Optimization of Internal Cooling Fins for Metal Hydride Reactors
Abstract
:1. Introduction
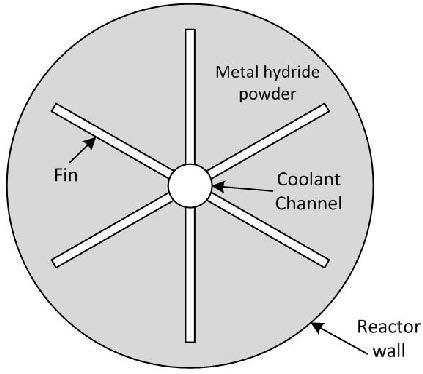
2. The Metal Hydride Reactor Model
2.1. Hydriding Process
2.2. Heat Generation Model
3. Fin Optimization
3.1. Optimization of Aspect Ratio
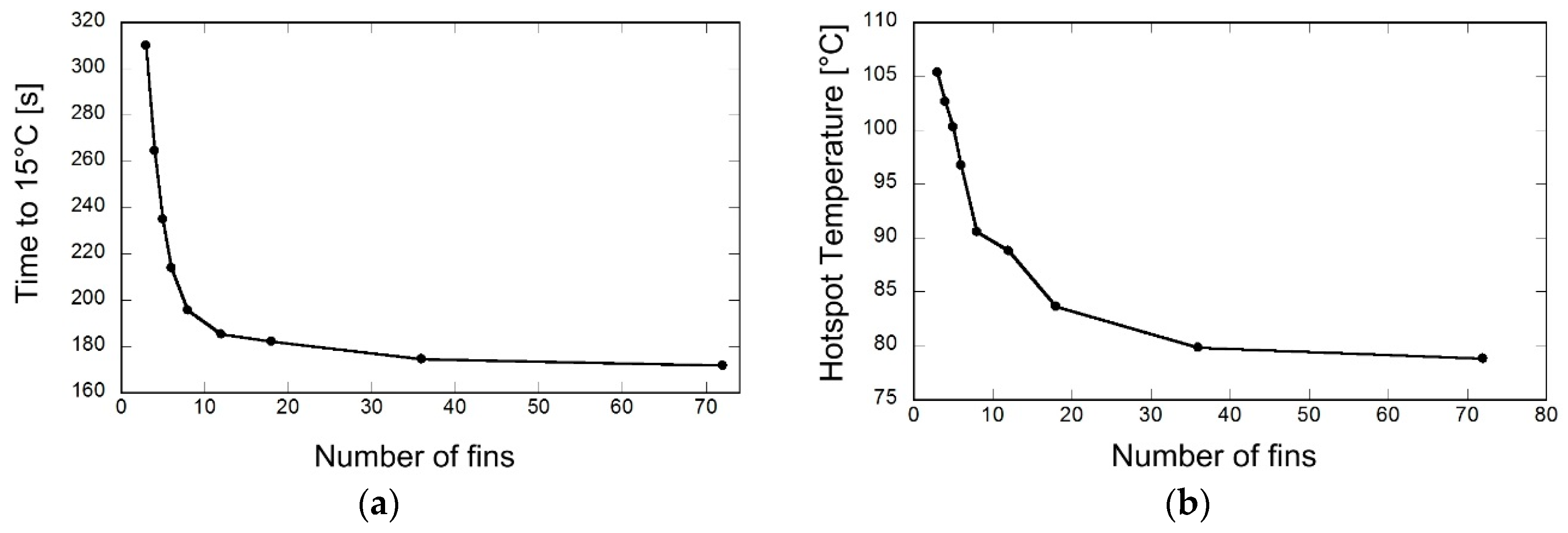
3.2. Optimization of Number of Fins
3.3. External Convective Cooling
4. Conclusions and End Remarks
- (1)
- The time required for the model to reach 15 °C for case (i) was measured at 397 s at fin aspect ratio of 0.0605, while case (ii), which incorporated external convection cooling, improved upon this time with a value of 195 s at a fin aspect ratio of 0.075. The simulation study also validates that there is an optimal aspect ratio, which must be considered in the design specifications in order to acquire substantial results in heat transfer performance.
- (2)
- Observations of the maximum hotspot temperature within the reactor revealed that case (i) generated a high value at 88 °C, while in case (ii) the external cooling convection model lowered this reading to 80 °C, thereby lowering overall temperatures within the reactor chamber itself.
- (3)
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RE | renewable energy |
| AR | aspect ratio |
| Nomenclature | |
| c | concentration of the hydrogen, mol·m−3 |
| cp | specific heat of metal hydride, J·kg−1·K−1 |
| Ca | constant |
| Ea | activation energy, J·mol−1 |
| Ha | activation enthalpy, eV |
| k | thermal conductivity, W·m−1·K−1 |
| kb | Boltzmann constant, eV·K−1 |
| m | mass, kg |
| pin | hydrogen supply pressure, Pa |
| R | reaction rate, mol·m−3·s−1 |
| Ru | universal gas constant, J·mol−1·K−1 |
| t | time, s |
| T | Temperature, K |
| Greek Letters | |
| ε | porosity |
| density, kg·m−3 | |
| Subscripts | |
| 0 | initial state |
| eq | equilibrium |
| g | hydrogen gas |
| m | metal hydride powder |
| sat | staturation |
References
- Leighty, W. Alaska village survival: Affordable energy independence via renewables firmed as hydrogen storage in liquid anhydrous ammonia. In Proceeding of NHA Conference and Hydrogen Expo 2009, Columbia, SC, USA, 30 March–2 April 2009.
- Isherwood, W.; Smith, J.R.; Aceves, S.M.; Berry, G.; Clark, W.; Johnson, R.; Das, D.; Goering, D.; Seifert, R. Remote power systems with advanced storage technologies for Alaskan villages. Energies 2000, 25, 1005–1020. [Google Scholar] [CrossRef]
- Rosen, M.A. The prospects for renewable energy through hydrogen energy systems. J. Power Energy Eng. 2015, 3, 373–377. [Google Scholar] [CrossRef]
- Gahleitner, G. Hydrogen from renewable electricity: An international review of power-to-gas pilot plants for stationary applications. Int. J. Hydrog. Energy 2013, 38, 2039–2061. [Google Scholar] [CrossRef]
- Dodds, P.E.; Staffell, I.; Hawkes, A.D.; Li, F.; Grünewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrog. Energy 2015, 40, 2065–2083. [Google Scholar] [CrossRef]
- Corgnale, C.; Hardy, B.J.; Tamburello, D.A.; Garrison, S.L.; Anton, D.L. Acceptability envelope for metal hydride-based hydrogen storage systems. Int. J. Hydrog. Energy 2012, 37, 2812–2824. [Google Scholar] [CrossRef]
- Nyamsi, S.N.; Yang, F.; Zhang, Z. An optimization study on the finned tube heat exchanger used in hydride hydrogen storage system—Analytical method and numerical simulation. Int. J. Hydrog. Energy 2012, 37, 16078–16092. [Google Scholar] [CrossRef]
- Souahlia, A.; Dhaou, H.; Askri, F.; Sofiene, M.; Jemni, A.; Ben Nasrallah, S. Experimental and comparative study of metal hydride hydrogen tanks. Int. J. Hydrog. Energy 2011, 36, 12918–12922. [Google Scholar] [CrossRef]
- Souahlia, A.; Dhaou, H.; Mellouli, S.; Askri, F.; Jemni, A.; Ben Nasrallah, S. Experimental study of metal hydride-based hydrogen storage tank at constant supply pressure. Int. J. Hydrog. Energy 2014, 39, 7365–7372. [Google Scholar] [CrossRef]
- Garrison, S.L.; Hardy, B.J.; Gorbounov, M.B.; Tamburello, D.A.; Corgnale, C.; Vanhassel, B.A.; Mosher, D.A.; Anton, D.L. Optimization of internal heat exchangers for hydrogen storage tanks utilizing metal hydrides. Int. J. Hydrog. Energy 2012, 37, 2850–2861. [Google Scholar] [CrossRef]
- Ronnebro, E.C.E.; Whyatt, G.; Powell, M.; Westman, M.; Zheng, F.; Fang, Z.Z. Metal hydrides for high-temperature power generation. Energies 2015, 8, 8406–8430. [Google Scholar] [CrossRef]
- McWhorter, S.; O’Malley, K.; Adams, J.; Ordaz, G.; Randolph, K.; Stetson, N.T. Moderate temperature dense phase hydrogen storage materials within the US department of energy (DOE) H2 storage program: Trends toward future development. Crystals 2012, 2, 413–445. [Google Scholar] [CrossRef]
- Felderhoff, M.; Bogdanović, B. High temperature metal hydrides as heat storage materials for solar and related applications. Int. J. Mol. Sci. 2009, 10, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Akiba, E.; Nomura, K.; Ono, S. Kinetics of the reaction between Mg-23.3%Ni eutectic alloy and hydrogen. J. Less Common Met. 1983, 89, 145–150. [Google Scholar] [CrossRef]
- Muthukumar, P.; Maiya, M.P.; Murthy, S.S. Experiments on a metal hydride based hydrogen compressor. Int. J. Hydrog. Energy 2005, 30, 879–892. [Google Scholar] [CrossRef]
- Lloyd, G.; Kim, K.J.; Razani, A.; Feldman, K.T., Jr. Thermal conductivity of porous metal hydrides. J. Thermophys. Heat Transf. 1998, 12, 132–137. [Google Scholar] [CrossRef]
- Ron, M.; Bershadsky, E.; Josephy, Y. Thermal conductivity of PMH compacts, measurements and evaluation. Int. J. Hydrog. Energy 1992, 17, 623–630. [Google Scholar] [CrossRef]
- Congdon, J.W. Metal Hydride Composition and Method of Making. U.S. Patent 1995. [Google Scholar]
- Dieterich, M.; Pohlmann, C.; Bürger, I.; Linder, M.; Röntzsch, L. Long-term cycle stability of metal hydride-graphite composites. Int. J. Hydrog. Energy 2015, 40, 16375–16382. [Google Scholar] [CrossRef]
- Kim, K.J.; Montoya, B.; Razani, A.; Lee, K.-H. Metal hydride compacts of improved thermal conductivity. Int. J. Hydrog. Energy 2001, 26, 609–613. [Google Scholar] [CrossRef]
- Klein, H.P.; Groll, M. Heat transfer characteristics of expanded graphite matrices in metal hydride beds. Int. J. Hydrog. Energy 2004, 29, 1503–1511. [Google Scholar] [CrossRef]
- Oi, T.; Maki, K.; Sakaki, Y. Heat transfer characteristics of the metal hydride vessel based on the plate-fin type heat exchanger. J. Power Sources 2004, 125, 52–61. [Google Scholar] [CrossRef]
- Raju, M.; Kumar, S. Optimization of heat exchanger designs in metal hydride based hydrogen storage systems. Int. J. Hydrog. Energy 2012, 37, 2767–2778. [Google Scholar] [CrossRef]
- Sekhar, B.S.; Lotoskyy, M.; Kolesnikov, A.; Moropeng, M.; Tarasov, B.; Pollet, B. Performance analysis of cylindrical metal hydride beds with various heat exchange options. J. Alloys Compd. 2015, 645, S89–S95. [Google Scholar] [CrossRef]
- Majer, G.; Kaess, U.; Bowman, R.C., Jr. Nucler magnetic resonance studies of hydrogen diffusion in LaNi5H6 and LaNi4.8Sn0.2H5.8. Phys. Rev. B 1998, 57, 13599–13603. [Google Scholar] [CrossRef]
- Inomata, A.; Aoki, H.; Miura, T. Measurement and modelling of hydriding and dehydriding kinetics. J. Alloys Compd. 1998, 278, 103–109. [Google Scholar] [CrossRef]
- COMSOL multiphysics modeling software. For more information about COMSOL multiphysics. Availabe online: https://www.comsol.com/ (accessed on 14 March 2016).








© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kukkapalli, V.K.; Kim, S. Optimization of Internal Cooling Fins for Metal Hydride Reactors. Energies 2016, 9, 447. https://doi.org/10.3390/en9060447
Kukkapalli VK, Kim S. Optimization of Internal Cooling Fins for Metal Hydride Reactors. Energies. 2016; 9(6):447. https://doi.org/10.3390/en9060447
Chicago/Turabian StyleKukkapalli, Vamsi Krishna, and Sunwoo Kim. 2016. "Optimization of Internal Cooling Fins for Metal Hydride Reactors" Energies 9, no. 6: 447. https://doi.org/10.3390/en9060447