Systematic Study of Separators in Air-Breathing Flat-Plate Microbial Fuel Cells—Part 2: Numerical Modeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Parameters Estimation and Model Validation
| Parameter | Definition | Value | Unit |
|---|---|---|---|
| Exchange current density of oxygen reduction in the biofilm | 0.36 | A·m−2 | |
| Exchange current density of ethanol oxidation in the biofilm | 0.40 | A·m−2 | |
| Mass transfer coefficient of oxygen in the biofiom | 2.7 × 10−6 | m·s−1 | |
| Mass transfer coefficient of ethanol in the biofilm | 9.3 × 10−6 | m·s−1 | |
| Anodic charge transfer coefficient of ethanol oxidation | 7 × 10−2 | - | |
| Anodic charge transfer coefficient of oxygen reduction | 0.24 | - | |
| Cathodic charge transfer coefficient of ethanol oxidation | 3 × 10−3 | - | |
| Cathodic charge transfer coefficient of oxygen reduction | 0.11 | - |
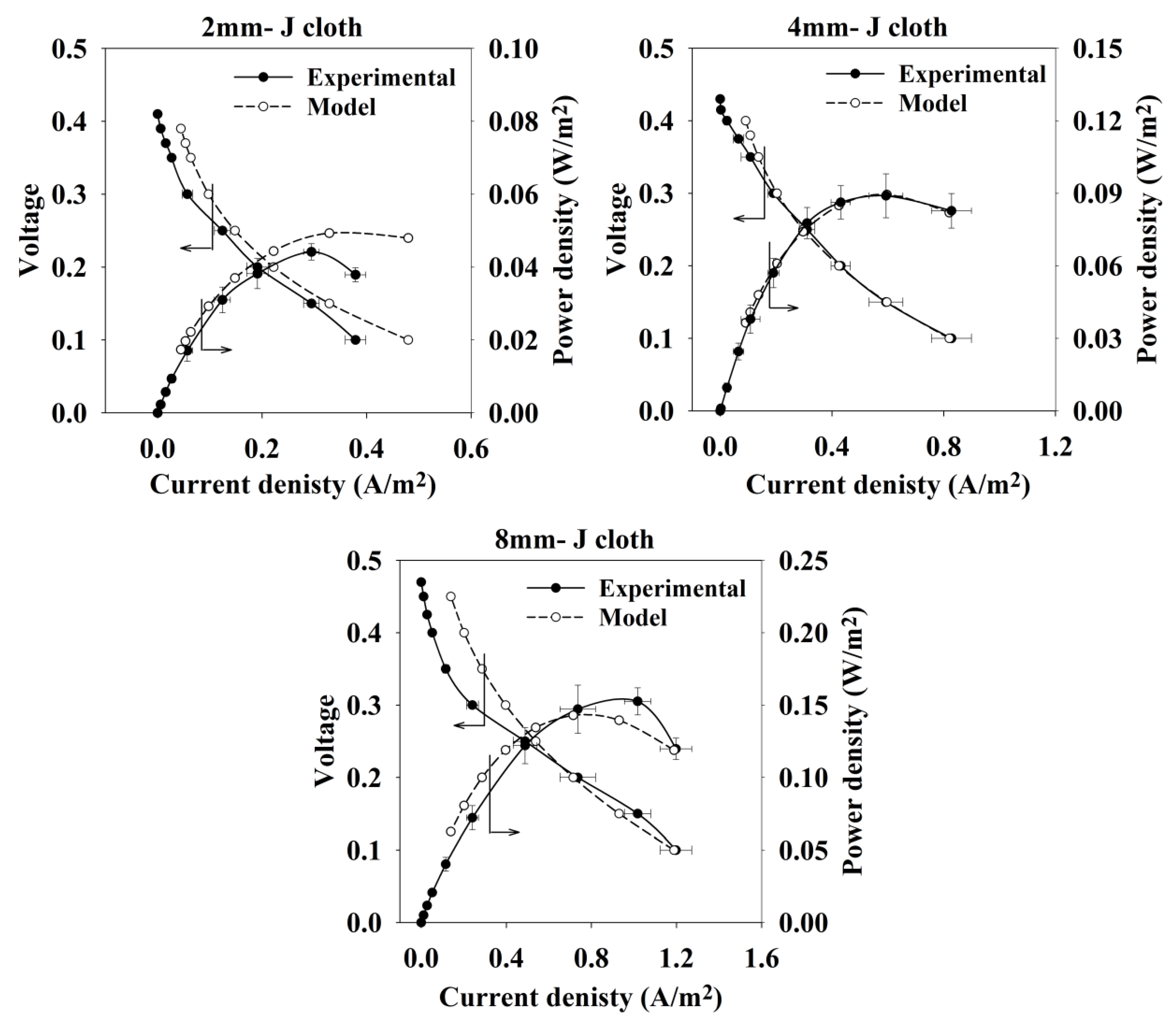

2.2. Sensitivity Analysis
2.2.1. Performance Sensitivity to Separator Characteristics
| Parameter/Value | Change in the Parameter | Change in the Superficial Peak Power Density | ||
|---|---|---|---|---|
| 2 mm | 4 mm | 8 mm | ||
| kO = 0.29 × 10−6 m·s−1 | −95% | +49% | +45% | +40% |
| +100% | −25% | −19% | −13% | |
| +1000% | −70% | −54% | −34% | |
| nH+ = 0.59 | −60% | −7% | −7% | −6% |
| +60% | +24% | +23% | +23% | |
| Parameter/Value | Change in the Parameter | Change in the Superficial Peak Power Density | ||
|---|---|---|---|---|
| 2 mm | 4 mm | 8 mm | ||
| kO = 9.5 × 10−6 m·s−1 | −95% | +286% | +121% | +48% |
| +100% | −10% | −5% | −2% | |
| nH+ = 0.78 | −20% | −10% | −9% | −8% |
| +20% | +31% | +25% | +20% | |
2.2.2. Performance Sensitivity to Electrode Spacing

3. Materials and Methods
3.1. Model Development

- The MFC system is similar to a chemical fuel cell, hence no terms are added to represent the growth and death rate of the microbial communities, the biomass generation, and the biofilm growth on the cathode, and the separator.
- The anode electrode is assumed to be covered by the bacteria. Hence, the ethanol oxidation and oxygen reduction reactions are catalyzed by the biofilm, and do not take place on the naked electrode.
- Transfer of electrons from the bacteria to the electrode is reversible and fast, therefore, considered as a Nernstian electrode process.
- Flow of ions through the biofilm is not limiting the electro-neutrality of the system, and the Ohmic loss is solely due to the distance between the surface of the anode and the separator.
- Complete ethanol oxidation and the oxygen reduction are the sole anodic and cathodic reactions occurring in the MFC. Hence, no by-products (e.g., acetate) are assumed to be generated, to simplify the model.
- Oxygen reduction by the bacteria is assumed to be an electrochemical reaction.
- The electrolyte is assumed to be stagnant, thus no effect of the electrolyte flow on the concentration profile is considered.
- The fuel (ethanol) is well-distributed within the 3D electrode, thus the concentration profile is neglected and no starvation is happening.
- The temperature variation within the anode chamber is negligible, hence the reactions are isothermal.
- The anode is well-buffered and there is no local pH drop within the biofilm. The temperature is also well-controlled at 303 K in both compartments.
- Due to the electro-activity of the 99% of the thickness of the 3D electrode [37], a uniform current and voltage distribution within the 3D electrode is assumed.
3.2. Parameter Estimation
| Separator | kO (×10−6 m·s−1) | kE (×10−6 m·s−1) | RS (×10−4 Ω·m2) | nH+ |
|---|---|---|---|---|
| Nafion®117 | 0.29 ± 0.02 | 0.49 ± 0.01 | 5.4 ± 0.1 | 0.59 ± 0.01 |
| Aquivion® | 0.77 ± 0.05 | 0.98 ± 0.01 | 0.8 ± 0.1 | 0.72 ± 0.01 |
| Celgard® | 1.2 ± 0.1 | 0.84 ± 0.01 | 4.4 ± 0.2 | 0.92 ± 0.01 |
| Zirfon® | 1.5 ± 0.1 | 0.58 ± 0.01 | 14 ± 0.4 | 0.92 ± 0.01 |
| Nylon mesh | 2.2 ± 0.1 | 2.2 ± 0.2 | 1.4 ± 0.1 | 0.89 ± 0.03 |
| Glass fiber filter | 0.87 ± 0.06 | 1.0 ± 0.1 | 7.8 ± 0.2 | 0.62 ± 0.02 |
| SciMat® | 2.6 ± 0.1 | 1.9 ± 0.1 | 3.1 ± 0.1 | 0.66 ± 0.03 |
| J-cloth | 9.5 ± 0.6 | 33 ± 3 | 6.2 ± 0.2 | 0.78 ± 0.02 |
| Symbol | Definition | Value | Unit |
|---|---|---|---|
| R | Universal gas constant | 8.314 | J·mol−1·K−1 |
| T | Temperature | 303 | K |
| F | Faraday constant | 96485.3 | C·mol−1 |
| Number of electrons transferred in ethanol full oxidation | 12 | - | |
| Number of electrons transferred in oxygen full reduction | 4 | - | |
| Outlet pH | 7 | - | |
| Inlet pH | 8.5 | - | |
| Exchange current density of oxygen reduction on Pt | 0.015 | A·m−2 | |
| Exchange current density of ethanol oxidation on Pt | 0.003 | A·m−2 | |
| Mass transfer coefficient of oxygen in the air cathode | 2.7 × 10−5 | m·s−1 | |
| Diffusion coefficient of oxygen in the electrolyte | 2 × 10−9 | m2·s−1 | |
| Ethanol concentration in the inlet stream | 0.085 | M | |
| Standard half-cell potential of ethanol oxidation | 0.084 | V vs. SHE | |
| Standard half-cell potential of oxygen reduction | 0.401 | V vs. SHE | |
| Partial pressure of oxygen in the air | 0.21 | atm | |
| Oxygen concentration at the cathode | 8.3 | mol.m−3 | |
| Ionic conductivity of the synthetic wastewater | 0.5 | S·m−1 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Abbreviation | Definition |
| MFC | Microbial Fuel Cell |
| FPMFC | Flat-Plate Microbial Fuel Cell |
| DMFC | Direct Methanol Fuel Cell |
| CE | Coulombic Efficiency |
| COD | Chemical Oxygen Demand |
| SQP | Sequential Quadratic Programming |
| SHE | Standard Hydrogen Electrode |
| 3D | 3 Dimensional |
| Symbol | Definition | Unit |
| R | Universal Gas Constant | J·mol−1·K−1 |
| T | Temperature | K |
| F | Faraday Constant | C·mol−1 |
| d | Electrode Spacing | m |
| Y | Separator thickness | m |
| Proton Transport Number of the Separator | - | |
| Conductivity of Wastewater | S·m−1 | |
| Ionic Resistivity of the Separator | Ω m2 | |
| Ionic Conductivity of the Separator | S·m−1 | |
| Cell Voltage | V | |
| Ohmic Overpotential | V | |
| E | Operating Electrode Potential | V vs. SHE |
| Cathode Potential | V vs. SHE | |
| Anode Potential | V vs. SHE | |
| Potential in the Solution Phase | V vs. SHE | |
| Standard Half-Cell Potential of the Reaction at 298 K | V vs. SHE | |
| Equilibrium Potential of the Reduction Reaction | V vs. SHE | |
| Standard half-cell Potential of Ethanol Oxidation at 298 K | V vs. SHE | |
| Equilibrium Potential of Ethanol Oxidation at the Anode | V vs. SHE | |
| Equilibrium Potential of Ethanol Oxidation at the Cathode | V vs. SHE | |
| Standard half-cell Potential of Oxygen Reduction at 298 K | V vs. SHE | |
| Equilibrium Potential of Oxygen Reduction at the Anode | V vs. SHE | |
| Equilibrium Potential of Oxygen Reduction at the Cathode | V vs. SHE | |
| n | Number of Electrons Exchanged in the redox Reaction | - |
| Number of Electrons Exchanged in Ethanol Oxidation | - | |
| Number of Electrons Exchanged in Oxygen Reduction | - | |
| Oxygen Concentration at the anode | M | |
| Oxygen Partial Pressure at the Cathode | atm | |
| Protons Concentration at the Anode | M | |
| Protons Concentration at the Cathode | M | |
| Hydroxyls Concentration at the Anode | M | |
| Hydroxyls Concentration at the Cathode | M | |
| Cathode Overpotential | V | |
| Anode Overpotential | V | |
| Overpotential of Ethanol Oxidation at the Anode | V | |
| Overpotential of Ethanol Oxidation at the Cathode | V | |
| Overpotential of Oxygen Reduction at the Anode | V | |
| Overpotential of Oxygen Reduction at the Cathode | V | |
| j | Local Faradic Current Density | A·m−2 |
| J′ | Measured Current Density | A·m−2 |
| J | Predicted Current Density | A·m−2 |
| Current Density of Ethanol Oxidation at the Cathode | A·m−2 | |
| Current Density of Ethanol Oxidation at the Anode | A·m−2 | |
| Current Density of Oxygen Reduction at the Cathode | A·m−2 | |
| Current Density of Oxygen Reduction at the Anode | A·m−2 | |
| Limiting Current Density of Ethanol Oxidation at the Anode | A·m−2 | |
| Kinetically Controlled Current Density of Ethanol Oxidation at the Anode | A·m−2 | |
| Limiting Current Density of Ethanol Oxidation at the Cathode | A·m−2 | |
| Kinetically Controlled Current Density of Ethanol Oxidation at the Cathode | A·m−2 | |
| Limiting Current Density of Oxygen Reduction at the Anode | A·m−2 | |
| Kinetically Controlled Current Density of Oxygen Reduction at the Anode | A·m−2 | |
| Limiting Current Density of Oxygen Reduction at the Cathode | A·m−2 | |
| Kinetically Controlled Current Density of Oxygen Reduction at the Cathode | A·m−2 | |
| Exchange Current Density | A·m−2 | |
| Exchange Current Density of Ethanol Oxidation in the biofilm | A·m−2 | |
| Exchange Current Density of Oxygen Reduction in the biofilm | A·m−2 | |
| Exchange Current Density of Ethanol Oxidation on Pt | A·m−2 | |
| Exchange Current Density of Oxygen Reduction on Pt | A·m−2 | |
| Diffusion Coefficient of Oxygen in the Separator | m2·s−1 | |
| Diffusion Coefficient of Ethanol in the Separator | m2·s−1 | |
| Mass Transfer Coefficient of Oxygen in the Separator | m2·s−1 | |
| Mass Transfer Coefficient of Ethanol in the Separator | m2·s−1 | |
| Effective Mass Transfer Coefficient of Ethanol at the Anode | m·s−1 | |
| Effective Mass Transfer Coefficient of Oxygen at the Cathode | m·s−1 | |
| Effective Mass Transfer Coefficient of Ethanol at the Anode | m·s−1 | |
| Effective Mass Transfer Coefficient of Oxygen at the Cathode | m·s−1 | |
| Mass Transfer Coefficient of Ethanol in the Biofilm | m·s−1 | |
| Mass Transfer Coefficient of Oxygen in the Biofilm | m·s−1 | |
| Diffusion Coefficient of Oxygen in the Electrolyte | m·s−1 | |
| Mass Transfer Coefficient of Oxygen in the Cathode | m·s−1 | |
| α | Electron Transfer Coefficient | - |
| Anodic Charge Transfer Coefficient of Ethanol Oxidation at the Anode | - | |
| Anodic Charge Transfer Coefficient of Ethanol Oxidation at the Cathode | - | |
| Cathodic Charge Transfer Coefficient of Ethanol Oxidation at the Anode | - | |
| Cathodic Charge Transfer Coefficient of Ethanol Oxidation at the Cathode | - | |
| Objective Function | - |
References
- Kim, B.; Chang, I.; Gadd, G. Challenges in microbial fuel cell development and operation. Appl. Microbiol. Biotechnol. 2007, 76, 485–494. [Google Scholar] [PubMed]
- Jung, R.K.; Cheng, S.; Oh, S.-E.; Logan, B.E. Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ. Sci. Technol. 2007, 41, 1004–1009. [Google Scholar]
- Rozendal, R.A.; Hamelers, H.V.M.; Rabaey, K.; Keller, J.; Buisman, C.J.N. Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol. 2008, 26, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.H.; Rabaey, K.; Aelterman, P.; Clauwaert, P.; de Schamphelaire, L.; Boon, N.; Verstraete, W. Microbial fuel cells in relation to conventional anaerobic digestion technology. Eng. Life Sci. 2006, 6, 285–292. [Google Scholar] [CrossRef]
- Aiyuk, S.; Forrez, I.; Lieven, D.K.; van Haandel, A.; Verstraete, W. Anaerobic and complementary treatment of domestic sewage in regions with hot climates—A review. Bioresour. Technol. 2006, 97, 2225–2241. [Google Scholar] [CrossRef] [PubMed]
- Kho, B.K.; Bae, B.; Scibioh, M.A.; Lee, J.; Ha, H.Y. On the consequences of methanol crossover in passive air-breathing direct methanol fuel cells. J. Power Sources 2005, 142, 50–55. [Google Scholar] [CrossRef]
- Saarinen, V.; Himanen, O.; Kallio, T.; Sundholm, G.; Kontturi, K. A 3D model for the free-breathing direct methanol fuel cell: Methanol crossover aspects and validations with current distribution measurements. J. Power Sources 2007, 172, 805–815. [Google Scholar] [CrossRef]
- Chen, R.; Zhao, T.S.; Liu, J.G. Effect of cell orientation on the performance of passive direct methanol fuel cells. J. Power Sources 2006, 157, 351–357. [Google Scholar] [CrossRef]
- Shimizu, T.; Momma, T.; Mohamedi, M.; Osaka, T.; Sarangapani, S. Design and fabrication of pumpless small direct methanol fuel cells for portable applications. J. Power Sources 2004, 137, 277–283. [Google Scholar] [CrossRef]
- Zhao, T.S.; Chen, R.; Yang, W.W.; Xu, C. Small direct methanol fuel cells with passive supply of reactants. J. Power Sources 2009, 191, 185–202. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous electricity generation from domestic wastewater and organic substrates in a flat plate microbial fuel cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, H.; Liang, P.; Huang, X.; Chen, X.; Logan, B.E. Air-cathode structure optimization in separator-coupled microbial fuel cells. Biosens. Bioelectron. 2011, 30, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Hu, H.; Liu, H. Enhanced Coulombic efficiency and power density of air-cathode microbial fuel cells with an improved cell configuration. J. Power Sources 2007, 171, 348–354. [Google Scholar] [CrossRef]
- Ortiz-Martínez, V.M.; Salar-García, M.J.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Egea, J.A.; Lozano, L.J. Developments in microbial fuel cell modeling. Chem. Eng. J. 2015, 271, 50–60. [Google Scholar] [CrossRef]
- Marcus, A.K.; Torres, C.I.; Rittmann, B.E. Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol. Bioeng. 2007, 98, 1171–1182. [Google Scholar] [CrossRef] [PubMed]
- Picioreanu, C.; van Loosdrecht, M.C.M.; Katuri, K.P.; Scott, K.; Head, I.M. Mathematical model for microbial fuel cells with anodic biofilms and anaerobic digestion. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2008, 57, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Picioreanu, C.; Head, I.M.; Katuri, K.P.; van Loosdrecht, M.C.M.; Scott, K. A computational model for biofilm-based microbial fuel cells. Water Res. 2007, 41, 2921–2940. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Halme, A. Modelling of a microbial fuel cell process. Biotechnol. Lett. 1995, 17, 809–814. [Google Scholar] [CrossRef]
- Sedaqatvand, R.; Esfahany, M.N.; Behzad, T.; Mohseni, M.; Mardanpour, M.M. Parameter estimation and characterization of a single-chamber microbial fuel cell for dairy wastewater treatment. Bioresour. Technol. 2013, 146, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Merkey, B.V.; Chopp, D.L. The performance of a microbial fuel cell depends strongly on anode geometry: A multidimensional modeling study. Bull. Math. Biol. 2012, 74, 834–857. [Google Scholar] [CrossRef] [PubMed]
- Sirinutsomboon, B. Modeling of a membraneless single-chamber microbial fuel cell with molasses as an energy source. Int. J. Energy Environ. Eng. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Oliveir, V.B.; Simões, M.; Melo, L.F.; Pinto, A.M.F.R. A 1D mathematical model for a microbial fuel cell. Energy 2013, 61, 463–471. [Google Scholar] [CrossRef]
- Zeng, Y.; Choob, Y.F.; Kim, B.; Wu, P. Modelling and simulation of two-chamber microbial fuel cell. J. Power Sources 2010, 195, 79–89. [Google Scholar] [CrossRef]
- Kazemi, S.; Fatih, M.M.K. Systematic study of separators in air-breathing flat-plate microbial fuel cells—Part 1: Structure, properties, and performance correlations. Energies 2016. [Google Scholar] [CrossRef]
- Pons, L.; Délia, M.-L.; Bergel, A. Effect of surface roughness, biofilm coverage and biofilm structure on the electrochemical efficiency of microbial cathodes. Bioresour. Technol. 2011, 102, 2678–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, C.; Basseguy, R.; Bergel, A. Microbial electrocatalysis with geobactersulfurreducens biofilm on stainless steel cathodes. Electrochim. Acta 2008, 53, 2494–2500. [Google Scholar] [CrossRef] [Green Version]
- Erable, B.; Etcheverry, L.; Bergel, A. Increased power from a two-chamber microbial fuel cell with a low-pH air-cathode compartment. Electrochem. Commun. 2009, 11, 619–622. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.L.; Lowy, D.A.; Baumann, R.G.; Tender, L.M. Influence of anode pretreatment on its microbial colonization. J. Appl. Microbiol. 2007, 102, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Mohan, S.V. Change in electrogenic activity of the microbial fuel cell (MFC) with the function of biocathode microenvironment as terminal electron accepting condition: Influence on overpotentials and bio-electro kinetics. Bioresour. Technol. 2012, 119, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ter Heijne, A.; Hamelers, H.V.M.; de Wilde, V.; Rozendal, R.A.; Buisman, C.J.N. A bipolar membrane combined with ferric iron reduction as an efficient cathode system in microbial fuel cells. Environ. Sci. Technol. 2006, 40, 5200–5205. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Min, B.; Logan, B.E. Cathode performance as a factor in electricity generation in microbial fuel cells. Environ. Sci. Technol. 2004, 38, 4900–4904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Cheng, S.; Huang, X.; Logan, B.E. The use of nylon and glass fiber filter separators with different pore sizes in air-cathode single-chamber microbial fuel cells. Energy Environ. Sci. 2010, 3, 659–664. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, S.; Liang, P.; Huang, X.; Logan, B.E. Scalable air cathode microbial fuel cells using glass fiber separators, plastic mesh supporters, and graphite fiber brush anodes. Bioresour. Technol. 2011, 102, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Recent development of non-platinum catalysts for oxygen reduction reaction. J. Power Sources 2005, 152, 1–15. [Google Scholar] [CrossRef]
- Wang, J.X.; Markovic, N.M.; Adzic, R.R. Kinetic analysis of oxygen reduction on Pt(111) in acid solutions: intrinsic kinetic parameters and anion adsorption effects. J. Phys. Chem. B 2004, 108, 4127–4133. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Kazemi, S.; Fatih, K.; Mohseni, M. Improved performance of a passive air breathing flat-plate microbial fuel cell. Can. J. Chem. Eng. 2015, 93, 479–485. [Google Scholar] [CrossRef]
- Harnisch, F.; Schröder, U.; Scholz, F. The suitability of monopolar and bipolar ion exchange membranes as separators for biological fuel cells. Environ. Sci. Technol. 2008, 42, 1740–1746. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazemi, S.; Barazandegan, M.; Mohseni, M.; Fatih, K. Systematic Study of Separators in Air-Breathing Flat-Plate Microbial Fuel Cells—Part 2: Numerical Modeling. Energies 2016, 9, 79. https://doi.org/10.3390/en9020079
Kazemi S, Barazandegan M, Mohseni M, Fatih K. Systematic Study of Separators in Air-Breathing Flat-Plate Microbial Fuel Cells—Part 2: Numerical Modeling. Energies. 2016; 9(2):79. https://doi.org/10.3390/en9020079
Chicago/Turabian StyleKazemi, Sona, Melissa Barazandegan, Madjid Mohseni, and Khalid Fatih. 2016. "Systematic Study of Separators in Air-Breathing Flat-Plate Microbial Fuel Cells—Part 2: Numerical Modeling" Energies 9, no. 2: 79. https://doi.org/10.3390/en9020079





