Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support
Abstract
:1. Introduction

2. Results and Discussion
| No. | Oil/Ethanol (mL/mL) | T (°C) | t (h) | FAEE (%) | MG + DG (%) | TG (%) | Yield (%) | Conv. (%) | TOF (mmol/h gPPL) |
|---|---|---|---|---|---|---|---|---|---|
| 0a | 12/6 | 40 | 10 | 57.7 | 34.2 | 8.1 | 57.7 | 91.9 | 57.68 |
| 0b | 12/6 | 40 | 10 | 13.4 | 65.1 | 21.5 | 13.4 | 78.5 | 58.26 |
| 1 | 12/12 | 40 | 24 | 6.1 | 23.3 | 70.6 | 6.1 | 29.4 | 0.67 |
| 2 | 12/6 | 25 | 24 | 11.7 | 71.0 | 17.3 | 11.7 | 82.7 | 1.28 |
| 3 | 12/6 | 30 | 69 | 53.3 | 46.7 | 0 | 53.3 | 100 | 2.03 |
| 4 | 12/6 | 35 | 48 | 64.5 | 31.3 | 0 | 64.5 | 100 | 3.53 |
| 5 | 12/6 | 40 | 31 | 67.0 | 27.7 | 5.3 | 67.0 | 94.7 | 5.68 |
| 6 | 12/6 | 45 | 20 | 59.9 | 40.1 | 0 | 59.9 | 100 | 7.88 |
| 7 | 45/4 | 25 | 24 | 16.9 | 41.8 | 41.3 | 16.9 | 58.7 | 6.94 |
| 8 | 45/4 | 30 | 44 | 40.9 | 15.4 | 43.7 | 40.9 | 56.3 | 9.17 |
| 9 | 45/4 | 35 | 30 | 34.4 | 24.8 | 40.8 | 34.4 | 59.2 | 11.32 |
| 10 | 45/4 | 40 | 18 | 27.2 | 51.2 | 21.6 | 27.2 | 78.4 | 14.91 |
| 11 | 45/4 | 45 | 24 | 43.7 | 23.9 | 32.4 | 43.7 | 67.6 | 17.97 |
| 12 | 45/4 | 50 | 23 | 44.3 | 33.6 | 22.1 | 45.3 | 77.9 | 19.43 |
| No. | Oil/EtOH (mL/mL) | T (°C) | t (h) | FAEE (%) | MG + DG (%) | TG (%) | Yield (%) | Conv. (%) | TOF (mmol/h gPPL) |
|---|---|---|---|---|---|---|---|---|---|
| 13 | 45:7 | 40 | 21 | 37.3 | 36.0 | 26.7 | 37.3 | 73.3 | 17.53 |
| 14 | 45:7 | 45 | 20 | 50.2 | 48.3 | 1.5 | 50.2 | 98.5 | 24.77 |
| 15 | 45:7 | 50 | 24 | 51.4 | 40.9 | 7.7 | 51.4 | 92.3 | 21.13 |
| 16 | 36:6 | 35 | 28 | 24.1 | 75.9 | 0 | 24.1 | 100 | 6.80 |
| 36:6 | 35 | 72 | 51.5 | 37.2 | 11.3 | 51.5 | 88.7 | 5.64 | |
| 17 | 36:6 | 40 | 17 | 58.4 | 41.6 | 0 | 58.4 | 100 | 27.94 |
| 36:6 | 40 | 25 | 54.3 | 41.2 | 4.5 | 54.3 | 95.5 | 17.15 | |
| 36:6 | 40 | 48 | 55.7 | 44.3 | 0 | 55.7 | 100 | 9.16 | |
| 18 | 36:6 | 45 | 4 | 49.9 | 45.1 | 5.0 | 49.9 | 95.0 | 98.49 |
| 36:6 | 40 | 11 | 54.8 | 40.6 | 4.6 | 54.8 | 95.4 | 39.33 | |
| 19 | 36:6 | 50 | 19 | 32.6 | 65.1 | 2.3 | 32.6 | 97.7 | 13.55 |
| 36:6 | 50 | 25 | 35.0 | 47.4 | 17.6 | 35.0 | 82.4 | 11.05 | |
| 36:6 | 50 | 46 | 56.4 | 34.7 | 8.9 | 56.4 | 91.1 | 9.67 | |
| 36:6 | 50 | 51 | 52.5 | 47.5 | 0 | 52.5 | 100 | 8.13 | |
| 20 | 24:4 | 25 | 65 | 57.1 | 42.9 | 0 | 57.1 | 100 | 4.62 |
| 21 | 24:4 | 30 | 38 | 62.2 | 37.8 | 0 | 62.2 | 100 | 8.61 |
| 22 | 24:4 | 35 | 6 | 61.5 | 38.5 | 0 | 61.5 | 100 | 52.95 |
| 24:4 | 35 | 10 | 61.9 | 38.1 | 0 | 61.9 | 100 | 32.58 | |
| 24:4 | 35 | 23 | 66.0 | 34.0 | 0 | 66.0 | 100 | 15.10 | |
| 23 | 24:4 | 40 | 7 | 62.0 | 38.0 | 0 | 62.0 | 100 | 46.62 |
| 24:4 | 40 | 14 | 61.1 | 38.9 | 0 | 61.1 | 100 | 22.97 | |
| 24 | 24:4 | 45 | 9 | 58.7 | 41.3 | 0 | 58.7 | 100 | 34.33 |
| 25 | 24:4 | 50 | 7 | 63.7 | 36.3 | 0 | 63.7 | 100 | 47.89 |
| 24:4 | 50 | 31 | 63.8 | 36.2 | 0 | 63.8 | 100 | 10.83 |
| Catalyst | Oil/Alcohol | FAE | MG + DG | Yield | Conv. | υ |
|---|---|---|---|---|---|---|
| NaOH | Waste/MeOH | 95.7 | 4.3 | 95.7 | 100.0 | 3.9 |
| KOH | Sunflower/ EtOH | 94.8 | 5.2 | 94.8 | 100.0 | 6.6 |
| PPL free | Sunflower/EtOH | 55.7 | 44.2 | 55.7 | 100.0 | 6.9 |
| - | Sunflower/EtOH | 44.3 | 33.6 | 45.3 | 77.9 | 19.6 |
| - | Waste/96% EtOH | 54.3 | 41.2 | 54.3 | 95.5 | 23.4 |
| - | Waste/96% EtOH | 51.4 | 40.9 | 51.4 | 92.3 | 24.5 |
| - | Waste/96% EtOH | 66.0 | 31.0 | 66.0 | 100.0 | 19.7 |
| - | Waste/96% EtOH | 58.4 | 41.6 | 58.4 | 100.0 | 15.0 |
| B20 | - | - | - | - | - | 4.4 |
| Sunflower oil | - | - | - | - | - | 31.9 |
| Waste oil | - | - | - | - | - | 44.6 |
| Diesel | - | - | - | - | - | 3.1 |
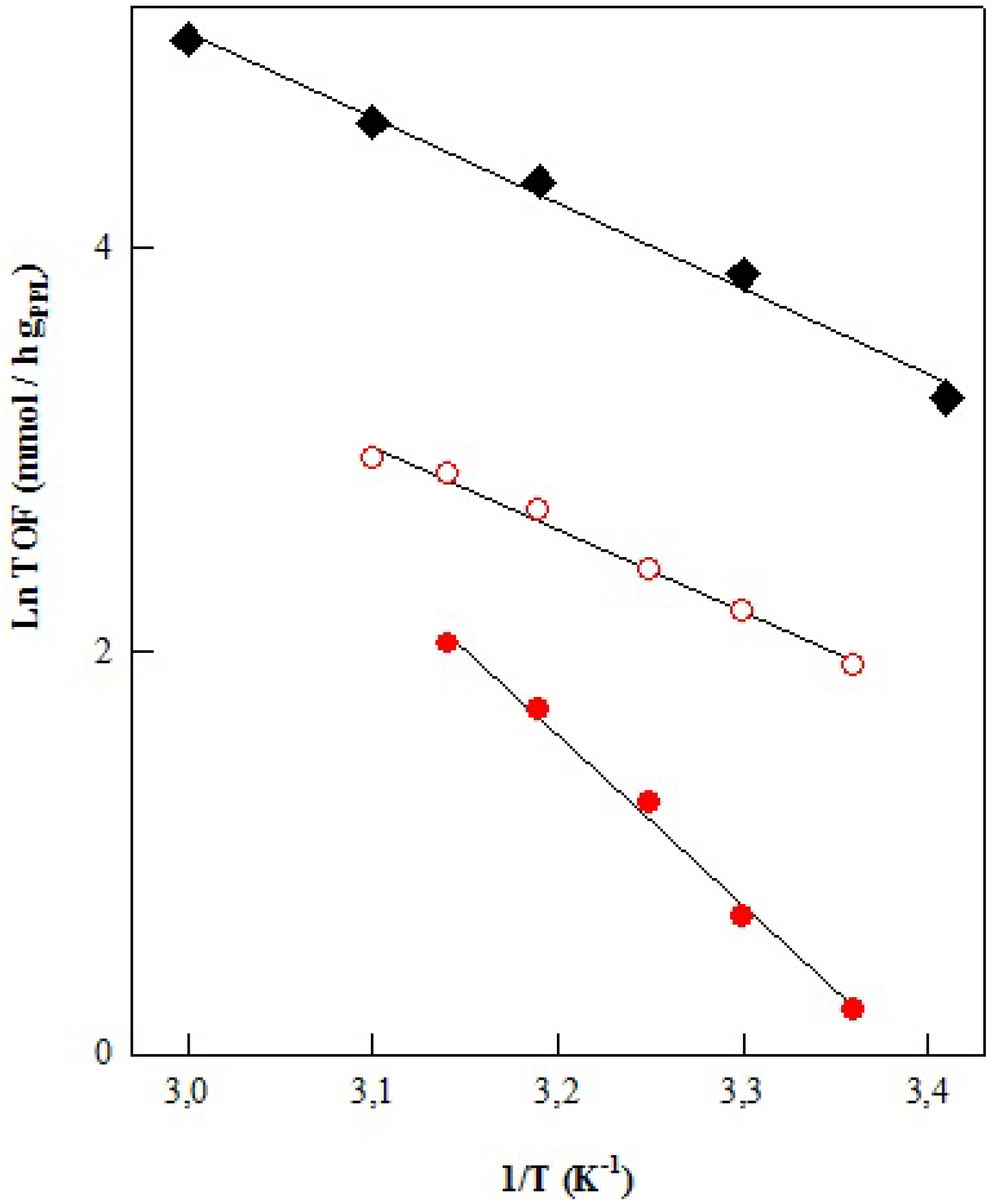
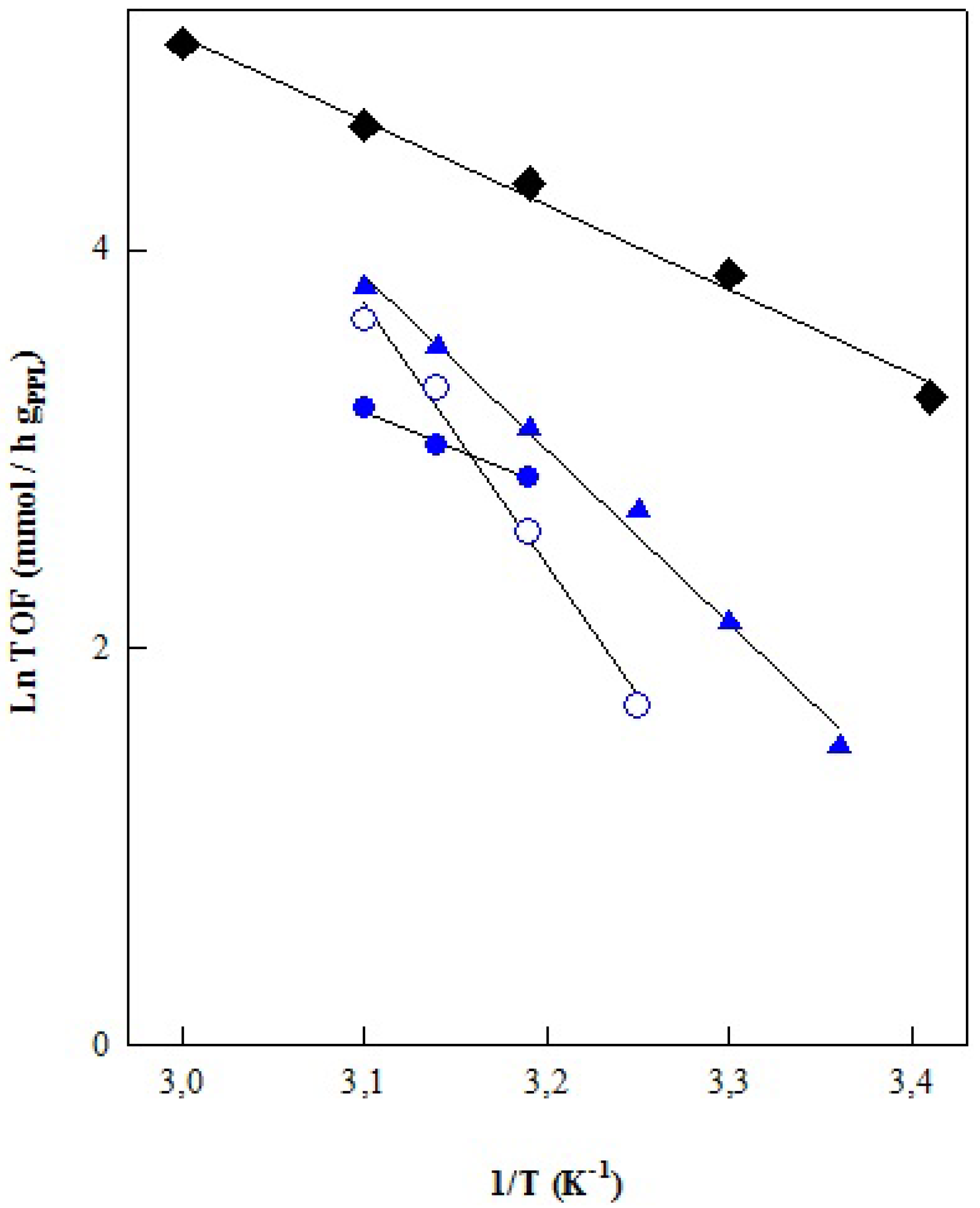
| PPL Enzyme | Oil/Vol (mL) | Acohol/Vol (mL) | Ea (Kcal/mol) | LnA (h−1) | r2 |
|---|---|---|---|---|---|
| Free | Sunflower/12 | EtOH abs./6 | 8.40 ± 0.24 | 17.76 ± 0.76 | 0.99 |
| Immobilized | Sunflower/12 | EtOH abs./6 | 16.80 ± 0.41 | 29.08 ± 1.33 | 0.99 |
| Immobilized | Sunflower/45 | EtOH abs./4 | 8.12 ± 0.20 | 15.89 ± 0.65 | 0.99 |
| Immobilized | Waste/45 | 96% EtOH/7 | 7.65 ± 0.39 | 15.33 ± 1.22 | 1 |
| Immobilized | Waste/36 | 96% EtOH/6 | 26.22 ± 0.89 | 45.28 ± 2.83 | 0.99 |
| Immobilized | Waste/24 | 96% EtOH/4 | 17.42 ± 0.39 | 31.49 ± 1.26 | 0.99 |
3. Experimental Section
3.1. Synthesis and Surface Functionalization of AlPO4 Used as Support
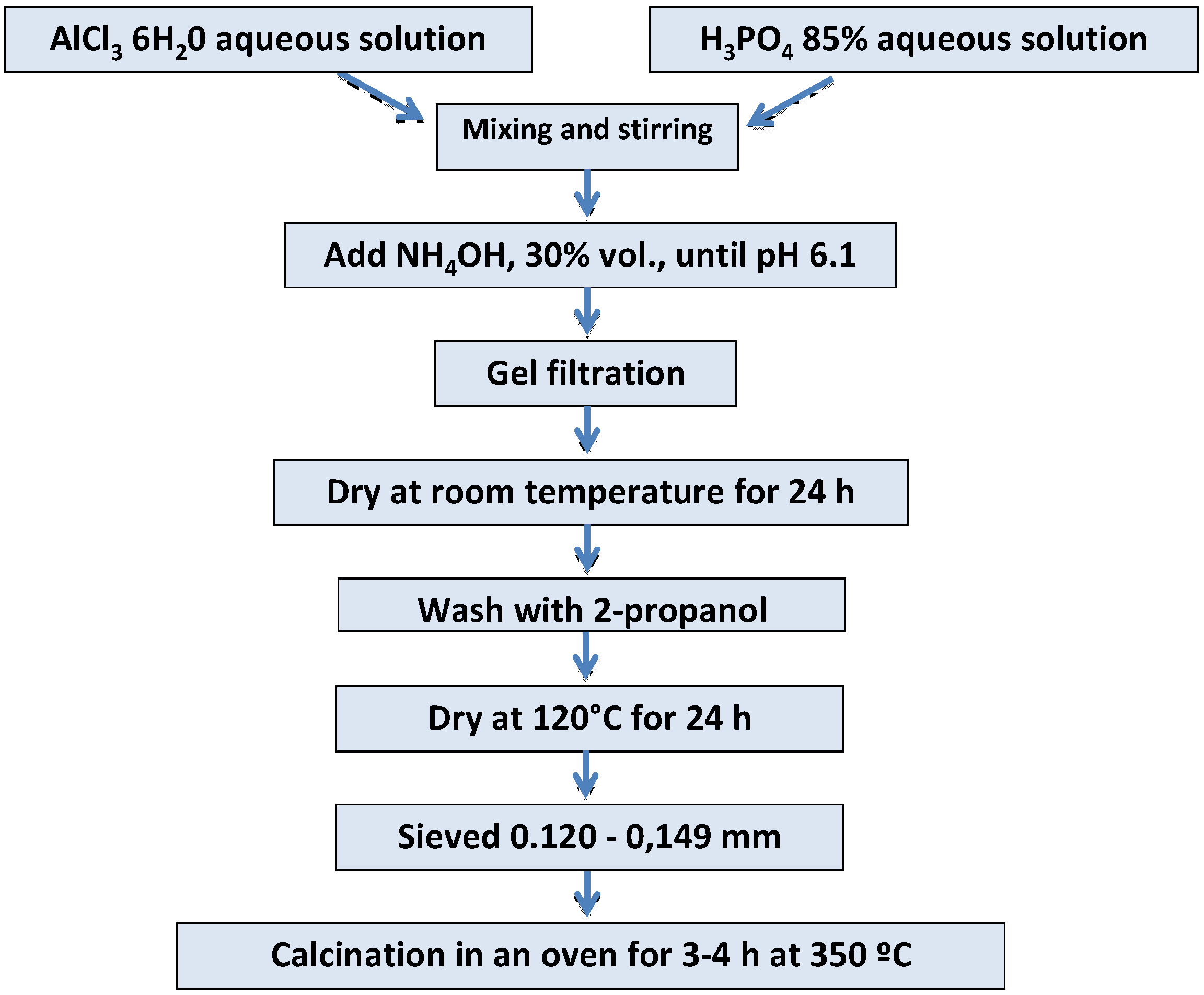
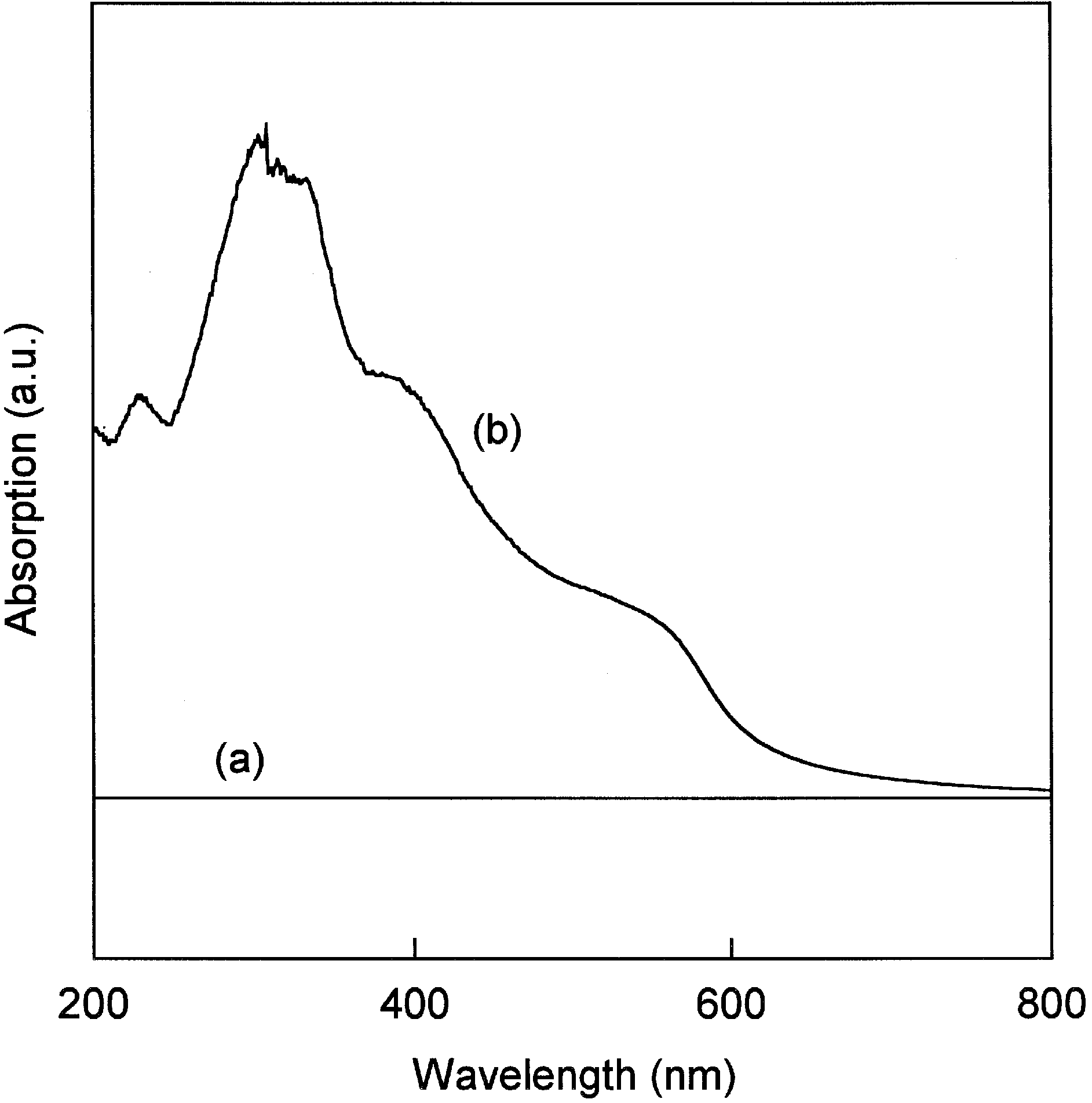
3.2. PPL Immobilization and Enzymatic Activity
3.3. Ethanolysis Reactions
3.4. Compositional Analysis of Reaction Products by Gas Chromatography
3.5. Viscosity Measurements
4. Conclusions
Acknowledgments
Conflict of Interest
References
- Demirbas, A. Political, economic and environmental impacts of biofuels: A review. Appl. Energy 2009, 86, 108–117. [Google Scholar] [CrossRef]
- Mercader, F.M.; Groeneveld, M.J.; Kersten, S.R.A.; Way, N.W.J.; Schaverien, C.J.; Hogendoorn, J.A. Production of advanced biofuels: Co-processing of upgraded pyrolysis oil in standard refinery units. Appl. Catal. B Environ. 2010, 96, 57–66. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Energy Rev. 2010, 14, 578–597. [Google Scholar]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Boer, K.; Bahri, P.A. Supercritical methanol for fatty acid methyl ester production: A review. Biomass Bioenergy 2011, 35, 983–991. [Google Scholar] [CrossRef]
- Chouhan, A.P.S.; Sarma, A.K. Modern heterogeneous catalysts for biodiesel production: A comprehensive review. Renew. Sustain. Energy Rev. 2011, 15, 4378–4399. [Google Scholar] [CrossRef]
- Li, E.; Xu, Z.P.; Rudolph, V. MgCoAl-LDH derived heterogeneous catalysts for the ethanol transesterification of canola oil to biodiesel. Appl. Catal. B Environ. 2009, 88, 42–49. [Google Scholar] [CrossRef]
- Gog, A.; Roman, M.; Tos, M.; Paizs, C.; Irimie, F.D. Biodiesel production using enzymatic transesterification—Current state and perspectives. Renew. Energy 2012, 39, 10–16. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G. Catalytic conversion of renewable source for biodiesel production: A comparison between biocatalysts and inorganic catalysts. Catal. Lett. 2013, 143, 159–168. [Google Scholar] [CrossRef]
- Nielsen, P.M.; Rancke-Madsen, A. Enzymatic large-scale production of biodiesel. Lipid Technol. 2011, 23, 230–233. [Google Scholar] [CrossRef]
- Yusuf, N.N.A.N.; Kamarudin, S.K.; Yaakub, Z. Overview on the current trends in biodiesel production. Energy Convers. Manag. 2011, 52, 2741–2751. [Google Scholar] [CrossRef]
- Atabania, A.E.; Silitonga, A.S.; Badruddin, I.A.; Mahlia, T.M.I.; Masjuki, H.H.; Mekhilef, S. A comprehensive review on biodiesel as an alternative energy resource and its characteristics. Renew. Sustain. Energy Rev. 2012, 16, 2070–2093. [Google Scholar] [CrossRef]
- Ganesan, D.; Rajendran, A.; Thangavelu, V. An overview on the recent advances in the transesterification of vegetable oils for biodiesel production using chemical and biocatalysts. Rev. Environ. Sci. Biotechnol. 2009, 8, 367–394. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.; Lu, J.; Nie, K.; Deng, L.; Wang, F. Biodiesel production with immobilized lipase: A review. Biotechnol. Adv. 2010, 28, 628–634. [Google Scholar] [CrossRef] [PubMed]
- De Diego, T.; Manjón, A.; Lozano, P.; Iborra, J.L. A recyclable enzymatic biodiesel production process in ionic liquids. Bioresour. Technol. 2011, 102, 6336–6339. [Google Scholar] [CrossRef] [PubMed]
- Mota, C.J.A.; da Silva, C.X.A.; Rosenbach, N., Jr.; Costa, J.; da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Da Silva, G.P.; Mack, M.; Contiero, J. Glycerol: A promising and abundant carbon source for industrial microbiology. Biotechnol. Adv. 2009, 27, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Vicente, G.; Morales, G.; Paniagua, M.; Bustamante, J. Oxygenated compounds derived from glycerol for biodiesel formulation: Influence on EN 14214 quality parameters. Fuel 2010, 89, 2011–2018. [Google Scholar] [CrossRef]
- Adamczak, M.; Bornscheuer, U.T.; Bednarski, W. The application of biotechnological methods for the synthesis of biodiesel. Eur. J. Lipid Sci. Technol. 2009, 111, 808–813. [Google Scholar] [CrossRef]
- Luque, R.; Herrero-Davila, L.; Campelo, J.M.; Clark, J.H.; Hidalgo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A. Biofuels: A technological perspective. Energy Environ. Sci. 2008, 1, 513–596. [Google Scholar] [CrossRef]
- Vasudevan, P.T.; Briggs, M. Biodiesel production—Current state of the art and challenges. J. Ind. Microbiol. Biotechnol. 2008, 35, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, D.; Bevoni, V.; Notari, M.; Rivetti, F. Properties of a potential biofuel obtained from soybean oil by transmethylation with dimethyl carbonate. Fuel 2007, 86, 690–697. [Google Scholar] [CrossRef]
- Kenar, J.A.; Knöthe, G.; Dunn, R.O.; Ryan, T.W.; Matheaus, A. Physical properties of oleochemical carbonates. J. Am. Oil Chem. Soc. 2005, 82, 201–205. [Google Scholar] [CrossRef]
- Su, E.Z.; Zhang, M.J.; Zhang, J.G.; Gao, J.F.; Wei, D.Z. Lipase-catalyzed irreversible transesterification of vegetable oils for fatty acid methyl esters production with dimethyl carbonate as the acyl acceptor. Biochem. Eng. J. 2007, 36, 167–173. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour. Technol. 2010, 101, 2735–2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Sheng, B.; Xin, Z.; Liu, Q.; Sun, S. Kinetics of transesterification of palm oil and dimethyl carbonate for biodiesel production at the catalysis of heterogeneous base catalyst. Bioresour. Technol. 2010, 101, 8144–8150. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Du, W.; Liu, D.; Zeng, J. A novel enzymatic route for biodiesel production from renewable oils in a solvent-free medium. Biotechnol. Lett. 2003, 25, 1239–1241. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.Y.; Du, W.; Liu, D.H. Study on the kinetics of enzymatic interesterification of triglycerides for biodiesel production with methyl acetate as the acyl acceptor. J. Mol. Catal. B Enzym. 2005, 32, 241–245. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.Y.; Liu, D.H.; Zeng, J. Comparative study on lipase-catalyzed transformation of soybean oil for biodiesel production with different acyl acceptors. J. Mol. Catal. B Enzym. 2004, 30, 125–129. [Google Scholar] [CrossRef]
- Akoh, C.C.; Chang, S.W.; Lee, G.C.; Shaw, J.F. Enzymatic approach to biodiesel production. J. Agric. Food Chem. 2007, 55, 8995–9005. [Google Scholar] [CrossRef] [PubMed]
- Ognjanovic, N.; Bezbradica, D.; Knezevic-Jugovic, Z. Enzymatic conversion of sunflower oil to biodiesel in a solvent-free system: Process optimization and the immobilized system stability. Bioresour. Technol. 2009, 100, 5146–5154. [Google Scholar] [CrossRef] [PubMed]
- Modi, M.K.; Reddy, J.R.C.; Rao, B.; Prasad, R.B.N. Lipase-mediated conversion of vegetable oils into biodiesel using ethyl acetate as acyl acceptor. Bioresour. Technol. 2007, 98, 1260–1264. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Jung, S.M.; Park, Y.C.; Park, K. Lipase catalyzed transesterification of soybean oil using ethyl acetate, an alternative acyl acceptor. Biotechnol. Bioprocess Eng. 2007, 12, 441–445. [Google Scholar] [CrossRef]
- Casas, A.; Ruiz, J.R.; Ramos, M.J.; Perez, A. Effects of triacetin on biodiesel quality. Energy Fuels 2010, 24, 4481–4489. [Google Scholar] [CrossRef]
- Sangsri, P.; Ratanawilai, S.; Meyer, P.; Tongurai, C. Feasibility of Biodiesel Production from Transmethylation of Used Cooking Oil. In Proceedings of the 5th PSU-UNS International Conference on Engineering and Technology (ICET-2011), Prince of Songkla University, Songkhla, Thailand, 2–3 May 2011; pp. 92–95.
- Caballero, V.; Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Hidalgo, J.M.; Luque, R.; Macario, A.; Giordano, G. Sustainable preparation of a novel glycerol-free biofuel by using pig pancreatic lipase: partial 1,3-regiospecific alcoholysis of sunflower oil. Process Biochem. 2009, 44, 334–342. [Google Scholar] [CrossRef]
- Verdugo, C.; Luque, R.; Luna, D.; Hidalgo, J.M.; Posadillo, A.; Sancho, E.; Rodríguez, S.; Ferreira-Días, S.; Bautista, F.M.; Romero, A.A. A comprehensive study of reaction parameters in the enzymatic production of novel biofuels integrating glycerol into their composition. Bioresour. Technol. 2010, 101, 6657–6662. [Google Scholar] [CrossRef] [PubMed]
- Luna, D.; Bautista, F.M.; Caballero, V.; Campelo, J.M.; Marinas, J.M.; Romero, A.A. Method for Producing Biodiesel Using Porcine Pancreatic Lipase as An Enzymatic Catalyst. Eur. Pat. EP 2050823 A1, 2009. [Google Scholar]
- Verdugo, C.; Luna, D.; Posadillo, A.; Sancho, E.; Rodríguez, S.; Bautista, F.M.; Luque, R.; Marinas, J.M.; Romero, A.A. Production of a new second generation biodiesel with a low cost lipase derived from Thermomyces lanuginosus: Optimization by response surface methodology. Catal. Today 2011, 167, 107–112. [Google Scholar] [CrossRef]
- Knöthe, G.; Steidley, K.R. Kinematic viscosity of biodiesel components (fatty acid alkyl esters) and related compounds at low temperatures. Fuel 2007, 86, 2560–2567. [Google Scholar] [CrossRef]
- Wadumesthrige, K.; Ara, M.; Salley, S.O.; Ng, K.Y.S. Investigation of lubricity characteristics of biodiesel in petroleum and synthetic fuel. Energy Fuels 2009, 23, 2229–2234. [Google Scholar] [CrossRef]
- Xu, Y.F.; Wang, Q.J.; Hu, X.G.; Li, C.; Zhu, X.F. Characterization of the lubricity of bio-oil/diesel fuel blends by high frequency reciprocating test rig. Energy 2010, 35, 283–287. [Google Scholar] [CrossRef]
- Sarin, R.; Arora, A.K.; Ranjan, R.; Gupta, A.A.; Malhotra, R.K. Biodiesel lubricity: Correlation study with residual acidity. Lubr. Sci. 2007, 19, 151–157. [Google Scholar] [CrossRef]
- Haseeb, A.S.M.A.; Sia, S.Y.; Fazal, M.A.; Masjuki, H.H. Effect of temperature on tribological properties of palm biodiesel. Energy 2010, 35, 1460–1464. [Google Scholar] [CrossRef]
- Macario, A.; Verri, F.; Diaz, U.; Corma, A.; Giordano, G. Pure silica nanoparticles for liposome/lipase system encapsulation: application in biodiesel production. Catal. Today 2013, 204, 148–155. [Google Scholar] [CrossRef]
- Dong, H.; Li, J.; Li, Y.; Hu, L.; Luo, D. Improvement of catalytic activity and stability of lipase by immobilization on organobentonite. Chem. Eng. J. 2012, 181, 590–596. [Google Scholar] [CrossRef]
- Bautista, F.M.; Bravo, M.C.; Campelo, J.M.; Garcia, A.; Luna, D.; Marinas, J.M.; Romero, A.A. Covalent immobilization of porcine pancreatic lipase on amorphous AlPO4 and other inorganic supports. J. Chem. Technol. Biotechnol. 1998, 72, 249–254. [Google Scholar] [CrossRef]
- Macario, A.; Giordano, G.; Setti, L.; Parise, A.; Campelo, J.M.; Marinas, J.M.; Luna, D. Study of lipase immobilization on zeolitic support and transesterification reaction in a solvent free-system. Biocatal. Biotransformation 2007, 25, 328–335. [Google Scholar] [CrossRef]
- Nunes, P.A.; Pires-Cabral, P.; Guillen, M.; Valero, F.; Luna, D.; Ferreira-Dias, S. Production of MLM-Type structured lipids catalyzed by immobilized heterologous Rhizopus oryzae lipase. J. Am. Oil Chem. Soc. 2011, 88, 473–480. [Google Scholar] [CrossRef]
- Osorio, N.; Maeiro, I.; Luna, D.; Ferreira-Dias, S. Interesterification of fat blends rich in omega-3 polyunsaturated fatty acids catalyzed by immobilized lipase on modified sepiolite. New Biotechnol. 2009, 255, 5111–5112. [Google Scholar]
- Bautista, F.M.; Bravo, M.C.; Campelo, J.M.; Garcia, A.; Luna, D.; Marinas, J.M.; Romero, A.A. Covalent immobilization of acid phosphatase on amorphous AlPO4 support. J. Mol. Catal. B Enzym. 1999, 6, 473–481. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Garcia, A.; Jurado, A.; Luna, D.; Marinas, J.M.; Romero, A.A. Properties of a glucose oxidase covalently immobilized on amorphous AlPO4 support. J. Mol. Catal. B Enzym. 2001, 11, 567–577. [Google Scholar] [CrossRef]
- Luna, D.; Bautista, F.M.; Garcia, A.; Campelo, J.M.; Marinas, J.M.; Romero, A.A.; Llobet, A.; Romero, I.; Serrano, I. Method for the Chemical Binding of Homogeneous Catalysts to Inorganic Solids Supports, Products thus Obtained and Application of Same. International Publication No. WO 2004/096442 A1, 2004. [Google Scholar]
- Bautista, F.M.; Caballero, V.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Romero, A.A.; Romero, I.; Serrano, I.; Llobet, A. Heterogeneization of a new Ru(II) homogeneous asymmetric hydrogenation catalyst containing BINAP and the N-tridentate BPEA ligand, through covalent attachment on amorphous AlPO4 support. Top. Catal. 2006, 40, 193–205. [Google Scholar] [CrossRef]
- Sheldon, R.A. Enzyme immobilization: The quest for optimum performance. Adv. Synth. Catal. 2007, 349, 1289–1307. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Garcia, A.; Luna, D.; Marinas, J.M.; Romero, A.A. Compensation effects in the liquid-phase regioselective hydrogenation of functionalized alkenes on supported rhodium catalysts. Stud. Surf. Sci. Catal. 2001, 138, 213–223. [Google Scholar]
- Bautista, F.M.; Campelo, J.M.; Luna, D.; Marinas, J.M.; Quiros, R.A.; Romero, A.A. Screening of amorphous metal-phosphate catalysts for the oxidative dehydrogenation of ethylbenzene to styrene. Appl. Catal. B Environ. 2007, 70, 611–620. [Google Scholar] [CrossRef]
- Bautista, F.M.; Campelo, J.M.; Garcia, A.; Luna, D.; Marinas, J.M.; Quiros, R.A.; Romero, A.A. Influence of acid-base properties of catalysts in the gas-phase dehydration-dehydrogenation of cyclohexanol on amorphous AlPO4 and several inorganic solids. Appl. Catal. A Gen. 2003, 243, 93–107. [Google Scholar] [CrossRef]
- Campelo, J.M.; Garcia, A.; Luna, D.; Marinas, J.M.; Romero, A.A.; Navio, J.A.; Macias, M. The effect of phosphate precursor and organic additives on the structural and catalytic properties of amorphous mesoporous AlPO4 materials. Chem. Mater. 2003, 15, 3352–3364. [Google Scholar] [CrossRef]
- Bornscheuer, U.T. Lipase-catalyzed syntheses of monoacylglycerols. Enzym. Microb. Technol. 1995, 17, 578–586. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Liu, D. Rhizopus oryzae whole-cell-catalyzed biodiesel production from oleic acid in tert-butanol medium. Energy Fuels 2008, 22, 155–158. [Google Scholar] [CrossRef]
- Tüter, M.; Babali, B.; Köse, Ö.; Dural, S.; Aksoy, H.A. Solvent-free glycerolysis of palm and palm kernel oils catalyzed by a 1,3-specific lipase and fatty acid composition of glycerolysis products. Biotechnol. Lett. 1999, 21, 245–248. [Google Scholar] [CrossRef]
- Rathore, V.; Madras, G. Synthesis of biodiesel from edible and non-edible oils in supercritical alcohols and enzymatic synthesis in supercritical carbon dioxide. Fuel 2007, 86, 2650–2659. [Google Scholar] [CrossRef]
- Hernandez-Martin, E.; Otero, C. Different enzyme requirements for the synthesis of biodiesel: Novozym® 435 and Lipozyme® TL IM. Bioresour. Technol. 2008, 99, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Oda, M.; Kaieda, M.; Hama, S.; Yamaji, H.; Kondo, A.; Izumoto, E.; Fukuda, H. Facilitatory effect of immobilized lipase-producing Rhizopus oryzae cells on acyl migration in biodiesel-fuel production. Biochem. Eng. J. 2005, 23, 45–51. [Google Scholar] [CrossRef]
- Du, W.; Xu, Y.Y.; Liu, D.H.; Li, Z.B. Study on acyl migration in immobilized lipozyme TL-catalyzed transesterification of soybean oil for biodiesel production. J. Mol. Catal. B Enzym. 2005, 37, 68–71. [Google Scholar] [CrossRef]
- Sjursnes, B.J.; Anthonsen, T. Acyl migration in 1,2-dibutyrin, dependence on solvent and water activity. Biocatalysis 1994, 9, 285–297. [Google Scholar] [CrossRef]
- Li, W.; Du, W.; Li, Q.; Sun, T.; Liu, D. Study on acyl migration kinetics of partial glycerides: Dependence on temperature and water activity. J. Mol. Catal. B Enzym. 2010, 63, 17–22. [Google Scholar] [CrossRef]
- Celikten, I. The effect of biodiesel, ethanol and diesel fuel blends on the performance and exhaust emissions in a DI diesel engine. Gazi Univ. J. Sci. 2011, 24, 341–345. [Google Scholar]
- Cheenkachorn, K.; Fungtammasan, B. Biodiesel as an additive for diesohol. Int. J. Green Energy 2009, 6, 57–72. [Google Scholar] [CrossRef]
- Jaganjac, M.; Prah, I.O.; Cipak, A.; Cindric, M.; Mrakovcic, L.; Tatzber, F.; Ilincic, P.; Rukavina, V.; Spehar, B.; Vukovic, J.P.; Telen, S.; Uchida, K.; Lulic, Z.; Zarkovic, N. Effects of bioreactive acrolein from automotive exhaust gases on human cells in vitro. Environ. Toxicol. 2012, 27, 644–652. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Mu, Y.; Yuan, J.; He, H. Carbonyls emission from ethanol-blended gasoline and biodiesel-ethanol-diesel used in engines. Atmos. Environ. 2008, 42, 1349–1358. [Google Scholar] [CrossRef]
- Rekondo, A.; Irusta, L.; Fernandez-Berridi, M.J. Characterization of silanized poly(ether-urethane) hybrid systems using thermogravimetric analysis (TG). J. Therm. Anal. Calorim. 2010, 101, 331–337. [Google Scholar] [CrossRef]
- Fukushima, H.; Kohri, M.; Kojima, T.; Taniguchi, T.; Saito, K.; Nakahira, T. Surface-initiated enzymatic vinyl polymerization: Synthesis of polymer-grafted silica particles using horseradish peroxidase as catalyst. Polym. Chem. 2012, 3, 1123–1125. [Google Scholar] [CrossRef]
- Galvin, C.J.; Genzer, I. Applications of surface-grafted macromolecules derived from post-polymerization modification reactions. Prog. Polym. Sci. 2012, 37, 871–906. [Google Scholar] [CrossRef]
- David, A.E.; Wang, N.S.; Yang, V.C.; Yang, A.J. Chemically surface modified gel (CSMG): An excellent enzyme-immobilization matrix for industrial processes. J. Biotechnol. 2006, 125, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Luna, D.; Posadillo, A.; Caballero, V.; Verdugo, C.; Bautista, F.M.; Romero, A.A.; Sancho, E.D.; Luna, C.; Calero, J. New biofuel integrating glycerol into their composition through the use of covalent immobilized pig pancreatic lipase. Int. J. Mol. Sci. 2012, 13, 10091–10112. [Google Scholar] [CrossRef] [PubMed]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luna, C.; Sancho, E.; Luna, D.; Caballero, V.; Calero, J.; Posadillo, A.; Verdugo, C.; Bautista, F.M.; Romero, A.A. Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support. Energies 2013, 6, 3879-3900. https://doi.org/10.3390/en6083879
Luna C, Sancho E, Luna D, Caballero V, Calero J, Posadillo A, Verdugo C, Bautista FM, Romero AA. Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support. Energies. 2013; 6(8):3879-3900. https://doi.org/10.3390/en6083879
Chicago/Turabian StyleLuna, Carlos, Enrique Sancho, Diego Luna, Verónica Caballero, Juan Calero, Alejandro Posadillo, Cristóbal Verdugo, Felipa M. Bautista, and Antonio A. Romero. 2013. "Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support" Energies 6, no. 8: 3879-3900. https://doi.org/10.3390/en6083879
APA StyleLuna, C., Sancho, E., Luna, D., Caballero, V., Calero, J., Posadillo, A., Verdugo, C., Bautista, F. M., & Romero, A. A. (2013). Biofuel that Keeps Glycerol as Monoglyceride by 1,3-Selective Ethanolysis with Pig Pancreatic Lipase Covalently Immobilized on AlPO4 Support. Energies, 6(8), 3879-3900. https://doi.org/10.3390/en6083879






