Thermodynamic Stability of Structure H Hydrates Based on the Molecular Properties of Large Guest Molecules
Abstract
:1. Introduction
2. Computational Method

3. Results and Discussion
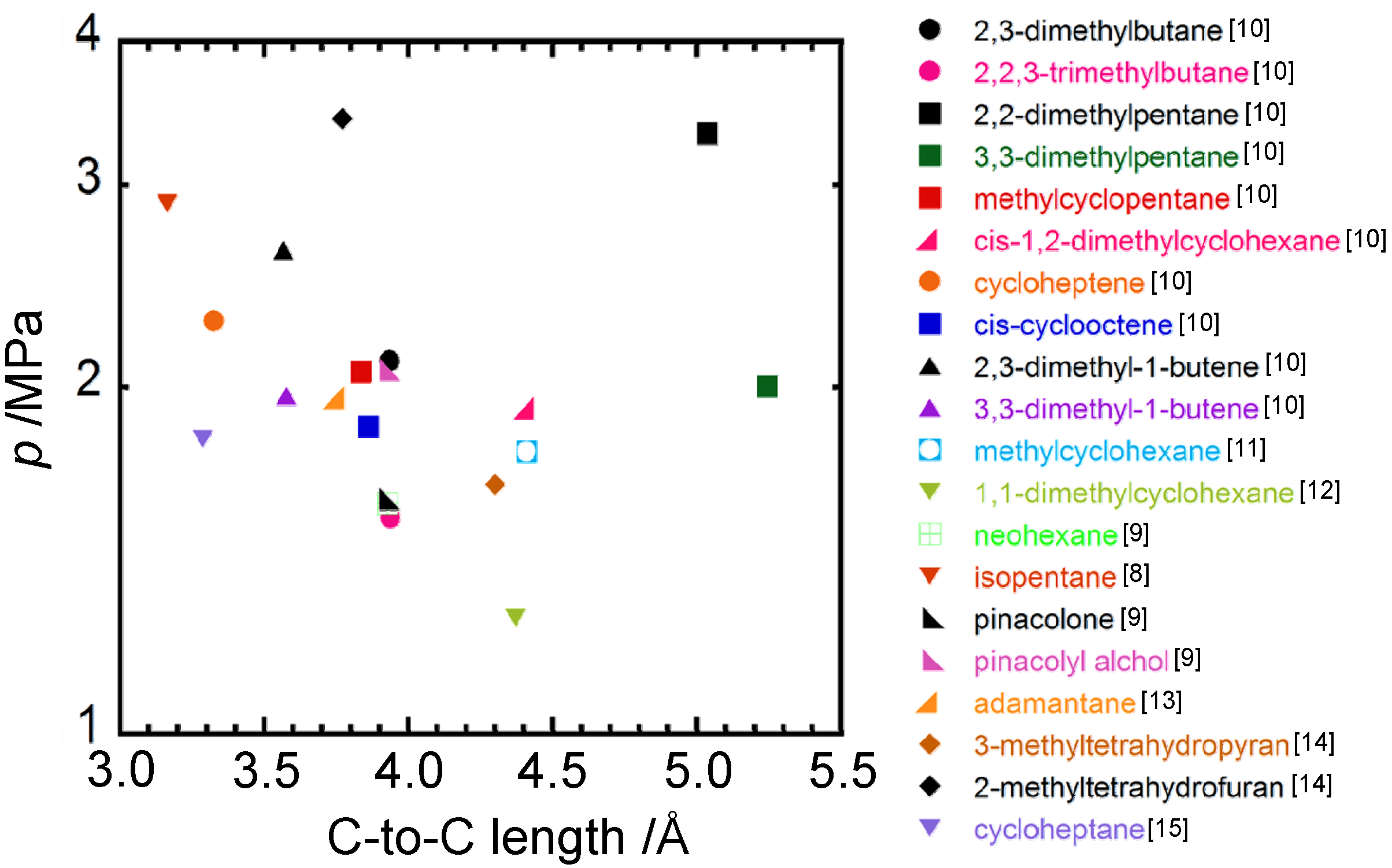


4. Concluding Remarks
Acknowledgments
References
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Ripmeester, J.A.; Ratcliffe, C.I. 129Xe NMR studies of clathrate hydrates: New guests for structure I1 and structure H. J. Phys. Chem. 1990, 94, 8773–8776. [Google Scholar] [CrossRef]
- Mori, Y.H. Recent advances in hydrate-based technologies for natural gas storage—A review. J. Chem. Ind. Eng. China 2003, 54, 1–17. [Google Scholar]
- Mao, W.L.; Mao, H. Hydrogen storage in molecular compounds. Proc. Natl. Acad. Sci. USA 2004, 101, 708–710. [Google Scholar] [CrossRef] [PubMed]
- Duarte, A.R.C.; Shariati, A.; Rovetto, L.J.; Peters, C.J. Water cavities of sH clathrate hydrate stabilized by molecular hydrogen: Phase equilibrium measurements. J. Phys. Chem. B 2008, 112, 1888–1889. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.T.; Moudravski, I.L.; Ripmeester, J.A.; Lee, J.W.; Lee, H. Efficient recovery of CO2 from flue gas by clathrate hydrate formation in porous silica gels. Environ. Sci. Technol. 2005, 39, 2315–2319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, T.; Ito, T.; Watanabe, K.; Tahara, K.; Hiraoka, R.; Ochiai, J.; Ohmura, R.; Mori, Y.H. Development of a novel hydrate-based refrigeration system: A preliminary overview. Appl. Therm. Eng. 2006, 26, 2157–2167. [Google Scholar] [CrossRef]
- Mehta, A.P.; Sloan, E.D. Structure H hydrate phase equilibria of methane + liquid hydrocarbon mixtures. J. Chem. Eng. Data 1993, 38, 580–582. [Google Scholar] [CrossRef]
- Ohmura, R.; Uchida, T.; Takeya, S.; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H. Phase equilibrium for structure-H hydrates formed with methane and either pinacolone (3,3-dimethyl-2-butanone) or pinacolyl alcohol (3,3-dimethyl-2-butanol). J. Chem. Eng. Data 2004, 48, 1337–1340. [Google Scholar] [CrossRef]
- Mehta, A.P.; Sloan, E.D. Structure H hydrate phase equilibria of paraffins naphthenes, and olefins with methane. J. Chem. Eng. Data 1994, 39, 887–890. [Google Scholar] [CrossRef]
- Ohmura, R.; Uchida, T.; Takeya, S.; Nagao, J.; Minagawa, H.; Ebinuma, T.; Narita, H. Clathrate hydrate formation in (methane + water + methylcyclohexanone) systems: The first phase equilibrium data. J. Chem. Thermdyn. 2003, 35, 2045–2054. [Google Scholar] [CrossRef]
- Hara, T.; Hashimoto, S.; Sugahara, T.; Ohgaki, K. Large pressure depression of methane hydrate by adding 1,1-dimethylcyclohexane. Chem. Eng. Sci. 2005, 60, 3117–3119. [Google Scholar] [CrossRef]
- Hütz, U.; Englezos, P. Measurement of structure H hydrate phase equilibrium and effect of electrolytes. Fluid Phase Equilib. 1996, 117, 178–185. [Google Scholar] [CrossRef]
- Ohmura, R.; Matsuda, S.; Takeya, S.; Ebinuma, T.; Narita, H. Phase equilibrium for structure-H hydrates formed with methane and methyl-substituted cyclic ether. Int. J. Thermophys. 2005, 5, 1515–1523. [Google Scholar] [CrossRef]
- Kozaki, T.; Taguchi, T.; Takeya, S.; Ohmura, R. Phase equilibrium for structure H hydrates formed with methane plus cycloheptane, cycloheptanone or oxacycloheptane. J. Chem. Eng. Data 2010, 55, 3059–3062. [Google Scholar] [CrossRef]
- Miyoshi, T.; Imai, M.; Ohmura, R.; Yasuoka, K. Thermodynamic stability of type-I and type-II clathrate hydrates depending on the chemical species of the guest substances. J. Chem. Phys. 2007, 126, 234506:1–234506:6. [Google Scholar] [CrossRef]
- Alavi, S.; Susilo, R.; Ripmeester, J.A. Linking microscopic guest properties to macroscope observables in clathrate hydrates: Guest-host hydrogen bonding. J. Chem. Phys. 2009, 170, 174501:1–174501:9. [Google Scholar]
- Okano, Y.; Yasuoka, K. Free-energy calculation of structure-H hydrates. J. Chem. Phys. 2006, 124, 024510:1–024510:9. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Kroes, G.J. A new method for screening potential sII and sH hydrogen clathrate hydrate promoters with model potentials. Phys. Chem. Chem. Phys. 2011, 13, 13410–13420. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. GAUSSIAN 03; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tezuka, K.; Taguchi, T.; Alavi, S.; Sum, A.K.; Ohmura, R. Thermodynamic Stability of Structure H Hydrates Based on the Molecular Properties of Large Guest Molecules. Energies 2012, 5, 459-465. https://doi.org/10.3390/en5020459
Tezuka K, Taguchi T, Alavi S, Sum AK, Ohmura R. Thermodynamic Stability of Structure H Hydrates Based on the Molecular Properties of Large Guest Molecules. Energies. 2012; 5(2):459-465. https://doi.org/10.3390/en5020459
Chicago/Turabian StyleTezuka, Kyoichi, Tatsuhiko Taguchi, Saman Alavi, Amadeu K. Sum, and Ryo Ohmura. 2012. "Thermodynamic Stability of Structure H Hydrates Based on the Molecular Properties of Large Guest Molecules" Energies 5, no. 2: 459-465. https://doi.org/10.3390/en5020459




