Hydrogen fuel station Location: Erlangen Target: First German LOHC fuel station from green hydrogen (PV) Consortium: Hydrogenious LOHC Technologies, H2 Mobility Financing: No information available Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: Dibenzyltoluene Electrolysis: Use of solar energy Hydrogenisation: No information available Transport: Road, tank trucks Dehydrogenisation: No information available Usage: Mobility (Fuel station) |
| 7 | Hydrogen Lab Bremerhaven [40] –2022 | Description: Investigating the potential of green hydrogen in four selected applications Location: Bremerhaven Target: Implementation of a test area for electrolysis; development of a complete hydrogen supply chain under the usage of the LOHC technology Consortium: Fraunhofer IWES, University Bremerhaven Financing: 20 million EUR from Bremen and EU Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: Dibenzyltoluene Electrolysis: Use of wind energy Hydrogenisation: No information available Transport: No information available Dehydrogenisation: No information available |
| 8 | LOHC train [41] 2019–2023 | Description: Equip trains with LOHC technology Location: Jülich Target: Development of a LOHC-powered commuter train Consortium: Helmholtz Institute Erlangen/Nuremberg, Research Center Jülich, Hydrogenious LOHC Technologies, FAU Erlangen/Nuremberg, Fraunhofer HHI Financing: 28 million EUR from Bavarian State Ministry of Economic Affairs, Regional Development and Energy Supply chain: Dehydrogenisation, usage LOHC compound: No information available Dehydrogenisation: No information available Usage: Mobility (commuter train) |
| 9 | Hydrogenlogistics [42] 2017–2019 | Description: The Hydrogenlogistics project has developed, built, and tested a modular dehydrogenation system for hydrogen carrier materials on an industrial scale, which will be used to supply hydrogen refuelling stations Location: Germany (non-specific) Target: Cost reduction of hydrogen transport by 80%, development of modular dehydrogenation system (ReleaseBOX/ReleaseUNIT) for hydrogen fuel stations Consortium: Hydrogenious LOHC Technologies, EU H2020 Financing: EU 2.3 million EUR (3.3 million EUR in total volume) Supply chain: Transport, dehydrogenisation, usage LOHC compound: Dibenzyltoluene Transport: Road, tank trucks Dehydrogenisation: No information available Usage: Mobility |
| 10 | Helmholtz Hydrogen Cluster [43] 2021 onwards | Description: The The Helmholtz Cluster for Sustainable and Infrastructurally Compatible Hydrogen Economy (HC H2) will act as a nucleus for a hydrogen model region, research future innovative hydrogen technologies and accompany them into practical application Location: Jülich Target: Research possibilities for a LOHC-driven hydrogen infrastructure Consortium: Research Center Jülich, DLR, Campus Power-to-X, RWTH Aachen, FH Aachen, iNew, FAU Erlangen/Nuremberg Financing: No information available Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: No information available Electrolysis: Use of excess renewable energy Hydrogenisation: No information available Transport: Road, tank trucks; use of existing logistics Dehydrogenisation: No information available Usage: E.g. train with LOHC technology |
| 11 | HECTOR [44] Until 2023 | Description: Pilot plant for storage of green hydrogen in LOHC Location: Dormagen Target: 2nd biggest plant worldwide for storage of green hydrogen with LOHC technology Consortium: LOHC Industrial Solutions NRW, Hydrogenious LOHC Technologies, Covestro, Research center Jülich, Royal Vopak Financing: 9 million EUR from progres.nrw fund (20 MEUR in total volume) Supply chain: Hydrogenisation LOHC compound: Dibenzyltoluene Hydrogenisation: Usage of excess hydrogenation heat for local energy supply |
| No. | Project Name | Project Specifications |
|---|---|---|
| 1 | IPCEI Green Hydrogen @ Blue Danube [45] 2022– | Description: In the “Blue Danube” project, green hydrogen produced in Bulgaria and Romania will be stored in LOHCs and transported via the Danube to customers in Austria and Germany Location: Romania and Bulgaria to Germany and Austria Target: European supply chain for green hydrogen; project roll-out in Bavaria with 3000 tons of green hydrogen via LOHCs Consortium: VERBUND, Hydrogenious LOHC Technologies, Bosch, DB Schenker, Donau Tankschifffahrtsgesellschaft, Siemens Energy Financing: No information available Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: (Di-)Benzyltoluene Electrolysis: Solar, hydro, and wind energy, on-site electrolysis Hydrogenisation: No information available Transport: Water (Danube), road, tank ships, tank trucks; inland vessels from fossil fuel transport Dehydrogenisation: No information available Usage: Industry, mobility |
| 2 | HySTOC [46] 2018–2022 | Description: Complete hydrogen logistics up to use at the refuelling station is being researched Location: Finland (Espoo) Target: Research applicability of LOHC technology for the distribution and storage of hydrogen refuelling stations Consortium: Institute of Chemical Reaction Engineering, Oy VTT Technical Research Centre of Finland Ltd., Hydrogenious LOHC Technologies, HyGear B.V., Oy Woikoski Ab, FAU Erlangen/Nuremberg Financing: EU funded (2.5 million EUR ) Supply chain: Hydrogenisation, transport, usage LOHC compound: Dibenzyltoluene Hydrogenisation: No information available Transport: Road, tank trucks, 1000 l electrically heated IBC containers Usage: Mobility (refuelling station) |
| 3 | Orion Project [47] 2026–2050+ | Description: Production and transport of hydrogen Location: Shetland Target: Annual production of 32 TWh blue/green hydrogen with partial exports to GB and other countries Consortium: Shetland Islands Council, OGTC, Highlands and Islands Enterprise Financing: 11.5 million EUR Shetland Islands Council Supply chain: Electrolysis, hydrogenisation, transport LOHC compound: No information available Electrolysis: Use of on- and offshore wind Hydrogenisation: No information available Transport: Water, tank ship; export via existing facilities possible |
| 4 | Green Crane [48] 2019–2024 | Description: Transport LOHCs from Spanish seaports to the Netherlands and establish large LOHC storage facilities around the ports Location: La Robla (Spain) Target: Hydrogen transport from Spain to central Europe Consortium: Enagás Renovable, Hydrogenious LOHC Technologies, Vopak, Térega, Petronor, Arcelor-Mittal I+D, Bosch, McPhy, Vestas, Falck Renewables, IGNIS Energia, H2V, Gransolar Financing: Partly EU funded Supply chain: Electrolysis, hydrogenisation, transport, dehydrogenisation, usage LOHC compound: No information available Electrolysis: Water electrolysis with PEM electrolyser; water electrolysis with ALK electrolyser; by-product Hydrogenisation: No information available Transport: Water, ship Dehydrogenisation: No information available Usage: Mobility, energy, industry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, X.; Zhang, C.; Ding, Z.; Jin, X. Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects. Energies 2024, 17, 1940. https://doi.org/10.3390/en17081940
Li H, Zhang X, Zhang C, Ding Z, Jin X. Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects. Energies. 2024; 17(8):1940. https://doi.org/10.3390/en17081940
Chicago/Turabian StyleLi, Hanqi, Xi Zhang, Chenjun Zhang, Zhenfeng Ding, and Xu Jin. 2024. "Application and Analysis of Liquid Organic Hydrogen Carrier (LOHC) Technology in Practical Projects" Energies 17, no. 8: 1940. https://doi.org/10.3390/en17081940




