Electrostatic Self-Assembly of Fe3O4 Nanoparticles on Graphene Oxides for High Capacity Lithium-Ion Battery Anodes
Abstract
:1. Introduction
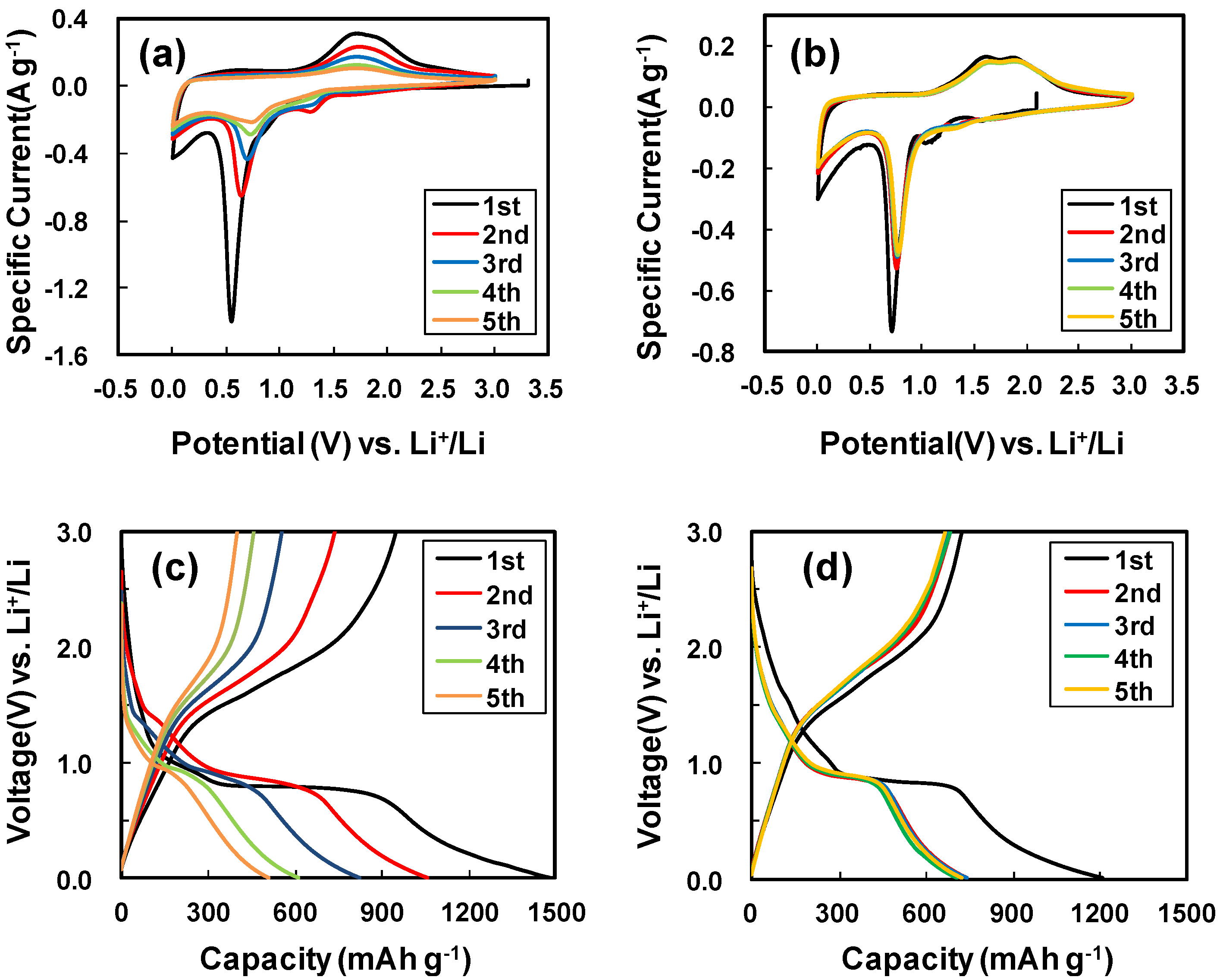
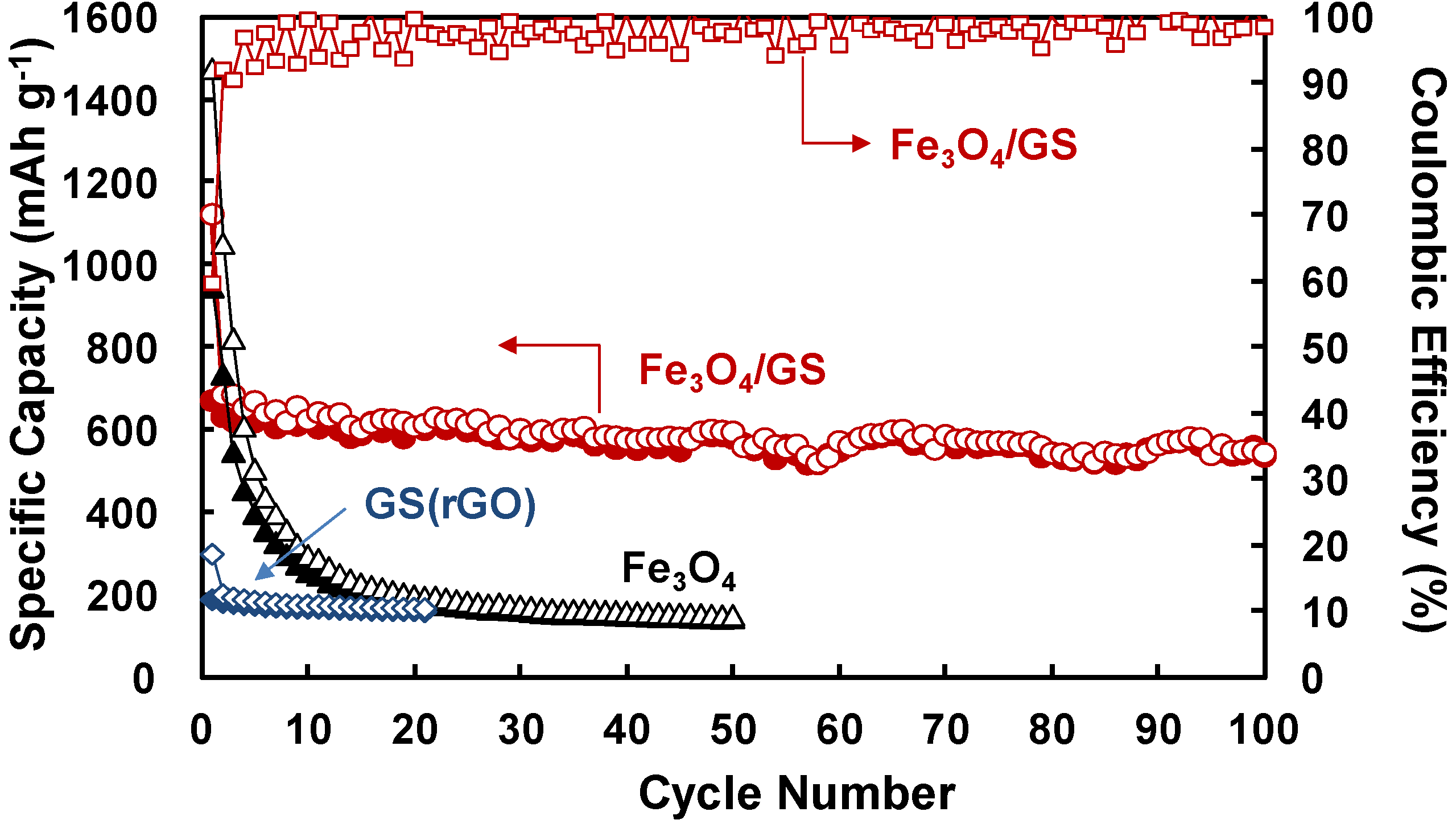
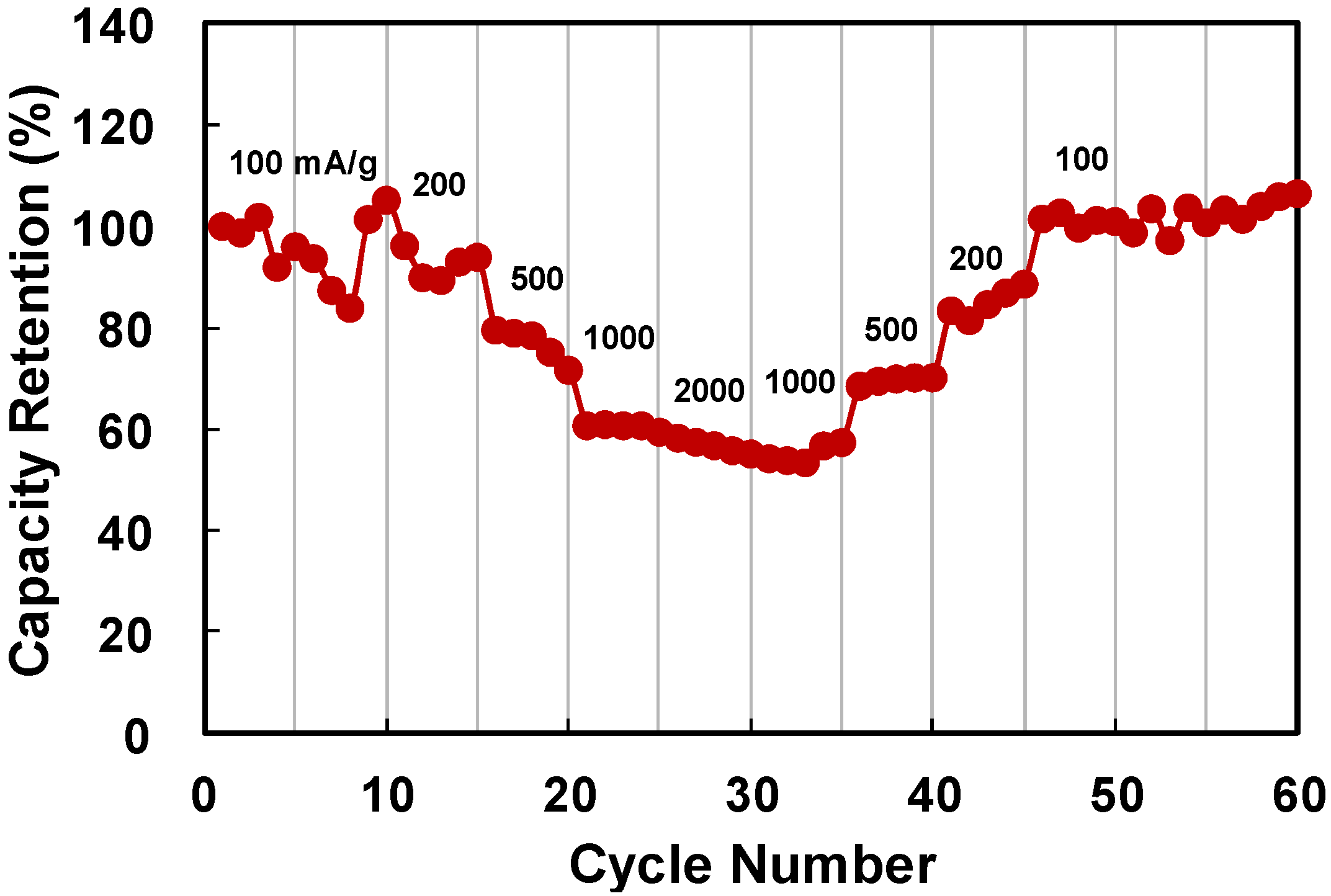
2. Results and Discussion
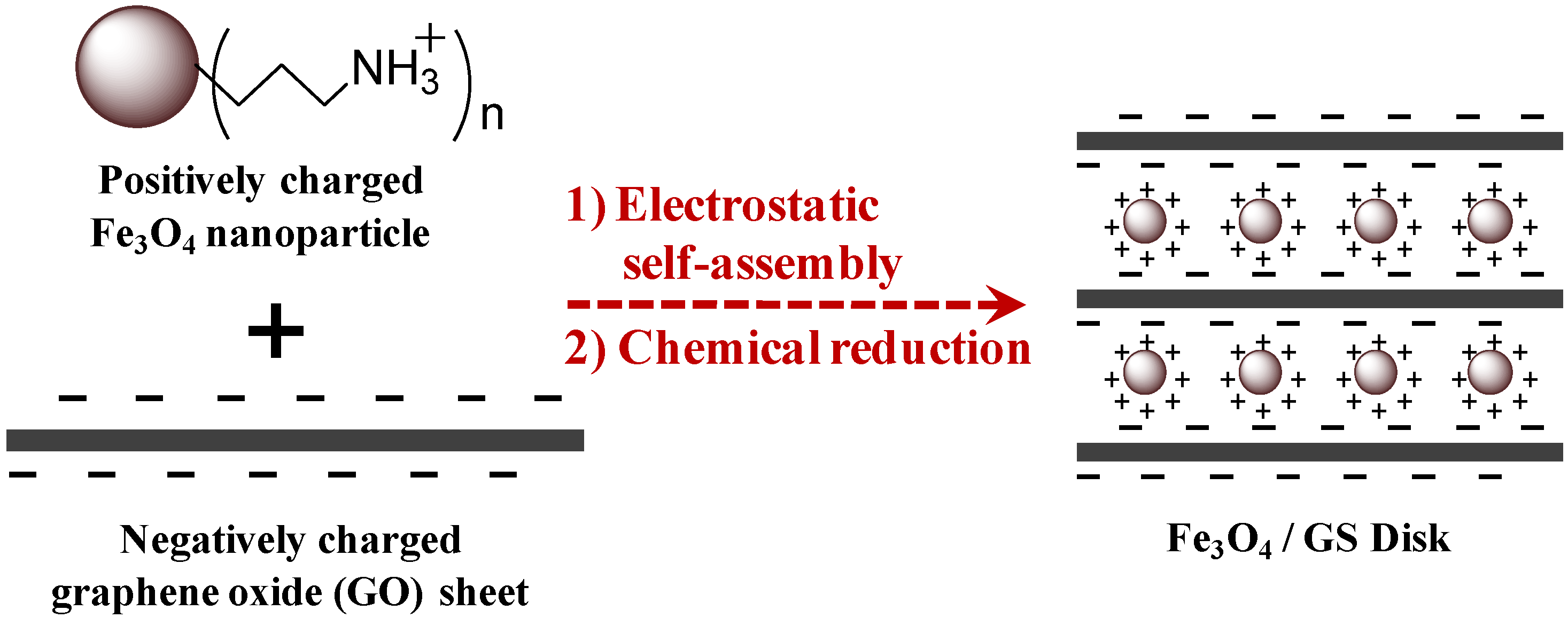
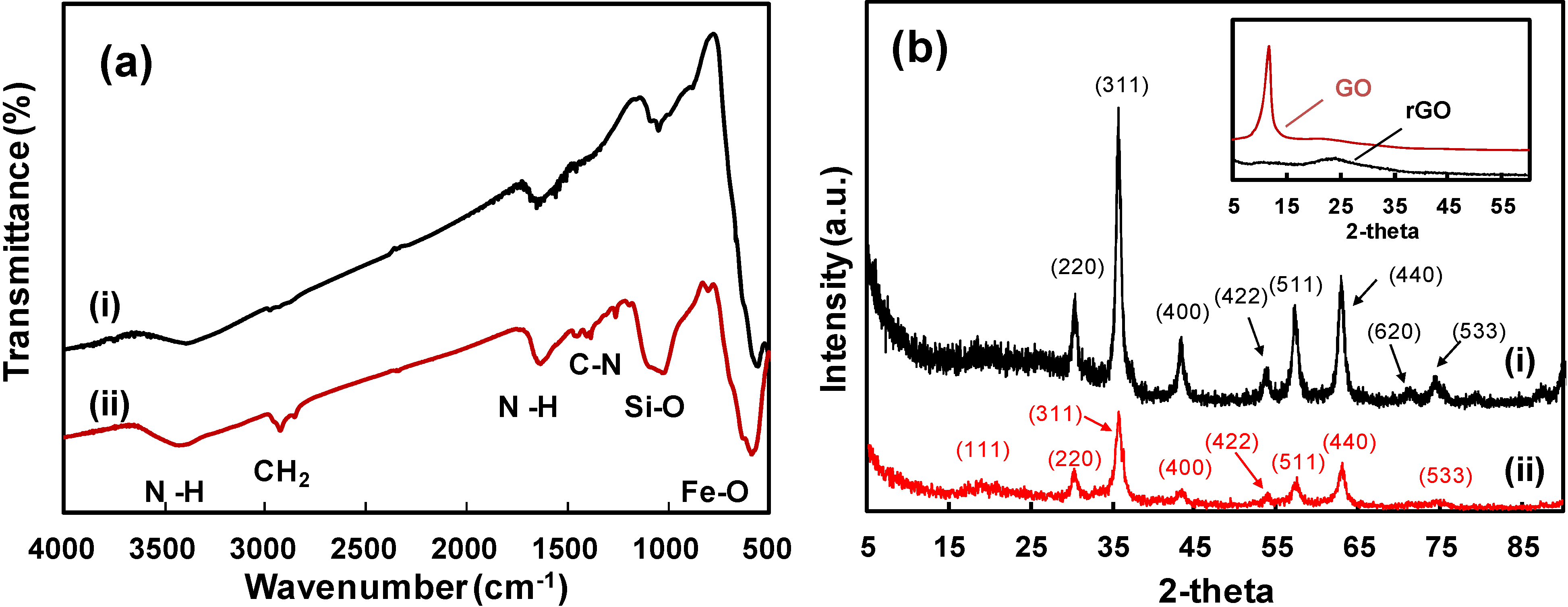

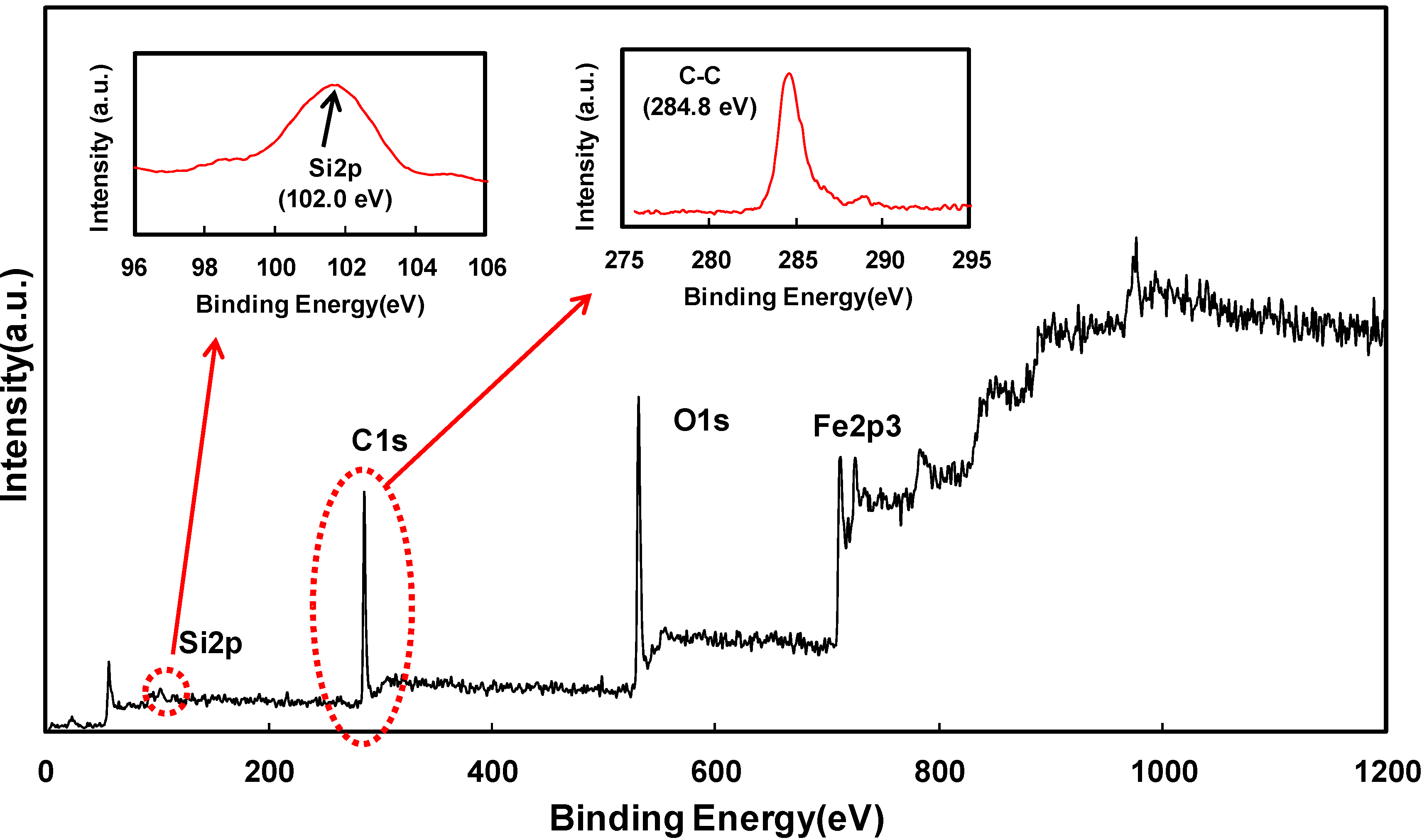
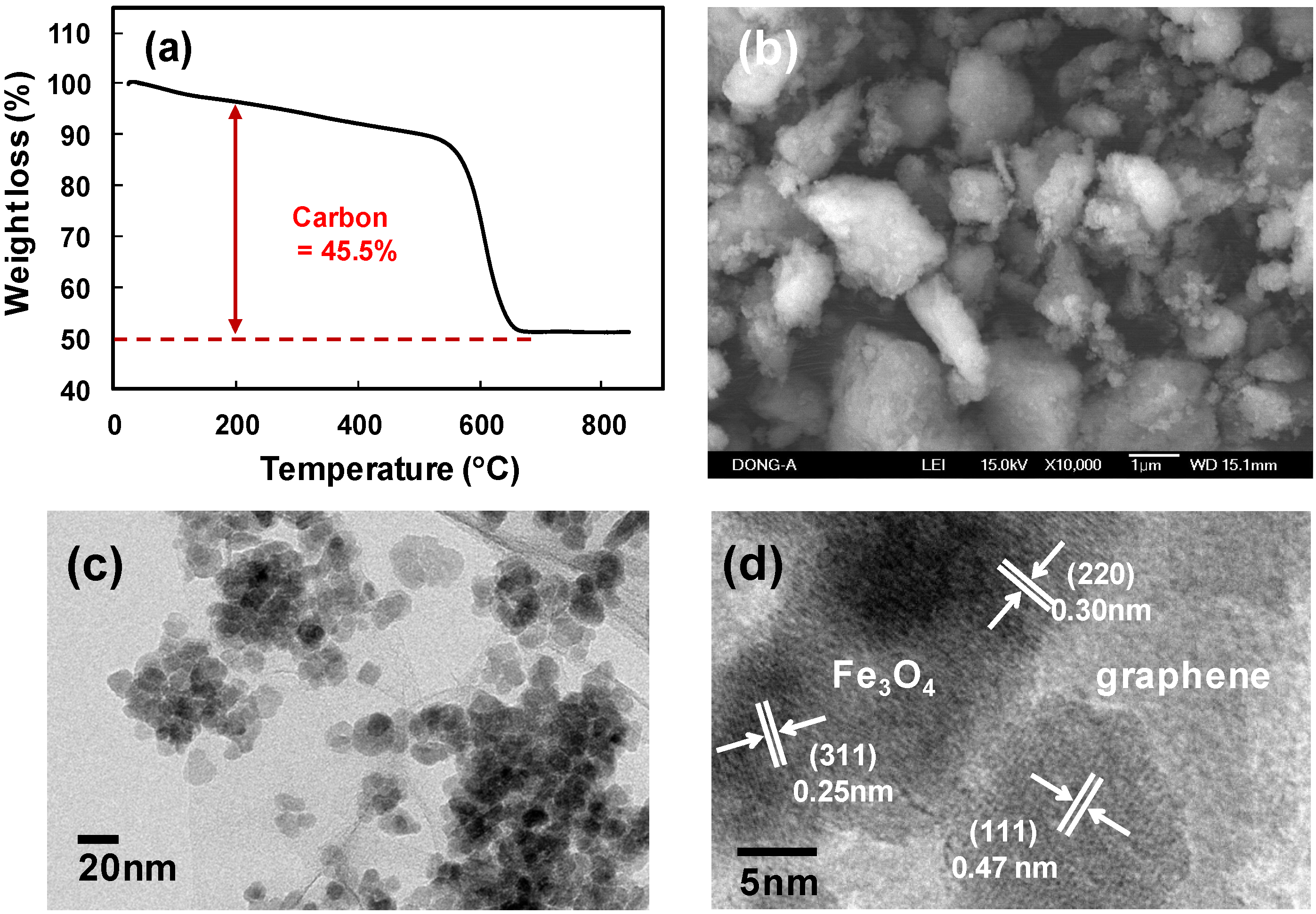
3. Experimental Section
3.1. Synthesis of Fe3O4 Nanoparticles and GO
3.2. Preparation of Fe3O4-APTMS and Fe3O4/GS Composite
3.3. Materials Characterization
3.4. Electrochemical Measurements
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Tarascon, J.M. Key challenges in future Li-battery research. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3227–3241. [Google Scholar] [CrossRef]
- Hassoun, J.; Lee, K.S.; Sun, Y.K.; Scrosati, B. An advanced lithium ion battery based on high performance electrode materials. J. Am. Chem. Soc. 2011, 133, 3139–3143. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Wu, X.L.; Hu, J.S.; Guo, Y.G.; Wan, L.J. Carbon coated Fe3O4 nanospindles as a superior anode material for lithium-ion batteries. Adv. Funct. Mater. 2008, 18, 3941–3946. [Google Scholar] [CrossRef]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacin, M.R. Beyond intercalation-based Li-ion batteries: The state of the art and challenges of electrode materials reacting through conversion reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.M.; Wang, D.W.; Li, F.; Zhang, L.L.; Li, N.; Wu, Z.S.; Wen, L.; Lu, G.Q.; Cheng, H.M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Lian, P.C.; Zhu, X.F.; Xiang, H.F.; Li, Z.; Yang, W.S.; Wang, H.H. Enhanced cycling performance of Fe3O4-graphene nanocomposite as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 56, 834–840. [Google Scholar] [CrossRef]
- Yoon, T.; Chae, C.; Sun, Y.K.; Zhao, X.; Kung, H.H.; Lee, J.K. Bottom-up in situ formation of Fe3O4 nanocrystals in a porous carbon foam for lithium-ion battery anodes. J. Mater. Chem. 2011, 21, 17325–17330. [Google Scholar] [CrossRef]
- Hassoun, J.; Croce, F.; Hong, I.; Scrosati, B. Lithium-iron battery: Fe2O3 anode versus LiFePO4 cathode. Electrochem. Commun. 2011, 13, 228–231. [Google Scholar] [CrossRef]
- Ji, L.W.; Lin, Z.; Alcoutlabi, M.; Zhang, X.W. Recent developments in nanostructured anode materials for rechargeable lithium-ion batteries. Energy Environ. Sci. 2011, 4, 2682–2699. [Google Scholar] [CrossRef]
- Ji, L.W.; Tan, Z.K.; Kuykendall, T.R.; Aloni, S.; Xun, S.D.; Lin, E.; Battaglia, V.; Zhang, Y.G. Fe3O4 nanoparticle-integrated graphene sheets for high-performance half and full lithium ion cells. Phys. Chem. Chem. Phys. 2011, 13, 7139–7146. [Google Scholar] [CrossRef]
- Ji, L.W.; Toprakci, O.; Alcoutlabi, M.; Yao, Y.F.; Li, Y.; Zhang, S.; Guo, B.K.; Lin, Z.; Zhang, X.W. α-Fe2O3 nanoparticle-loaded carbon nanofibers as stable and high-capacity anodes for rechargeable lithium-ion batteries. ACS Appl. Mater. Interfaces 2012, 4, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, L.; Cai, J.S.; Zheng, X.M.; Sun, S.G. Structure and electrochemical performance of nanostructured Fe3O4/carbon nanotube composites as anodes for lithium ion batteries. Electrochim. Acta 2011, 55, 1140–1144. [Google Scholar] [CrossRef]
- Zhu, X.J.; Zhu, Y.W.; Murali, S.; Stollers, M.D.; Ruoff, R.S. Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS Nano 2011, 5, 3333–3338. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.L.; Cui, L.F.; Yang, Y.A.; Casalongue, H.S.; Robinson, J.T.; Liang, Y.Y.; Cui, Y.; Dai, H.J. Mn3O4-graphene hybrid as a high-capacity anode material for lithium ion batteries. J. Am. Chem. Soc. 2010, 132, 13978–13980. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Lee, K.P.; Gopalan, A.Y.; Kang, H.D. Organosilane modified magnetite nanoparticles/poly(aniline-co-o/m-aminobenzenesulfonic acid) composites: Synthesis and characterization. React. Funct. Polym. 2007, 67, 943–954. [Google Scholar] [CrossRef]
- Seckin, T.; Vural, S.; Koytepe, S. Preparation and structural properties of Fe3O4-polyimide hybrid nanocomposites. Polym. Bull. 2010, 64, 115–126. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Chun, L.; Wu, X.Z.; Lou, X.M.; Zhang, Y.X. Hematite nanoflakes as anode electrode materials for rechargeable lithium-ion batteries. Electrochim. Acta 2010, 55, 3089–3092. [Google Scholar] [CrossRef]
- Jin, S.L.; Deng, H.G.; Long, D.H.; Liu, X.J.; Zhan, L.A.; Liang, X.Y.; Qiao, W.M.; Ling, L.C. Facile synthesis of hierarchically structured Fe3O4/carbon micro-flowers and their application to lithium-ion battery anodes. J. Power Sources 2011, 196, 3887–3893. [Google Scholar] [CrossRef]
- Wu, X.L.; Guo, Y.G.; Wan, L.J.; Hu, C.W. α-Fe2O3 nanostructures: Inorganic salt-controlled synthesis and their electrochemical performance toward lithium storage. J. Phys. Chem. C 2008, 112, 16824–16829. [Google Scholar] [CrossRef]
- Kovtyukhova, N.I.; Ollivier, P.J.; Martin, B.R.; Mallouk, T.E.; Chizhik, S.A.; Buzaneva, E.V.; Gorchinskiy, A.D. Layer-by-layer assembly of ultrathin composite films from micron-sized graphite oxide sheets and polycations. Chem. Mater. 1999, 11, 771–778. [Google Scholar] [CrossRef]
- Lee, J.K.; Smith, K.B.; Hayner, C.M.; Kung, H.H. Silicon nanoparticles-graphene paper composites for Li ion battery anodes. Chem. Commun. 2010, 46, 2025–2027. [Google Scholar] [CrossRef]
- Yoon, T.; Cho, M.; Suh, Y.W.; Oh, E.S.; Lee, J.K. Reassembled graphene-platelets encapsulated silicon nanoparticles for Li-ion battery anodes. J. Nanosci. Nanotech. 2011, 11, 10193–10200. [Google Scholar] [CrossRef]
- Yang, S.B.; Feng, X.L.; Ivanovici, S.; Mullen, K. Fabrication of graphene-encapsulated oxide nanoparticles: Towards high-performance anode materials for lithium storage. Angew. Chem. Int. Ed. 2010, 49, 8408–8411. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yoon, T.; Kim, J.; Kim, J.; Lee, J.K. Electrostatic Self-Assembly of Fe3O4 Nanoparticles on Graphene Oxides for High Capacity Lithium-Ion Battery Anodes. Energies 2013, 6, 4830-4840. https://doi.org/10.3390/en6094830
Yoon T, Kim J, Kim J, Lee JK. Electrostatic Self-Assembly of Fe3O4 Nanoparticles on Graphene Oxides for High Capacity Lithium-Ion Battery Anodes. Energies. 2013; 6(9):4830-4840. https://doi.org/10.3390/en6094830
Chicago/Turabian StyleYoon, Taegyune, Jaegyeong Kim, Jinku Kim, and Jung Kyoo Lee. 2013. "Electrostatic Self-Assembly of Fe3O4 Nanoparticles on Graphene Oxides for High Capacity Lithium-Ion Battery Anodes" Energies 6, no. 9: 4830-4840. https://doi.org/10.3390/en6094830



