Estimation of Fuel Properties for the Heavy Fraction of Biomass Pyrolysis Oil Consisting of Proposed Structures for Pyrolytic Lignin and Humins
Abstract
:1. Introduction
2. Materials and Methods
2.1. High Molecular Weight Biomass Pyrolysis Oil Molecules
2.2. Elemental Analysis and Heat of Combustion Estimation
2.3. Group Contribution Regularized Regression for Normal Boiling Point Estimation
2.4. Heat of Vaporization Estimation
2.5. Flash Point Estimation
3. Results
3.1. Elemental Analysis and Heat of Combustion
3.2. Normal Boiling Point
3.3. Heat of Vaporization
3.4. Flash Point
4. Discussion
| Property | Standard Method | Example Application | Reference |
|---|---|---|---|
| Elemental analysis | ASTM D5291 | Carbon, hydrogen, and nitrogen content is measured for vacuum gas oil, dry bio-oil, catalytic pyrolysis oil, and hydrotreated bio-oil. Oxygen content is calculated by difference. | [101] |
| Heat of combustion | ASTM D5865, ASTM D4809 | HHV measurement for biomass feedstocks and pyrolysis liquids in a study comparing accelerated aging procedures to assess bio-oil stability. | [102] |
| Boiling point a | ASTM D86, ASTM D2892; NIST ADC method(s) | Fast pyrolysis bio-oil from sawmill residues is analyzed with ADC methods to measure and simulate distillation curves (temperature vs. distillate volume fraction). Limitations of the ASTM methods (designed for petroleum) when utilized for bio-oils are discussed. | [103,104,105,106,107,108] |
| Heat of vaporization | ASTM E1782 | Vapor pressure measurements were carried out using differential scanning calorimetry for phenolic compounds, with heat of vaporization being calculated by the Clausius-Clapeyron equation. An alternative method using thermogravimetic analysis for (petroleum) oils is presented in work by Rannaveski and Oja. | [73,109] |
| Flash point | ASTM D93 | Flash point measurement using Pensky–Martens closed cup method for bio-oil produced from pyrolysis of bay laurel biomass. | [110] |
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gernaat, D.E.H.J.; de Boer, H.S.; Daioglou, V.; Yalew, S.G.; Müller, C.; van Vuuren, D.P. Climate change impacts on renewable energy supply. Nat. Clim. Chang. 2021, 11, 119–125. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, H.W.; Lee, S.M.; Jae, J.; Park, Y.K. Overview of the recent advances in lignocellulose liquefaction for producing biofuels, bio-based materials and chemicals. Bioresour. Technol. 2019, 279, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Elkasabi, Y.; Mullen, C.A. Progress on Biobased Industrial Carbons as Thermochemical Biorefinery Coproducts. Energy Fuels 2021, 35, 5627–5642. [Google Scholar] [CrossRef]
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Vikram, S.; Rosha, P.; Kumar, S. Recent Modeling Approaches to Biomass Pyrolysis: A Review. Energy Fuels 2021, 35, 7406–7433. [Google Scholar] [CrossRef]
- Kostetskyy, P.; Broadbelt, L.J. Progress in Modeling of Biomass Fast Pyrolysis: A Review. Energy Fuels 2020, 34, 15195–15216. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Biomass pyrolysis: A review of the process development and challenges from initial researches up to the commercialisation stage. J. Energy Chem. 2019, 39, 109–143. [Google Scholar] [CrossRef]
- Guedes, R.E.; Luna, A.S.; Torres, A.R. Operating parameters for bio-oil production in biomass pyrolysis: A review. J. Anal. Appl. Pyrolysis 2018, 129, 134–149. [Google Scholar] [CrossRef]
- Wang, G.; Dai, Y.; Yang, H.; Xiong, Q.; Wang, K.; Zhou, J.; Li, Y.; Wang, S. A Review of Recent Advances in Biomass Pyrolysis. Energy Fuels 2020, 34, 15557–15578. [Google Scholar] [CrossRef]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Jiang, S.-F.; Sheng, G.-P.; Jiang, H. Advances in the Characterization Methods of Biomass Pyrolysis Products. ACS Sustain. Chem. Eng. 2019, 7, 12639–12655. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Pecha, M.B.; Arbelaez, J.I.M.; Garcia-Perez, M.; Chejne, F.; Ciesielski, P.N. Progress in understanding the four dominant intra-particle phenomena of lignocellulose pyrolysis: Chemical reactions, heat transfer, mass transfer, and phase change. Green. Chem. 2019, 21, 2868–2898. [Google Scholar] [CrossRef]
- Donaldson, B.; Hughes, B.; Coker, E.N.; Yilmaz, N. Pyrolysis of Oils from Unconventional Resources. Energies 2023, 16, 3455. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Fonts, I.; Atienza-Martínez, M.; Carstensen, H.-H.; Benés, M.; Pinheiro Pires, A.P.; Garcia-Perez, M.; Bilbao, R. Thermodynamic and Physical Property Estimation of Compounds Derived from the Fast Pyrolysis of Lignocellulosic Materials. Energy Fuels 2021, 35, 17114–17137. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, S.; Hu, X.; Gholizadeh, M. Progress in application of the pyrolytic lignin from pyrolysis of biomass. Chem. Eng. J. 2021, 419, 129560. [Google Scholar] [CrossRef]
- Stankovikj, F.; McDonald, A.G.; Helms, G.L.; Garcia-Perez, M. Quantification of Bio-Oil Functional Groups and Evidences of the Presence of Pyrolytic Humins. Energy Fuels 2016, 30, 6505–6524. [Google Scholar] [CrossRef]
- Rover, M.R.; Johnston, P.A.; Whitmer, L.E.; Smith, R.G.; Brown, R.C. The effect of pyrolysis temperature on recovery of bio-oil as distinctive stage fractions. J. Anal. Appl. Pyrolysis 2014, 105, 262–268. [Google Scholar] [CrossRef]
- Marathe, P.S.; Westerhof, R.J.M.; Kersten, S.R.A. Effect of Pressure and Hot Vapor Residence Time on the Fast Pyrolysis of Biomass: Experiments and Modeling. Energy Fuels 2020, 34, 1773–1780. [Google Scholar] [CrossRef]
- Marathe, P.S.; Westerhof, R.J.M.; Kersten, S.R.A. Fast pyrolysis of lignins with different molecular weight: Experiments and modelling. Appl. Energy 2019, 236, 1125–1137. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Martinez-Valencia, L.; Tanzil, A.H.; Garcia-Perez, M.; García-Ojeda, J.C.; Bertok, B.; Heckl, I.; Argoti, A.; Friedler, F. Synthesis and Techno-Economic Analysis of Pyrolysis-Oil-Based Biorefineries Using P-Graph. Energy Fuels 2021, 35, 13159–13169. [Google Scholar] [CrossRef]
- Han, Y.; Gholizadeh, M.; Tran, C.-C.; Kaliaguine, S.; Li, C.-Z.; Olarte, M.; Garcia-Perez, M. Hydrotreatment of pyrolysis bio-oil: A review. Fuel Process. Technol. 2019, 195, 106140. [Google Scholar] [CrossRef]
- Kloekhorst, A.; Wildschut, J.; Heeres, H.J. Catalytic hydrotreatment of pyrolytic lignins to give alkylphenolics and aromatics using a supported Ru catalyst. Catal. Sci. Technol. 2014, 4, 2367–2377. [Google Scholar] [CrossRef]
- Figueirêdo, M.B.; Jotic, Z.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Hydrotreatment of pyrolytic lignins to aromatics and phenolics using heterogeneous catalysts. Fuel Process. Technol. 2019, 189, 28–38. [Google Scholar] [CrossRef]
- Lange, J.P. Lignocellulose Liquefaction to Biocrude: A Tutorial Review. ChemSusChem 2018, 11, 997–1014. [Google Scholar] [CrossRef]
- Buffi, M.; Cappelletti, A.; Rizzo, A.M.; Martelli, F.; Chiaramonti, D. Combustion of fast pyrolysis bio-oil and blends in a micro gas turbine. Biomass Bioenergy 2018, 115, 174–185. [Google Scholar] [CrossRef]
- Kass, M.D.; Armstrong, B.L.; Kaul, B.C.; Connatser, R.M.; Lewis, S.; Keiser, J.R.; Jun, J.; Warrington, G.; Sulejmanovic, D. Stability, Combustion, and Compatibility of High-Viscosity Heavy Fuel Oil Blends with a Fast Pyrolysis Bio-Oil. Energy Fuels 2020, 34, 8403–8413. [Google Scholar] [CrossRef]
- Erdoğan, S. LHV and HHV prediction model using regression analysis with the help of bond energies for biodiesel. Fuel 2021, 301, 121065. [Google Scholar] [CrossRef]
- Santos, S.M.; Nascimento, D.C.; Costa, M.C.; Neto, A.M.B.; Fregolente, L.V. Flash point prediction: Reviewing empirical models for hydrocarbons, petroleum fraction, biodiesel, and blends. Fuel 2020, 263, 116375. [Google Scholar] [CrossRef]
- Jia, Q.; Wang, Q.; Ma, P. Prediction of the Enthalpy of Vaporization of Organic Compounds at Their Normal Boiling Point with the Positional Distributive Contribution Method. J. Chem. Eng. Data 2010, 55, 5614–5620. [Google Scholar] [CrossRef]
- Abdi, S.; Movagharnejad, K.; Ghasemitabar, H. Estimation of the enthalpy of vaporization at normal boiling temperature of organic compounds by a new group contribution method. Fluid. Phase Equilibria 2018, 473, 166–174. [Google Scholar] [CrossRef]
- Terrell, E.; Garcia-Perez, M. Novel Strategy to Analyze Fourier Transform Ion Cyclotron Resonance Mass Spectrometry Data of Biomass Pyrolysis Oil for Oligomeric Structure Assignment. Energy Fuels 2020, 34, 8466–8481. [Google Scholar] [CrossRef]
- Fu, X.; Li, Q.; Hu, C. Identification and structural characterization of oligomers formed from the pyrolysis of biomass. J. Anal. Appl. Pyrolysis 2019, 144, 104696. [Google Scholar] [CrossRef]
- Terrell, E.; Garcia-Perez, M. Vacuum Pyrolysis of Hybrid Poplar Milled Wood Lignin with Fourier Transform-Ion Cyclotron Resonance Mass Spectrometry Analysis of Feedstock and Products for the Elucidation of Reaction Mechanisms. Energy Fuels 2020, 34, 14249–14263. [Google Scholar] [CrossRef]
- Hosokai, S.; Matsuoka, K.; Kuramoto, K.; Suzuki, Y. Modification of Dulong’s formula to estimate heating value of gas, liquid and solid fuels. Fuel Process. Technol. 2016, 152, 399–405. [Google Scholar] [CrossRef]
- Lopes, S.M.; Furey, R.; Geng, P. Calculation of Heating Value for Diesel Fuels Containing Biodiesel. SAE Int. J. Fuels Lubr. 2013, 6, 407–418. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Lo, S.-L. Predicting heating value of lignocellulosic biomass based on elemental analysis. Energy 2020, 191, 116501. [Google Scholar] [CrossRef]
- Terrell, E. Estimation of Hansen solubility parameters with regularized regression for biomass conversion products: An application of adaptable group contribution. Chem. Eng. Sci. 2022, 248, 117184. [Google Scholar] [CrossRef]
- Tsibanogiannis, I.N.; Kalospiros, N.S.; Tassios, D.P. Prediction of Normal Boiling Point Temperature of Medium/High Molecular Weight Compounds. Ind. Eng. Chem. Res. 1995, 34, 997–1002. [Google Scholar] [CrossRef]
- Askonas, C.F.; Daubert, T.E. Vapor pressure determination of eight oxygenated compounds. J. Chem. Eng. Data 1988, 33, 225–229. [Google Scholar] [CrossRef]
- Ambrose, D. Critical temperatures of some phenols and other organic compounds. Trans. Faraday Soc. 1963, 59, 1988–1993. [Google Scholar] [CrossRef]
- Taft, R.; Stareck, J. Relationship between Melting-Points, Normal Boiling-Points and Critical Temperatures. J. Phys. Chem. 2002, 34, 2307–2317. [Google Scholar] [CrossRef]
- Nikitin, E.D.; Popov, A.P. Vapour–liquid critical properties of components of biodiesel. 1. Methyl esters of n-alkanoic acids. Fuel 2015, 153, 634–639. [Google Scholar] [CrossRef]
- Astra, H.-L.; Oja, V. Vapour pressure data for 2-n-propylresorcinol, 4-ethylresorcinol and 4-hexylresorcinol near their normal boiling points measured by differential scanning calorimetry. J. Chem. Thermodyn. 2019, 134, 119–126. [Google Scholar] [CrossRef]
- Verevkin, S.P. Determination of vapor pressures and enthalpies of vaporization of 1,2-alkanediols. Fluid. Phase Equilibria 2004, 224, 23–29. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Yermalayeu, A.V.; Portnova, S.V.; Pimerzin, A.A.; Verevkin, S.P. Renewable platform chemicals: Evaluation of thermochemical data of alkyl lactates with complementary experimental and computational methods. J. Chem. Thermodyn. 2019, 128, 55–67. [Google Scholar] [CrossRef]
- Preprocessing Data. Available online: https://scikit-learn.org/stable/modules/preprocessing.html (accessed on 12 January 2022).
- Linear Models. Available online: https://scikit-learn.org/stable/modules/linear_model.html (accessed on 12 January 2022).
- Melkumova, L.E.; Shatskikh, S.Y. Comparing Ridge and LASSO estimators for data analysis. Procedia Eng. 2017, 201, 746–755. [Google Scholar] [CrossRef]
- Ogutu, J.; Schultz-Streeck, T.; Piepho, H.-P. Genomic selection using regularized linear regression models: Ridge regression, lasso, elastic net and their extensions. BMC Proc. 2012, 6, S10. [Google Scholar] [CrossRef] [PubMed]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B (Methodol.) 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Nishimura, T.; Hibe, Y.; Nagai, M.; Sato, H.; Johnston, I. Regularized regression analysis of digitized molecular structures in organic reactions for quantification of steric effects. J. Comput. Chem. 2017, 38, 1825–1833. [Google Scholar] [CrossRef] [PubMed]
- Stein, S.E.; Brown, R.L. Estimation of normal boiling points from group contributions. J. Chem. Inf. Comput. Sci. 2002, 34, 581–587. [Google Scholar] [CrossRef]
- Krimizis-Tsatsoulis, C. Trouton’s rule mysteries: An attempt to a better understanding. J. Chem. Thermodyn. 2021, 152, 106256. [Google Scholar] [CrossRef]
- Nash, L.K. Trouton and T-H-E rule. J. Chem. Educ. 1984, 61, 981. [Google Scholar] [CrossRef]
- Catoire, L.; Naudet, V. A Unique Equation to Estimate Flash Points of Selected Pure Liquids Application to the Correction of Probably Erroneous Flash Point Values. J. Phys. Chem. Ref. Data 2004, 33, 1083–1111. [Google Scholar] [CrossRef]
- Gharagheizi, F.; Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Empirical Method for Representing the Flash-Point Temperature of Pure Compounds. Ind. Eng. Chem. Res. 2011, 50, 5877–5880. [Google Scholar] [CrossRef]
- Patil, G.S. Estimation of flash point. Fire Mater. 1988, 12, 127–131. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Z. Research Progress on Flash Point Prediction. J. Chem. Eng. Data 2010, 55, 2943–2950. [Google Scholar] [CrossRef]
- Phoon, L.Y.; Mustaffa, A.A.; Hashim, H.; Mat, R. A Review of Flash Point Prediction Models for Flammable Liquid Mixtures. Ind. Eng. Chem. Res. 2014, 53, 12553–12565. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile production from pyrolysis of cellulose, hemicellulose and lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Yin, R.; Liu, R.; Mei, Y.; Fei, W.; Sun, X. Characterization of bio-oil and bio-char obtained from sweet sorghum bagasse fast pyrolysis with fractional condensers. Fuel 2013, 112, 96–104. [Google Scholar] [CrossRef]
- Trinh, T.N.; Jensen, P.A.; Sárossy, Z.; Dam-Johansen, K.; Knudsen, N.O.; Sørensen, H.R.; Egsgaard, H. Fast Pyrolysis of Lignin Using a Pyrolysis Centrifuge Reactor. Energy Fuels 2013, 27, 3802–3810. [Google Scholar] [CrossRef]
- Cao, J.P.; Xiao, X.B.; Zhang, S.Y.; Zhao, X.Y.; Sato, K.; Ogawa, Y.; Wei, X.Y.; Takarada, T. Preparation and characterization of bio-oils from internally circulating fluidized-bed pyrolyses of municipal, livestock, and wood waste. Bioresour. Technol. 2011, 102, 2009–2015. [Google Scholar] [CrossRef] [PubMed]
- Cross-validation: Evaluating Estimator Performance. Available online: https://scikit-learn.org/stable/modules/cross_validation.html (accessed on 13 January 2022).
- Twu, C.H. An internally consistent correlation for predicting the critical properties and molecular weights of petroleum and coal-tar liquids. Fluid. Phase Equilibria 1984, 16, 137–150. [Google Scholar] [CrossRef]
- Boduszynski, M.M.; Altgelt, K.H. Composition of heavy petroleums. 4. Significance of the extended atmospheric equivalent boiling point (AEBP) scale. Energy Fuels 1992, 6, 72–76. [Google Scholar] [CrossRef]
- Rannaveski, R.; Oja, V. A new thermogravimetric application for determination of vapour pressure curve corresponding to average boiling points of oil fractions with narrow boiling ranges. Thermochim. Acta 2020, 683, 178468. [Google Scholar] [CrossRef]
- White, C.M. Prediction of the boiling point, heat of vaporization, and vapor pressure at various temperatures for polycyclic aromatic hydrocarbons. J. Chem. Eng. Data 1986, 31, 198–203. [Google Scholar] [CrossRef]
- Gray, J.A.; Holder, G.D.; Brady, C.J.; Cunningham, J.R.; Freeman, J.R.; Wilson, G.M. Thermophysical properties of coal liquids. 3. Vapor pressure and heat of vaporization of narrow boiling coal liquid fractions. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 97–107. [Google Scholar] [CrossRef]
- Joback, K.G. A Unified Approach to Physical Property Estimation Using Multivariate Statistical Techniques. Master’s Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 1982. [Google Scholar]
- Riedel, L. Kritischer Koeffizient, Dichte des gesättigten Dampfes und Verdampfungswärme. Untersuchungen über eine Erweiterung des Theorems der übereinstimmenden Zustände Teil III. Chem. Ing. Tech. 1954, 26, 679–683. [Google Scholar] [CrossRef]
- Sipilä, K.; Kuoppala, E.; Fagernäs, L.; Oasmaa, A. Characterization of biomass-based flash pyrolysis oils. Biomass Bioenergy 1998, 14, 103–113. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J. Oil from biomass corncob tar as a fuel. Energy Convers. Manag. 2007, 48, 1751–1757. [Google Scholar] [CrossRef]
- Al-Soufi, H.H.; Savaya, Z.F.; Mohammed, H.K.; Al-Azawi, I.A. Thermal conversion (visbreaking) of heavy Iraqi residue. Fuel 1988, 67, 1714–1715. [Google Scholar] [CrossRef]
- Denson, M.D.; Terell, E.; Kostetskyy, P.; Broadbelt, L.; Olarte, M.; Garcia-Perez, M. Theoretical Insights on the Fragmentation of Cellulosic Oligomers to Form Hydroxyacetone and Hydroxyacetaldehyde. Energy Fuels 2023, 37, 13997–14005. [Google Scholar] [CrossRef]
- Denson, M.D.; Terrell, E.; Kostetskyy, P.; Olarte, M.; Broadbelt, L.; Garcia-Perez, M. Elucidation of Structure and Physical Properties of Pyrolytic Sugar Oligomers Derived from Cellulose Depolymerization/Dehydration Reactions: A Density Functional Theory Study. Energy Fuels 2023, 37, 7834–7847. [Google Scholar] [CrossRef]
- Manrique, R.; Terrell, E.; Kostetskyy, P.; Chejne, F.; Olarte, M.; Broadbelt, L.; García-Pérez, M. Elucidating Biomass-Derived Pyrolytic Lignin Structures from Demethylation Reactions through Density Functional Theory Calculations. Energy Fuels 2023, 37, 5189–5205. [Google Scholar] [CrossRef]
- Sanchez-Lengeling, B.; Roch, L.M.; Perea, J.D.; Langner, S.; Brabec, C.J.; Aspuru-Guzik, A. A Bayesian Approach to Predict Solubility Parameters. Adv. Theory Simul. 2018, 2, 1800069. [Google Scholar] [CrossRef]
- Gourdin, W.H. Estimate of the Vapor Pressure of Squalane at Approximately 293 K Using a Knudsen Cell Method. J. Chem. Eng. Data 2021, 66, 1630–1639. [Google Scholar] [CrossRef]
- Suuberg, E.M.; Oja, V. Vapor Pressures and Heats of Vaporization of Primary Coal Tars; U.S. Department of Energy: Washington, DC, USA, 1997. [Google Scholar]
- ASTM D5291-21; Standard Test Methods for Instrumental Determination of Carbon, Hydrogen, and Nitrogen in Petroleum Products and Lubricants. ASTM International: West Conshohocken, PA, USA, 2021.
- ASTM D5865/D5865M-19; Standard Test Method for Gross Calorific Value of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2019.
- ASTM D4809-18; Standard Test Method for Heat of Combustion of Liquid Hydrocarbon Fuels by Bomb Calorimeter (Precision Method). ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM D86-20b; Standard Test Method for Distillation of Petroleum Products and Liquid Fuels at Atmospheric Pressure. ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM D2892-20; Standard Test Method for Distillation of Crude Petroleum (15-Theoretical Plate Column). ASTM International: West Conshohocken, PA, USA, 2020.
- ASTM E1782-14; Standard Test Method for Determining Vapor Pressure by Thermal Analysis. ASTM International: West Conshohocken, PA, USA, 2016.
- ASTM D93-20; Standard Test Methods for Flash Point by Pensky-Martens Closed Cup Tester. ASTM International: West Conshohocken, PA, USA, 2020.
- Oasmaa, A.; Källi, A.; Lindfors, C.; Elliott, D.C.; Springer, D.; Peacocke, C.; Chiaramonti, D. Guidelines for Transportation, Handling, and Use of Fast Pyrolysis Bio-Oil. 1. Flammability and Toxicity. Energy Fuels 2012, 26, 3864–3873. [Google Scholar] [CrossRef]
- Lehto, J.; Oasmaa, A.; Solantausta, Y.; Kytö, M.; Chiaramonti, D. Review of fuel oil quality and combustion of fast pyrolysis bio-oils from lignocellulosic biomass. Appl. Energy 2014, 116, 178–190. [Google Scholar] [CrossRef]
- Lindfors, C.; Kuoppala, E.; Oasmaa, A.; Solantausta, Y.; Arpiainen, V. Fractionation of Bio-Oil. Energy Fuels 2014, 28, 5785–5791. [Google Scholar] [CrossRef]
- Oasmaa, A.; van de Beld, B.; Saari, P.; Elliott, D.C.; Solantausta, Y. Norms, Standards, and Legislation for Fast Pyrolysis Bio-oils from Lignocellulosic Biomass. Energy Fuels 2015, 29, 2471–2484. [Google Scholar] [CrossRef]
- Oasmaa, A.; Fonts, I.; Pelaez-Samaniego, M.R.; Garcia-Perez, M.E.; Garcia-Perez, M. Pyrolysis Oil Multiphase Behavior and Phase Stability: A Review. Energy Fuels 2016, 30, 6179–6200. [Google Scholar] [CrossRef]
- Elliott, D.C.; Meier, D.; Oasmaa, A.; van de Beld, B.; Bridgwater, A.V.; Marklund, M. Results of the International Energy Agency Round Robin on Fast Pyrolysis Bio-oil Production. Energy Fuels 2017, 31, 5111–5119. [Google Scholar] [CrossRef]
- Oasmaa, A.; Lehto, J.; Solantausta, Y.; Kallio, S. Historical Review on VTT Fast Pyrolysis Bio-oil Production and Upgrading. Energy Fuels 2021, 35, 5683–5695. [Google Scholar] [CrossRef]
- Lindfors, C.; Paasikallio, V.; Kuoppala, E.; Reinikainen, M.; Oasmaa, A.; Solantausta, Y. Co-processing of Dry Bio-oil, Catalytic Pyrolysis Oil, and Hydrotreated Bio-oil in a Micro Activity Test Unit. Energy Fuels 2015, 29, 3707–3714. [Google Scholar] [CrossRef]
- Hilten, R.N.; Das, K.C. Comparison of three accelerated aging procedures to assess bio-oil stability. Fuel 2010, 89, 2741–2749. [Google Scholar] [CrossRef]
- Krutof, A.; Hawboldt, K.A. Thermodynamic model of fast pyrolysis bio-oil advanced distillation curves. Fuel 2020, 261, 116446. [Google Scholar] [CrossRef]
- Bruno, T.J. Improvements in the Measurement of Distillation Curves. 1. A Composition-Explicit Approach. Ind. Eng. Chem. Res. 2006, 45, 4371–4380. [Google Scholar] [CrossRef]
- Bruno, T.J.; Smith, B.L. Improvements in the Measurement of Distillation Curves. 2. Application to Aerospace/Aviation Fuels RP-1 and S-8. Ind. Eng. Chem. Res. 2006, 45, 4381–4388. [Google Scholar] [CrossRef]
- Smith, B.L.; Bruno, T.J. Improvements in the Measurement of Distillation Curves. 4. Application to the Aviation Turbine Fuel Jet-A. Ind. Eng. Chem. Res. 2006, 46, 310–320. [Google Scholar] [CrossRef]
- Smith, B.L.; Bruno, T.J. Improvements in the Measurement of Distillation Curves. 3. Application to Gasoline and Gasoline + Methanol Mixtures. Ind. Eng. Chem. Res. 2006, 46, 297–309. [Google Scholar] [CrossRef]
- Windom, B.C.; Bruno, T.J. Improvements in the Measurement of Distillation Curves. 5. Reduced Pressure Advanced Distillation Curve Method. Ind. Eng. Chem. Res. 2011, 50, 1115–1126. [Google Scholar] [CrossRef]
- Mozaffari, P.; Järvik, O.; Baird, Z.S. Vapor Pressures of Phenolic Compounds Found in Pyrolysis Oil. J. Chem. Eng. Data 2020, 65, 5559–5566. [Google Scholar] [CrossRef]
- Ertaş, M.; Hakkı Alma, M. Pyrolysis of laurel (Laurus nobilis L.) extraction residues in a fixed-bed reactor: Characterization of bio-oil and bio-char. J. Anal. Appl. Pyrolysis 2010, 88, 22–29. [Google Scholar] [CrossRef]
- Shoji, T.; Kawamoto, H.; Saka, S. Boiling point of levoglucosan and devolatilization temperatures in cellulose pyrolysis measured at different heating area temperatures. J. Anal. Appl. Pyrolysis 2014, 109, 185–195. [Google Scholar] [CrossRef]
- Pecha, M.B.; Montoya, J.I.; Chejne, F.; Garcia-Perez, M. Effect of a Vacuum on the Fast Pyrolysis of Cellulose: Nature of Secondary Reactions in a Liquid Intermediate. Ind. Eng. Chem. Res. 2017, 56, 4288–4301. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Ferrell, J.R. Methods and Challenges in the Determination of Molecular Weight Metrics of Bio-oils. Energy Fuels 2018, 32, 8905–8920. [Google Scholar] [CrossRef]
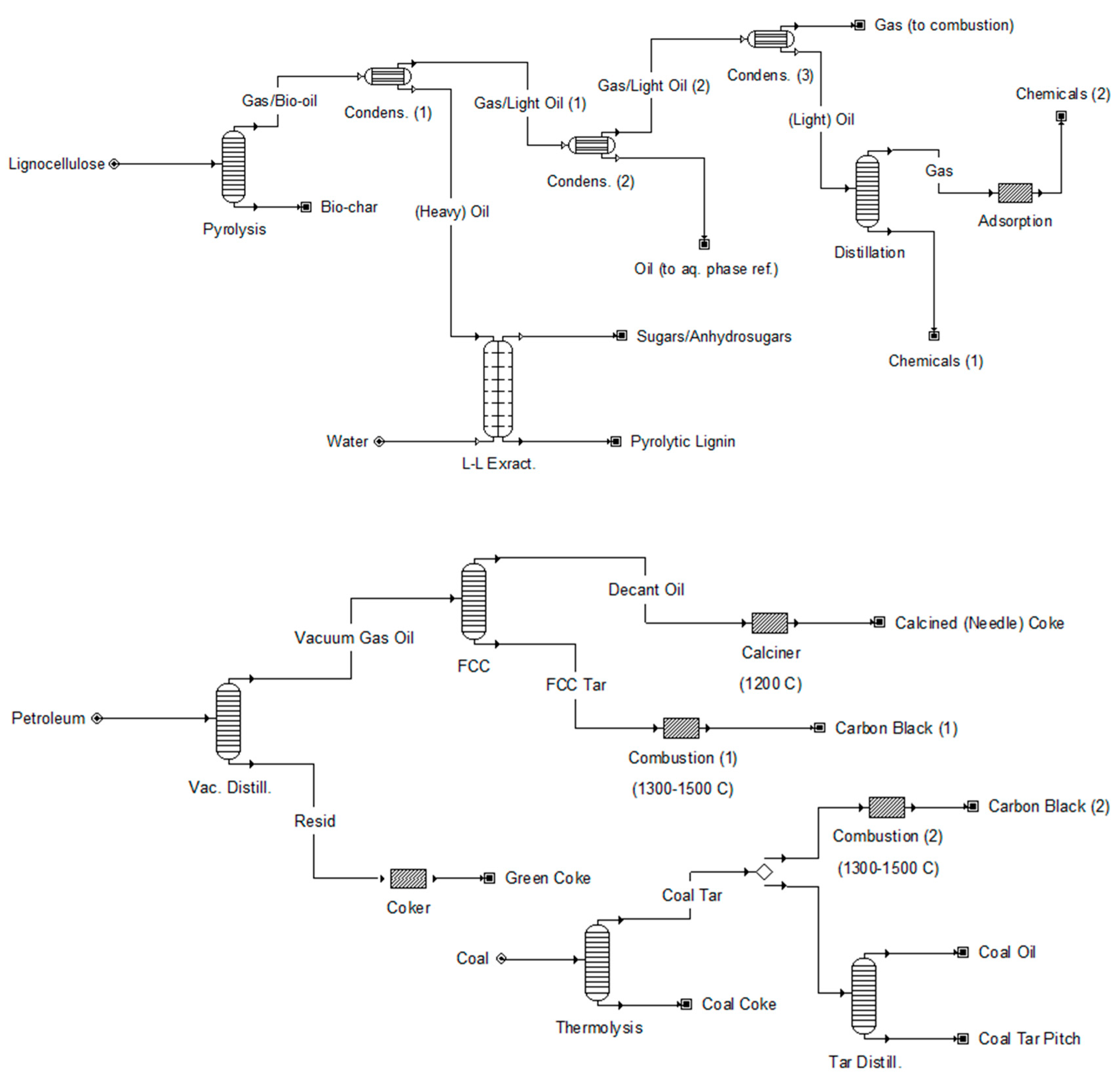
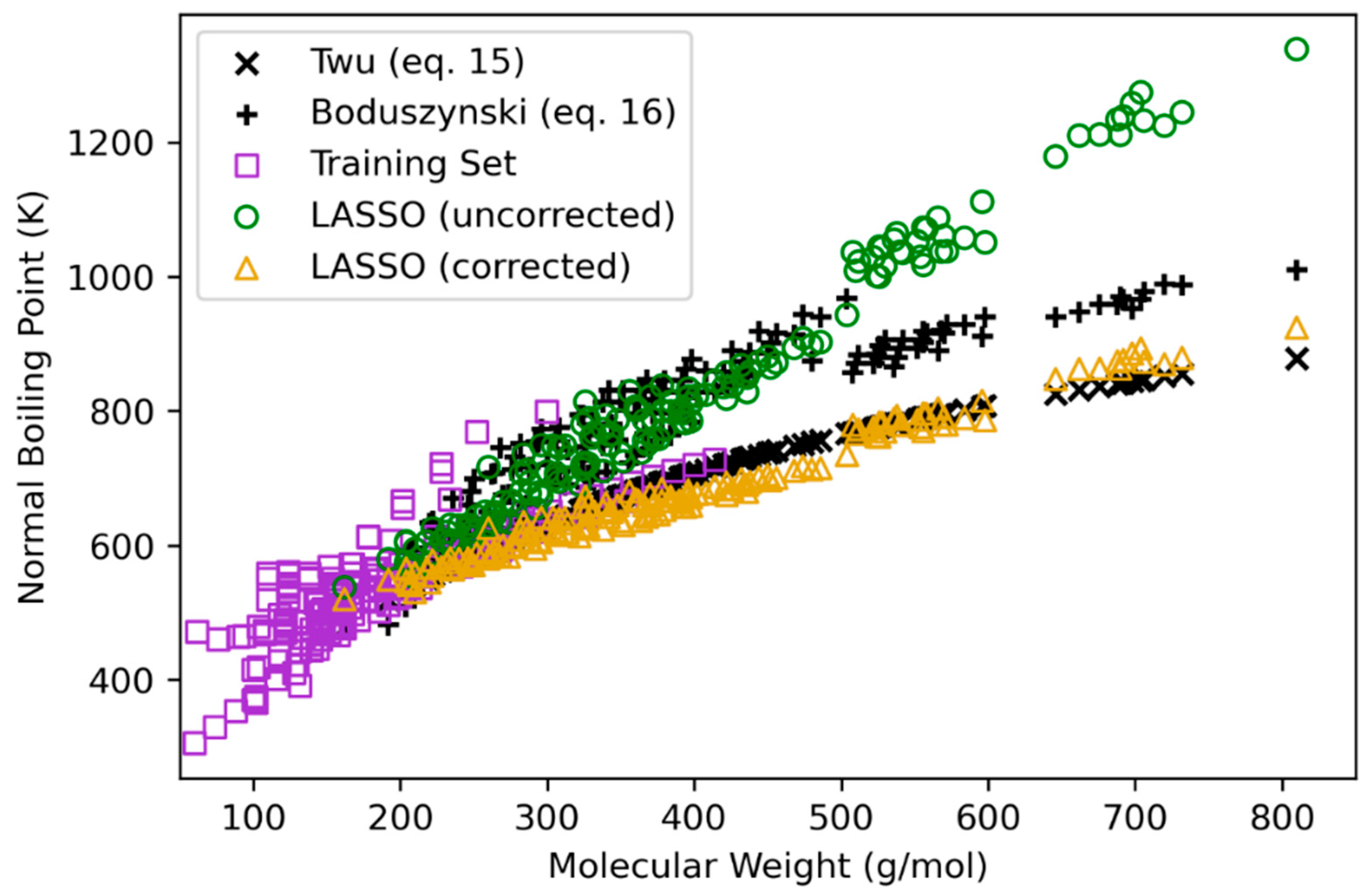

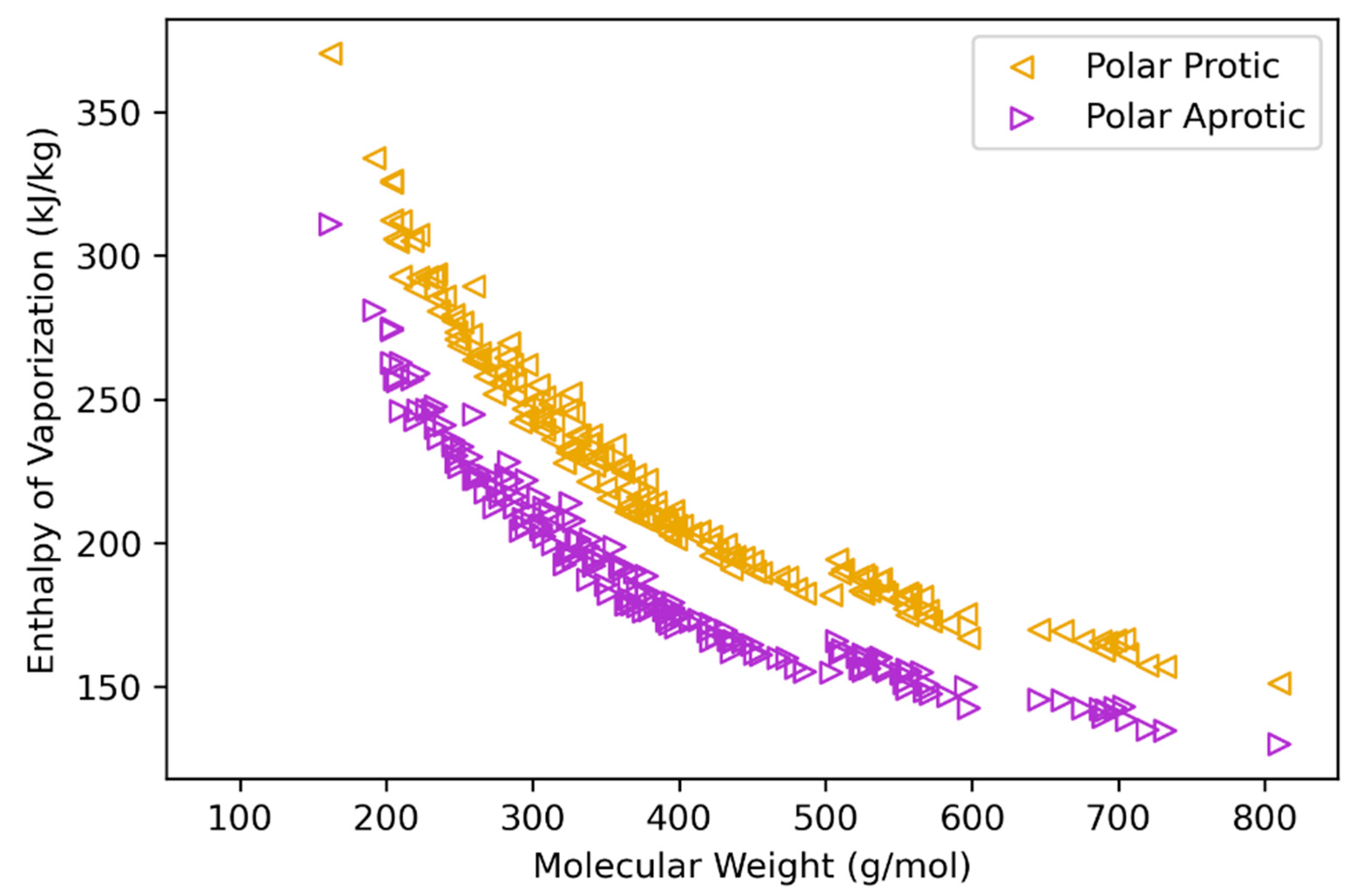
| Class | n a | MW Range b | C Range | H Range | O Range | Reference |
|---|---|---|---|---|---|---|
| Hexose | 66 | 162–504 g/mol | 6–22 | 10–32 | 4–16 | [36,37] |
| Pentose | 6 | 192–282 g/mol | 10 | 8–18 | 4–9 | [37] |
| Hex/Pent | 24 | 204–407 g/mol | 11–18 | 8–30 | 4–15 | [37] |
| LCC | 10 | 308–378 g/mol | 14–16 | 16–22 | 7–11 | [37] |
| Lignin | 57 | 260–810 g/mol | 14–40 | 12–44 | 4–18 | [37,38] |
| Total | 163 | 162–810 g/mol | 6–40 | 8–44 | 4–18 | -- |
| Abbreviation | Description | Abbreviation | Description |
|---|---|---|---|
| Hexose | Hexose-derived oligomers | LHV and HHV | Lower heating value and higher heating value, respectively (MJ/kg) |
| Pentose | Pentose-derived oligomers | TB | Normal boiling point (K); temperature of vapor-liquid equilibrium at atmospheric pressure |
| Hex/Pent | Hexose/pentose-derived oligomers | SVAP | Entropy of vaporization (J/mol-K) at the normal boiling point |
| LCC | Lignin carbohydrate complex compounds | HVAP | Enthalpy of vaporization (kJ/mol) at the normal boiling point |
| Lignin | Lignin-derived oligomers | TFL | Flash point (K); lowest temperature at which combustion can occur with an ignition source |
| Class | C Range (wt.%) | H Range (wt.%) | O Range (wt.%) | LHV Range (MJ/kg) | HHV Range (MJ/kg) |
|---|---|---|---|---|---|
| Hexose | 40.4–70.1 | 4.3–7.5 | 23.4–53.8 | 13.1–29.4 | 14.7–30.7 |
| Pentose | 42.6–62.5 | 4.2–6.4 | 33.3–51.1 | 14.8–23.4 | 16.5–24.0 |
| Hex/Pent | 42.1–64.7 | 3.9–6.4 | 31.4–51.5 | 14.7–24.2 | 16.3–24.7 |
| LCC | 47.6–58.4 | 4.9–6.1 | 36.4–46.6 | 16.5–22.4 | 18.1–23.1 |
| Lignin | 57.5–72.3 | 4.1–6.5 | 22.7–36.7 | 20.4–28.9 | 21.8–29.7 |
| Total | 40.4–72.3 | 3.9–7.5 | 22.7–53.8 | 13.1–29.4 | 14.7–30.7 |
| Ridge Coeff. | Ridge Coeff. | Ridge Coeff. | LASSO Coeff. | LASSO Coeff. | LASSO Coeff. |
|---|---|---|---|---|---|
| MW: 1.15 | CH3: −1.52 | OHcyclic: 0.90 | MW: 1.16 | CH3: −12.79 | OHcyclic: 4.53 |
| C%: 1.38 | CH2: −1.69 | OFnoncyc.: 0.51 | C%: 0 | CH2: −1.89 | OFnoncyc.: 0 |
| H%: −2.33 | CH: 1.48 | Oether: −0.68 | H%: 0 | CH: 0 | Oether: −6.44 |
| O%: 0.94 | C*: 2.00 | Ocarbonyl: −0.52 | O%: 0 | C*: 12.61 | Ocarbonyl: 0 |
| Ridge intercept: 234.14 | LASSO intercept: 331.62 | ||||
| Class | Minimum (K) | Median (K) | Maximum (K) | Minimum (°C) | Median (°C) | Maximum (°C) |
|---|---|---|---|---|---|---|
| Hexose | 375 | 447 | 550 | 102 | 173 | 277 |
| Pentose | 390 | 420 | 464 | 117 | 147 | 191 |
| Hex/Pent | 399 | 469 | 538 | 126 | 196 | 265 |
| LCC | 441 | 471 | 514 | 168 | 198 | 241 |
| Lignin | 428 | 524 | 674 | 155 | 251 | 400 |
| Total | 375 | 469 | 674 | 102 | 195 | 400 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrell, E. Estimation of Fuel Properties for the Heavy Fraction of Biomass Pyrolysis Oil Consisting of Proposed Structures for Pyrolytic Lignin and Humins. Energies 2024, 17, 2011. https://doi.org/10.3390/en17092011
Terrell E. Estimation of Fuel Properties for the Heavy Fraction of Biomass Pyrolysis Oil Consisting of Proposed Structures for Pyrolytic Lignin and Humins. Energies. 2024; 17(9):2011. https://doi.org/10.3390/en17092011
Chicago/Turabian StyleTerrell, Evan. 2024. "Estimation of Fuel Properties for the Heavy Fraction of Biomass Pyrolysis Oil Consisting of Proposed Structures for Pyrolytic Lignin and Humins" Energies 17, no. 9: 2011. https://doi.org/10.3390/en17092011





