Reorientation of Magnetic Graphene Oxide Nanosheets in Crosslinked Quaternized Polyvinyl Alcohol as Effective Solid Electrolyte
Abstract
:1. Introduction
2. Results and Discussion
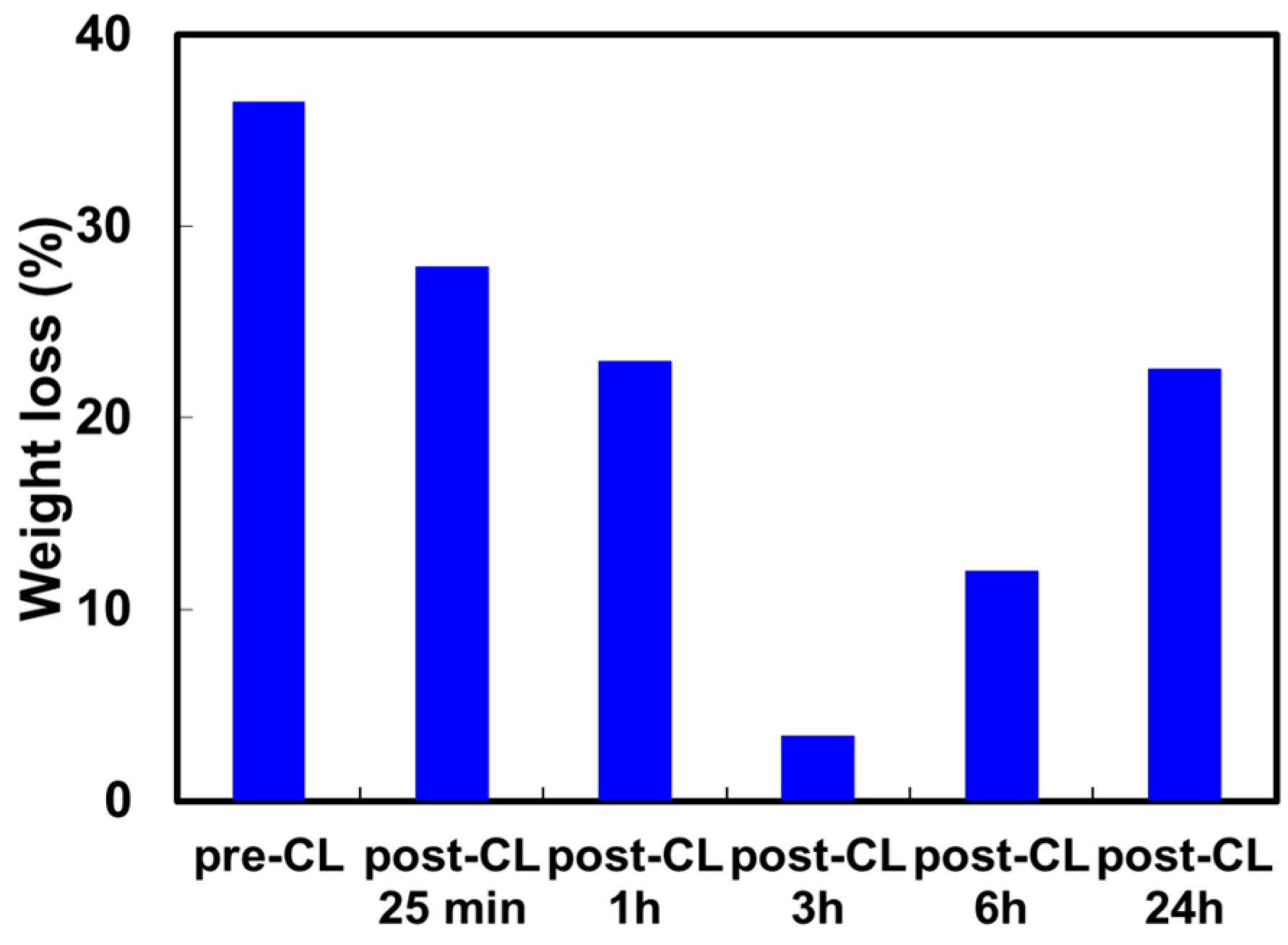
2.1. Stability of Crosslinked Quaternized Polyvinyl Alcohol Membrane
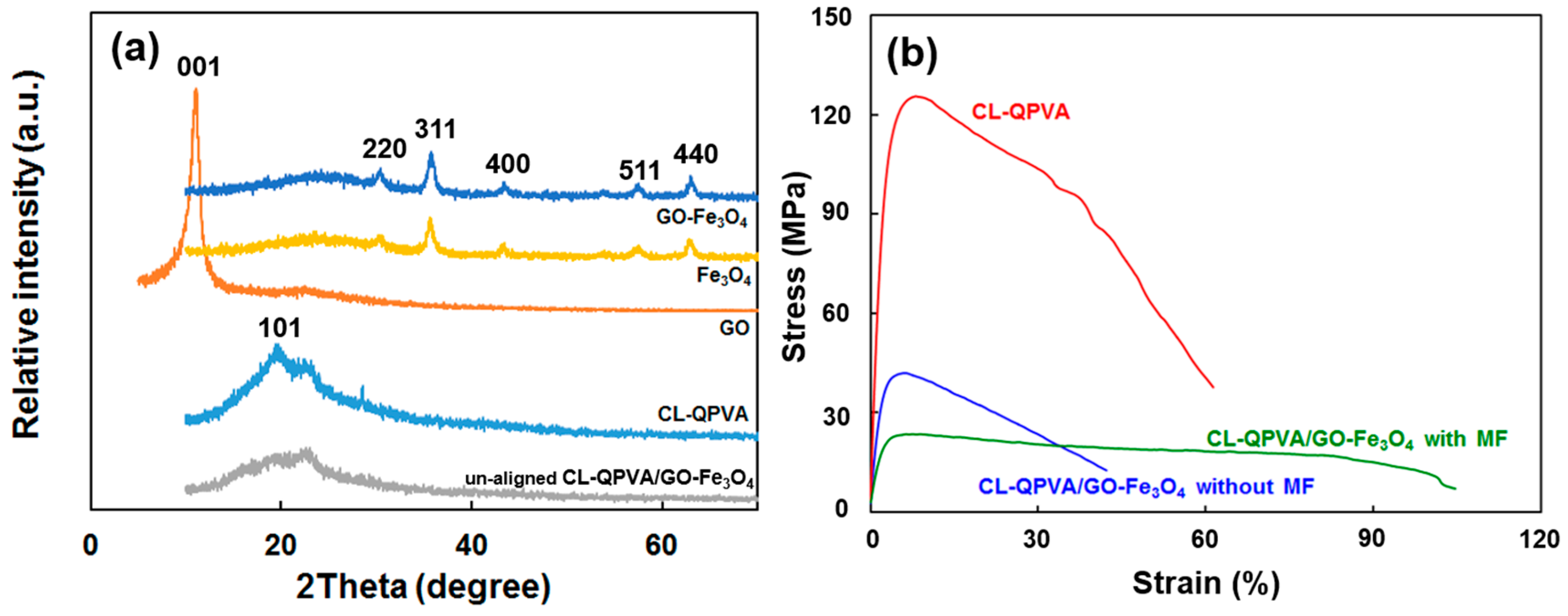
2.2. Crystallinity and Mechanical Properties of Quaternized Polyvinyl Alcohol and Nanocomposite Membrane
2.3. Methanol Permeability of Quaternized Polyvinyl Alcohol Composite Membranes
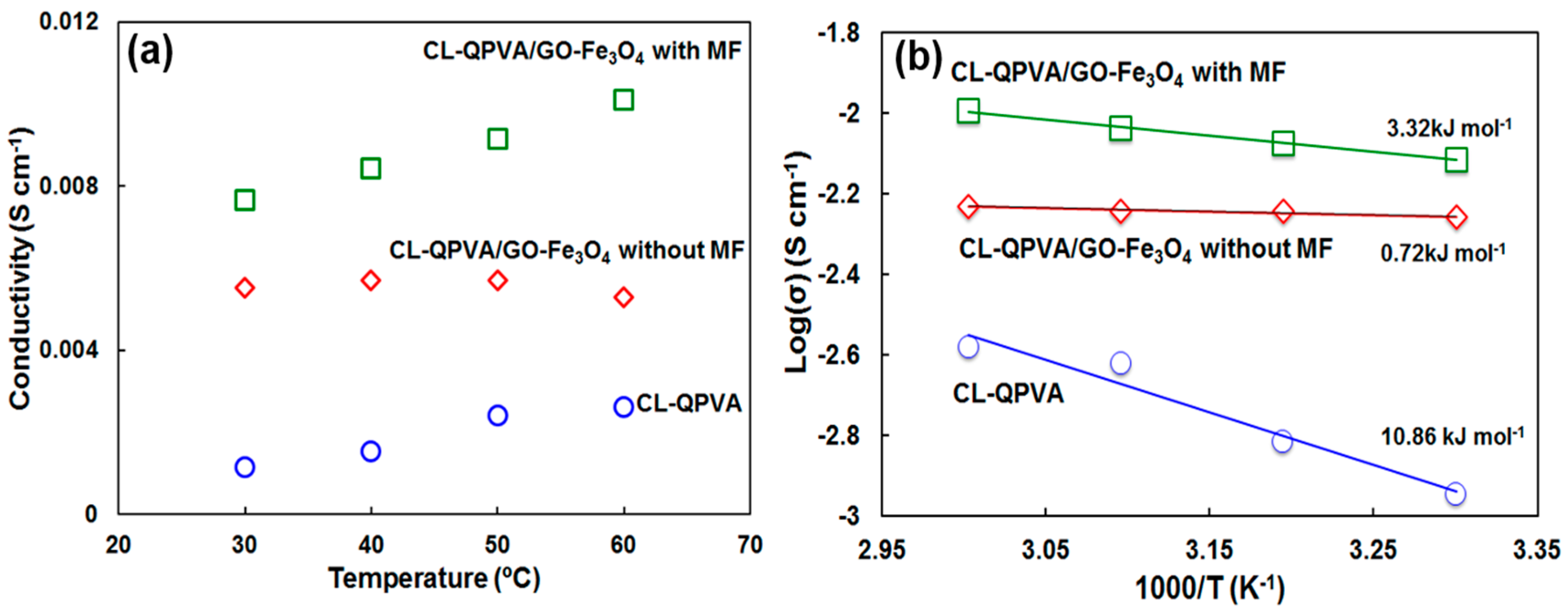
2.4. Ionic Conductivity of KOH-Doped Quaternized Polyvinyl Alcohol Nanocomposite Membranes
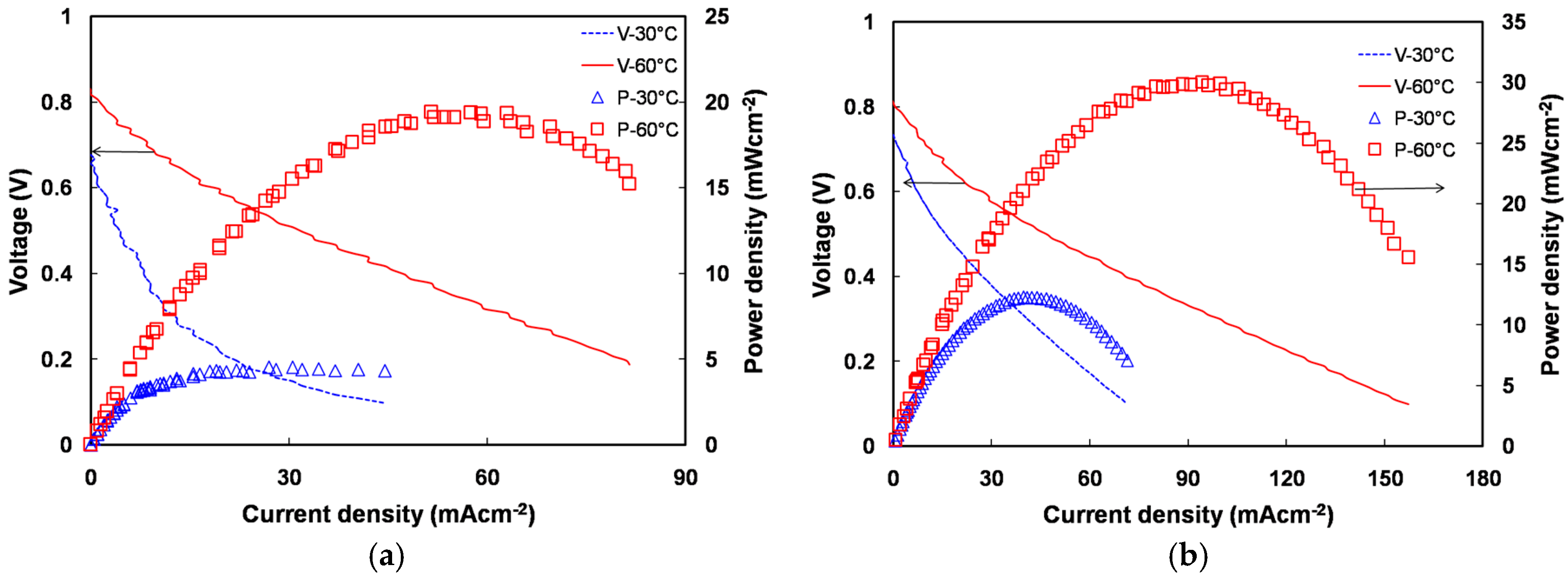
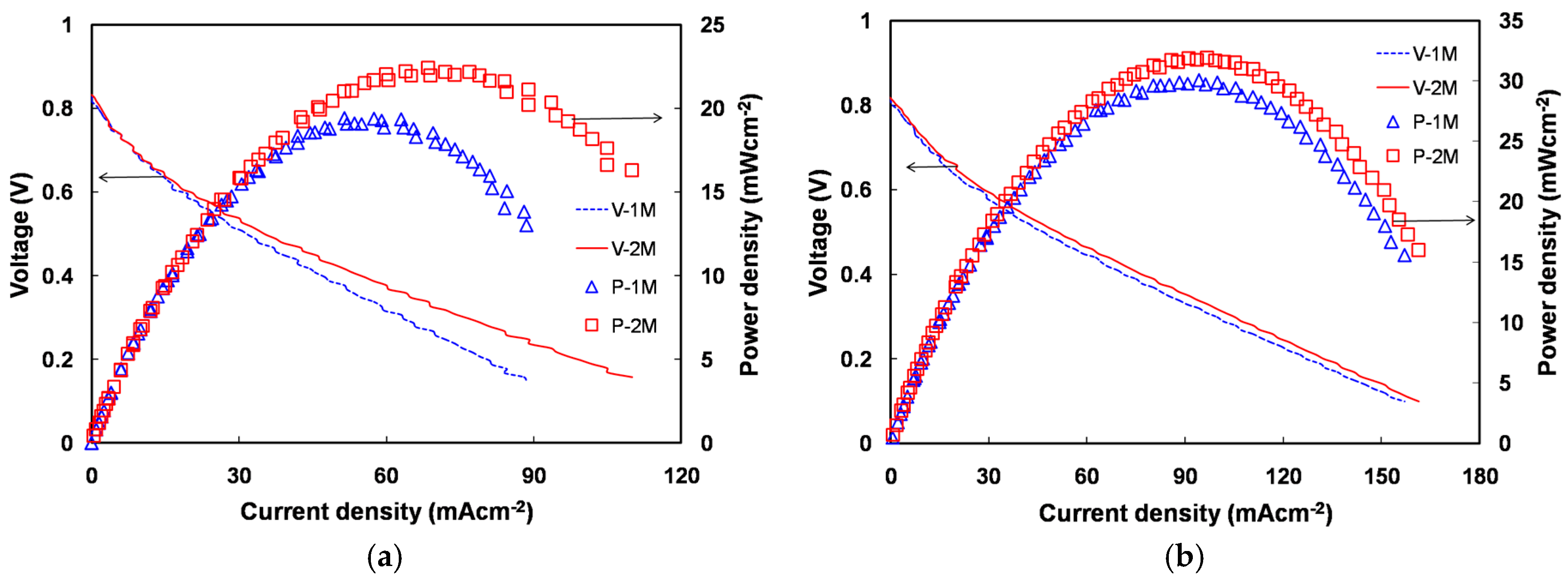
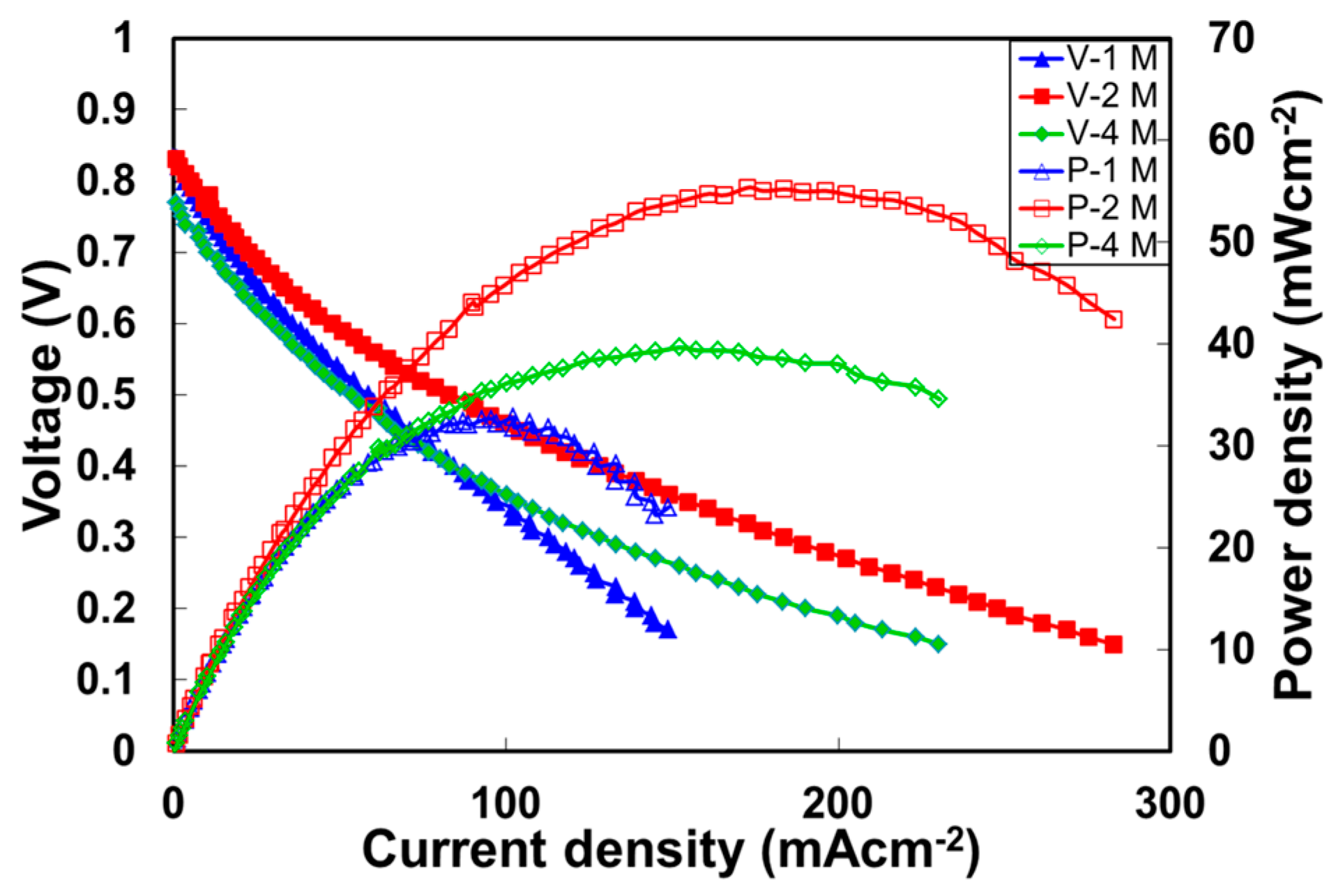
2.5. Direct Methanol Alkaline Fuel Cell Performance of Quaternized Polyvinyl Alcohol Composite Membranes
3. Materials and Methods
3.1. Materials
3.2. Preparation of Quaternized Polyvinyl Alcohol and Graphene Oxide-Iron Oxide
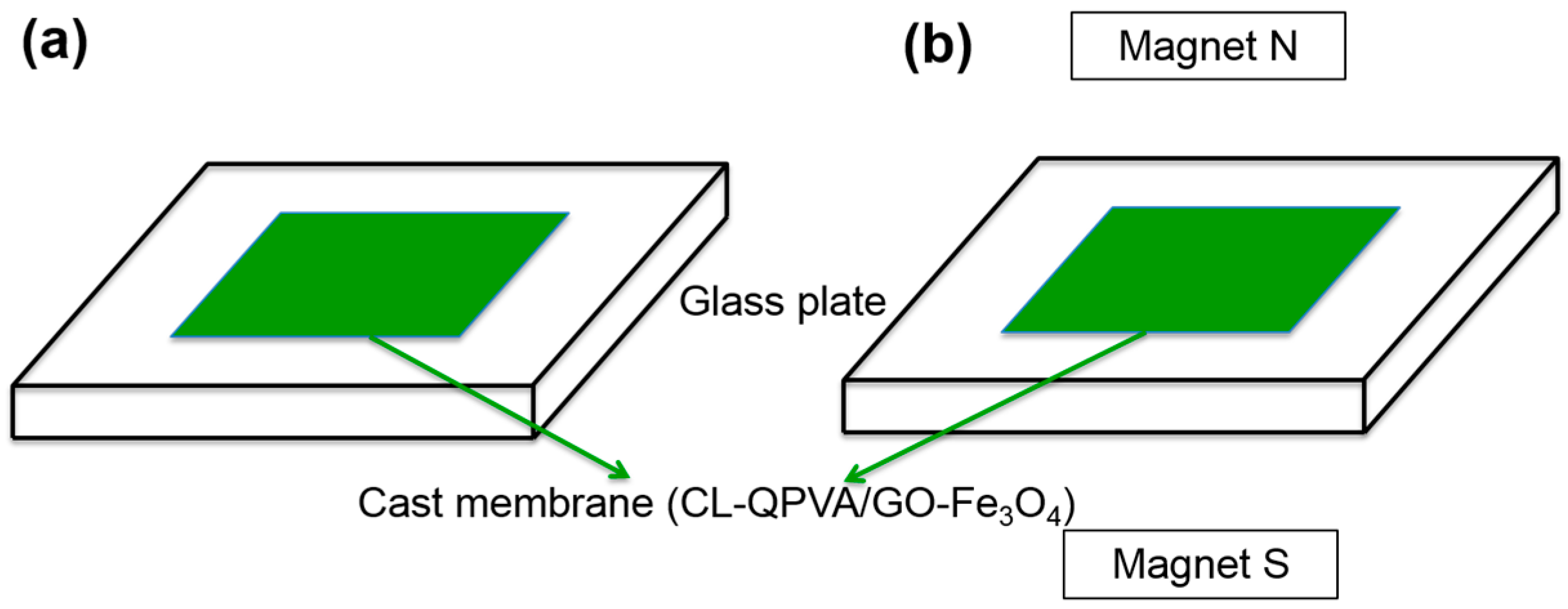
3.3. Preparation of Quaternized Polyvinyl Alcohol and Quaternized Polyvinyl Alcohol/Graphene Oxide-Iron Oxide Nanocomposite Membrane
3.4. Membrane Characterization
3.5. Cell Performance Measurement
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hardman, S.; Chandan, A.; Steinberger-Wilckens, R. Fuel cell added value for early market applications. J. Power Sources 2015, 287, 297–306. [Google Scholar] [CrossRef]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Dyer, C.K. Fuel cells for portable applications. J. Power Sources 2002, 106, 31–34. [Google Scholar] [CrossRef]
- Liao, G.M.; Li, P.C.; Lin, J.S.; Ma, W.T.; Yu, B.C.; Li, H.Y.; Liu, Y.L.; Yang, C.C.; Shih, C.M.; Lue, S.J. Highly conductive quasi-coaxial electrospun quaternized polyvinyl alcohol nanofibers and composite as high-performance solid electrolytes. J. Power Sources 2016, 304, 136–145. [Google Scholar] [CrossRef]
- Li, P.C.; Liao, G.M.; Kumar, S.R.; Shih, C.M.; Yang, C.C.; Wang, D.M.; Lue, S.J. Fabrication and characterization of chitosan nanoparticle-incorporated quaternized poly(vinyl alcohol) composite membranes as solid electrolytes for direct methanol alkaline fuel cells. Electrochim. Acta 2016, 187, 616–628. [Google Scholar] [CrossRef]
- Varcoe, J.R.; Atanassov, P.; Dekel, D.R.; Herring, A.M.; Hickner, M.A.; Kohl, P.A.; Kucernak, A.R.; Mustain, W.E.; Nijmeijer, K.; Scott, K.; et al. Anion-exchange membranes in electrochemical energy systems. Energy Environ. Sci. 2014, 7, 3135–3191. [Google Scholar] [CrossRef]
- Kumar, S.R.; Juan, C.H.; Liao, G.M.; Lin, J.S.; Yang, C.C.; Ma, W.T.; You, J.H.; Lue, S.J. Fumed silica nanoparticles incorporated in quaternized poly (vinyl alcohol) nanocomposite membrane for enhanced power densities in direct alcohol alkaline fuel cells. Energies 2015, 9, 15. [Google Scholar] [CrossRef]
- Wu, J.F.; Lo, C.F.; Li, L.Y.; Li, H.Y.; Chang, C.M.; Liao, K.S.; Hu, C.C.; Liu, Y.L.; Lue, S.J. Thermally stable polybenzimidazole/carbon nano-tube composites for alkaline direct methanol fuel cell applications. J. Power Sources 2014, 246, 39–48. [Google Scholar] [CrossRef]
- Li, L.Y.; Yu, B.C.; Shih, C.M.; Lue, S.J. Polybenzimidazole membranes for direct methanol fuel cell: Acid-doped or alkali-doped? J. Power Sources 2015, 287, 386–395. [Google Scholar] [CrossRef]
- Lo, C.F.; Wu, J.F.; Li, H.Y.; Hung, W.S.; Shih, C.M.; Hu, C.C.; Liu, Y.L.; Lue, S.J. Novel polyvinyl alcohol nanocomposites containing carbon nano-tubes with Fe3O4 pendants for alkaline fuel cell applications. J. Membr. Sci. 2013, 444, 41–49. [Google Scholar] [CrossRef]
- Tripković, A.V.; Popović, K.D.; Grgur, B.N.; Blizanac, B.; Ross, P.N.; Marković, N.M. Methanol electrooxidation on supported Pt and PtRu catalysts in acid and alkaline solutions. Electrochim. Acta 2002, 47, 3707–3714. [Google Scholar] [CrossRef]
- Lu, S.; Pan, J.; Huang, A.; Zhuang, L.; Lu, J. Alkaline polymer electrolyte fuel cells completely free from noble metal catalysts. Proc. Natl. Acad. Sci. USA 2008, 105, 20611–20614. [Google Scholar] [CrossRef]
- Huang, C.Y.; Lin, J.S.; Pan, W.H.; Shih, C.M.; Liu, Y.L.; Lue, S.J. Alkaline direct ethanol fuel cell performance using alkali-impregnated polyvinyl alcohol/functionalized carbon nano-tube solid electrolytes. J. Power Sources 2016, 303, 267–277. [Google Scholar] [CrossRef]
- Lue, S.J.; Mahesh, K.P.O.; Wang, W.T.; Chen, J.Y.; Yang, C.C. Permeant transport properties and cell performance of potassium hydroxide doped poly(vinyl alcohol)/fumed silica nanocomposites. J. Membr. Sci. 2011, 367, 256–264. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Chien, W.C.; Chiu, S.S. Quaternized poly(vinyl alcohol)/alumina composite polymer membranes for alkaline direct methanol fuel cells. J. Power Sources 2010, 195, 2212–2219. [Google Scholar] [CrossRef]
- Ma, W.T.; Wang, Y.C.; Yu, B.C.; Teng, L.W.; Shih, C.M.; Tsai, S.W.; Liu, Y.L.; Lue, S.J. Magnetic field-assisted alignment of graphene oxide nanosheets in polymer matrix for enhancing ionic conduction. J. Membr. Sci. 2016. in revision. [Google Scholar]
- Lue, S.J.; Lee, D.T.; Chen, J.Y.; Chiu, C.H.; Hu, C.C.; Jean, Y.C.; Lai, J.Y. Diffusivity enhancement of water vapor in poly(vinyl alcohol)-fumed silica nano-composite membranes: Correlation with polymer crystallinity and free-volume properties. J. Membr. Sci. 2008, 325, 831–839. [Google Scholar] [CrossRef]
- Yuan, T.; Pu, L.; Huang, Q.; Zhang, H.; Li, X.; Yang, H. An effective methanol-blocking membrane modified with graphene oxide nanosheets for passive direct methanol fuel cells. Electrochim. Acta 2014, 117, 393–397. [Google Scholar] [CrossRef]
- Yang, C.C.; Chiu, S.J.; Lee, K.T.; Chien, W.C.; Lin, C.T.; Huang, C.A. Study of poly(vinyl alcohol)/titanium oxide composite polymer membranes and their application on alkaline direct alcohol fuel cell. J. Power Sources 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Movil, O.; Frank, L.; Staser, J.A. Graphene oxide-polymer nanocomposite anion-exchange membranes. J. Electrochem. Soc. 2015, 162, F419–F426. [Google Scholar] [CrossRef]
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Lue, S.J.; Pai, Y.L.; Shih, C.M.; Wu, M.C.; Lai, S.M. Novel bilayer well-aligned Nafion/graphene oxide composite membranes prepared using spin coating method for direct liquid fuel cells. J. Membr. Sci. 2015, 493, 212–223. [Google Scholar] [CrossRef]
- Vadivel Murugan, A.; Muraliganth, T.; Manthiram, A. Rapid, facile microwave-solvothermal synthesis of graphene nanosheets and their polyaniline nanocomposites for energy strorage. Chem. Mater. 2009, 21, 5004–5006. [Google Scholar] [CrossRef]
- Zhao, G.; Li, J.; Ren, X.; Chen, C.; Wang, X. Few-layered graphene oxide nanosheets as superior sorbents for heavy metal ion pollution management. Environ. Sci. Technol. 2011, 45, 10454–10462. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Alabadi, A.; Razzaque, S.; Dong, Z.; Wang, W.; Tan, B. Graphene oxide-polythiophene derivative hybrid nanosheet for enhancing performance of supercapacitor. J. Power Sources 2016, 306, 241–247. [Google Scholar] [CrossRef]
- Pandey, R.P.; Shahi, V.K. Sulphonated imidized graphene oxide (SIGO) based polymer electrolyte membrane for improved water retention, stability and proton conductivity. J. Power Sources 2015, 299, 104–113. [Google Scholar] [CrossRef]
- Yuan, M.; Erdman, J.; Tang, C.; Ardebili, H. High performance solid polymer electrolyte with graphene oxide nanosheets. RSC Adv. 2014, 4, 59637–59642. [Google Scholar] [CrossRef]
- Wang, L.S.; Lai, A.N.; Lin, C.X.; Zhang, Q.G.; Zhu, A.M.; Liu, Q.L. Orderly sandwich-shaped graphene oxide/Nafion composite membranes for direct methanol fuel cells. J. Membr. Sci. 2015, 492, 58–66. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M. Aligned nanocomposite membranes containing sulfonated graphene oxide with superior ionic conductivity for direct methanol fuel cell application. Ind. Eng. Chem. Res. 2015, 28, 7028–7037. [Google Scholar] [CrossRef]
- Aricò, A.S.; Baglio, V.; Antonucci, V.; Nicotera, I.; Oliviero, C.; Coppola, L.; Antonucci, P.L. An NMR and SAXS investigation of DMFC composite recast Nafion membranes containing ceramic fillers. J. Membr. Sci. 2006, 270, 221–227. [Google Scholar] [CrossRef]
- Zhao, Y.; Fu, Y.; Hu, B.; Lv, C. Quaternized graphene oxide modified ionic cross-linked sulfonated polymer electrolyte composite proton exchange membranes with enhanced properties. Solid State Ion. 2016, 294, 43–53. [Google Scholar] [CrossRef]
- Nicotera, I.; Angjeli, K.; Coppola, L.; Aricò, A.S.; Baglio, V. NMR and electrochemical investigation of the transport properties of methanol and water in Nafion and clay-nanocomposites membranes for DMFCs. Membranes 2012, 2, 325–345. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C. Alkaline direct methanol fuel cell based on a novel anion-exchange composite polymer membrane. J. Appl. Electrochem. 2012, 42, 305–317. [Google Scholar] [CrossRef]
- Nicotera, I.; Simari, C.; Coppola, L.; Zygouri, P.; Gournis, D.; Brutti, S.; Minuto, F.D.; Aricò, A.S.; Sebastian, D.; Baglio, V. Sulfonated graphene oxide platelets in Nafion nanocomposite membrane: Advantages for application in direct methanol fuel cells. J. Phys. Chem. C 2014, 118, 24357–24368. [Google Scholar] [CrossRef]
- Paneri, A.; He, Y.; Ehlert, G.; Cottrill, A.; Sodano, H.; Pintauro, P.; Moghaddam, S. Proton selective ionic graphene-based membrane for high concentration direct methanol fuel cells. J. Membr. Sci. 2014, 467, 217–225. [Google Scholar] [CrossRef]
- Lin, C.W.; Lu, Y.S. Highly ordered graphene oxide paper laminated with a Nafion membrane for direct methanol fuel cells. J. Power Sources 2013, 237, 187–194. [Google Scholar] [CrossRef]
- Choi, B.G.; Huh, Y.S.; Park, Y.C.; Jung, D.H.; Hong, W.H.; Park, H. Enhanced transport properties in polymer electrolyte composite membranes with graphene oxide sheets. Carbon 2012, 50, 5395–5402. [Google Scholar] [CrossRef]
- Kumar, R.; Xu, C.; Scott, K. Graphite oxide/Nafion composite membranes for polymer electrolyte fuel cells. RSC Adv. 2012, 2, 8777–8782. [Google Scholar] [CrossRef]
- Liao, G.M.; Yang, C.C.; Hu, C.C.; Pai, Y.L.; Lue, S.J. Novel quaternized polyvinyl alcohol/quaternized chitosan nano-composite as an effective hydroxide-conducting electrolyte. J. Membr. Sci. 2015, 485, 17–29. [Google Scholar] [CrossRef]
- Peckham, T.J.; Holdcroft, S. Structure-morphology-property relationships of non-perfluorinated proton-conducting membranes. Adv. Mater. 2010, 22, 4667–4690. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Lin, H.K.; Liu, N.Y.; Mahesh, K.P.O.; Lue, S.J. Cell performance modeling of direct methanol fuel cells using proton-exchange solid electrolytes: Effective reactant diffusion coefficients in porous diffusion layers. J. Power Sources 2013, 227, 275–283. [Google Scholar] [CrossRef]
- Guo, H.; Wyart, Y.; Perot, J.; Nauleau, F.; Moulin, P. Magnetic nanoparticles for UF membrane integrity: Industrial scale. Membr. Water Treat. 2011, 2, 1–11. [Google Scholar]
- Xiong, Y.; Fang, J.; Zeng, Q.H.; Liu, Q.L. Preparation and characterization of cross-linked quaternized poly(vinyl alcohol) membranes for anion exchange membrane fuel cells. J. Membr. Sci. 2008, 311, 319–325. [Google Scholar] [CrossRef]
- Lue, S.J.; Wang, W.T.; Mahesh, K.P.O.; Yang, C.C. Enhanced performance of a direct methanol alkaline fuel cell (DMAFC) using a polyvinyl alcohol/fumed silica/KOH electrolyte. J. Power Sources 2010, 195, 7991–7999. [Google Scholar] [CrossRef]
- Lue, S.J.; Pan, W.H.; Chang, C.M.; Liu, Y.L. High-performance direct methanol alkaline fuel cells using potassium hydroxide-impregnated polyvinyl alcohol/carbon nano-tube electrolytes. J. Power Sources 2012, 202, 1–10. [Google Scholar] [CrossRef]
- Dragana, I.M.P.; Zugic, L.; Nikolic, V.M.; Maslovara, S.L.; Kaninski, M.P.M. Enhanced performance of the solid alkaline fuel cell using PVA-KOH membrane. Int. J. Electrochem. Sci. 2013, 8, 949–957. [Google Scholar]
- Fu, J.; Qiao, J.; Lv, H.; Ma, J.; Yuan, X.Z.; Wang, H. Alkali doped poly(vinyl alcohol) (PVA) for anion-exchange membrane fuel cells: Ionic conductivity, chemical stability and FT-IR characterizations. ECS Trans. 2010, 25, 15–23. [Google Scholar]
- Qiao, J.; Fu, J.; Lin, R.; Ma, J.; Liu, J. Alkaline solid polymer electrolyte membranes based on structurally modified PVA/PVP with improved alkali stability. Polymer 2010, 51, 4850–4859. [Google Scholar] [CrossRef]
- Li, H.Y.; Chang, C.M.; Hsu, K.Y.; Liu, Y.L. Poly(lactide)-functionalized and Fe3O4 nanoparticle-decorated multiwalled carbon nanotubes for preparation of electrically-conductive and magnetic poly(lactide) films and electrospun nanofibers. J. Mater. Chem. 2012, 22, 4855. [Google Scholar] [CrossRef]
- Shen, X.; Jiang, L.; Ji, Z.; Wu, J.; Zhou, H.; Zhu, G. Stable aqueous dispersions of graphene prepared with hexamethylenetetramine as a reductant. J. Colloid Interface Sci. 2011, 354, 493–497. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, M.E.; Patel, U.S.; Ranade, M.; Palumbo, P.S. p-Maleimidophenylisocyanate: A novel heterobifunctional linker for hydroxyl to thiol coupling. Bioconjugate Chem. 1993, 4, 212–218. [Google Scholar] [CrossRef]
- Lue, S.J.; Chien, C.F.; Mahesh, K.P.O. Pervaporative concentration of ethanol–water mixtures using heterogeneous polydimethylsiloxane (PDMS) mixed matrix membranes. J. Membr. Sci. 2011, 384, 17–26. [Google Scholar] [CrossRef]
- Lue, S.J.; Liaw, T.-H. Separation of xylene mixtures using polyurethane-zeolite composite membranes. Desalination 2006, 193, 137–143. [Google Scholar] [CrossRef]
- Lue, S.J.; Shih, T.S.; Wei, T.C. Plasma modification on a Nafion membrane for direct methanol fuel cell applications. Korean J. Chem. Eng. 2006, 23, 441–446. [Google Scholar] [CrossRef]
- Cheng, K.W.; Huang, C.M.; Pan, G.T.; Chang, W.S.; Lee, T.C.; Yang, T.C.K. The physical properties and photoresponse of AgIn5S8 polycrystalline film electrodes fabricated by chemical bath deposition. J. Photochem. Photobiol. A Chem. 2007, 190, 77–87. [Google Scholar] [CrossRef]
- Wang, B.Y.; Tseng, C.K.; Shih, C.M.; Pai, Y.L.; Kuo, H.P.; Lue, S.J. Polytetrafluoroethylene (PTFE)/silane cross-linked sulfonated poly(styrene-ethylene/butylene-styrene) (sSEBS) composite membrane for direct alcohol and formic acid fuel cells. J. Membr. Sci. 2014, 464, 43–54. [Google Scholar] [CrossRef]

| Samples | Permeability | |
|---|---|---|
| at 30 °C (cm2·s−1) | at 60 °C (cm2·s−1) | |
| CL-QPVA | 1.62 × 10−7 | 1.36 × 10−6 |
| Unaligned CL-QPVA/GO-Fe3O4 | 8.47 × 10−8 | 8.12 × 10−7 |
| Aligned CL-QPVA/GO-Fe3O4 | 6.89 × 10−8 | 2.96 × 10−7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-S.; Ma, W.-T.; Shih, C.-M.; Yu, B.-C.; Teng, L.-W.; Wang, Y.-C.; Cheng, K.-W.; Chiu, F.-C.; Lue, S.J. Reorientation of Magnetic Graphene Oxide Nanosheets in Crosslinked Quaternized Polyvinyl Alcohol as Effective Solid Electrolyte. Energies 2016, 9, 1003. https://doi.org/10.3390/en9121003
Lin J-S, Ma W-T, Shih C-M, Yu B-C, Teng L-W, Wang Y-C, Cheng K-W, Chiu F-C, Lue SJ. Reorientation of Magnetic Graphene Oxide Nanosheets in Crosslinked Quaternized Polyvinyl Alcohol as Effective Solid Electrolyte. Energies. 2016; 9(12):1003. https://doi.org/10.3390/en9121003
Chicago/Turabian StyleLin, Jia-Shuin, Wei-Ting Ma, Chao-Ming Shih, Bor-Chern Yu, Li-Wei Teng, Yi-Chun Wang, Kong-Wei Cheng, Fang-Chyou Chiu, and Shingjiang Jessie Lue. 2016. "Reorientation of Magnetic Graphene Oxide Nanosheets in Crosslinked Quaternized Polyvinyl Alcohol as Effective Solid Electrolyte" Energies 9, no. 12: 1003. https://doi.org/10.3390/en9121003






