Enhanced Electrocatalytic Activity for Water Splitting on NiO/Ni/Carbon Fiber Paper
Abstract
:1. Introduction
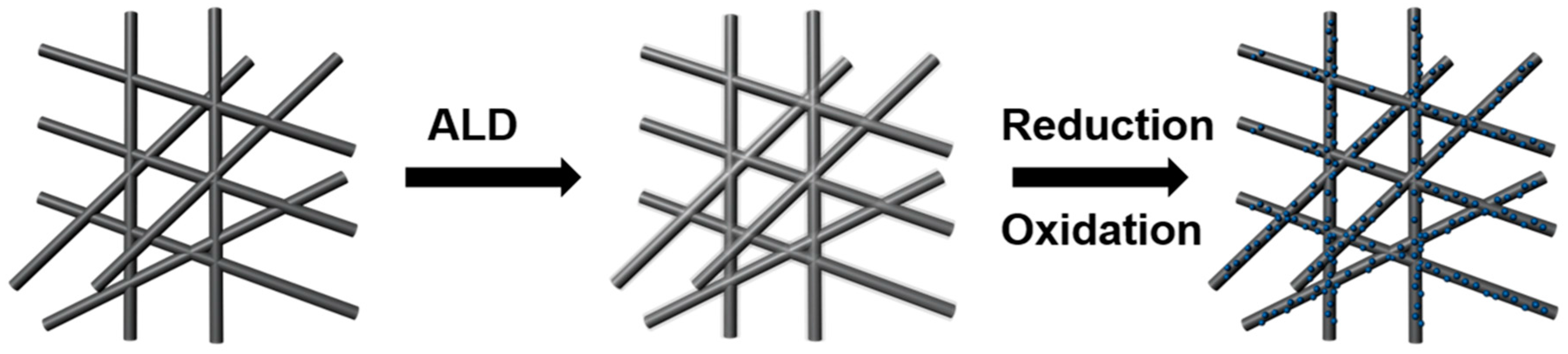
2. Experiment
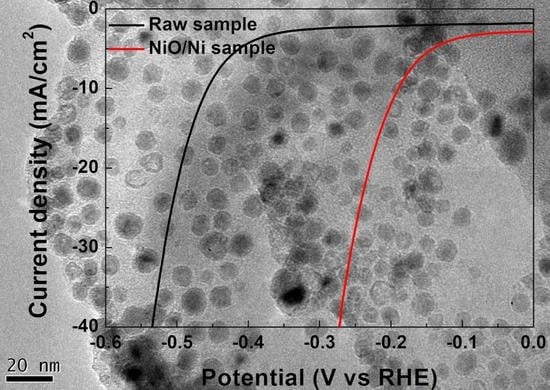
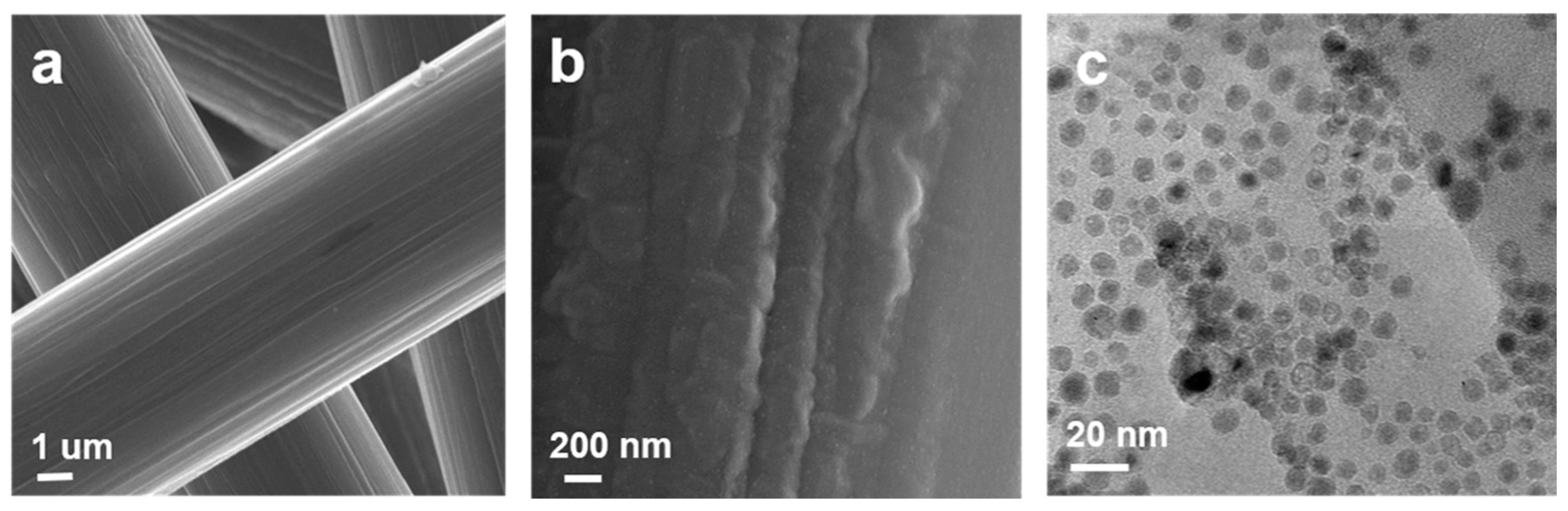
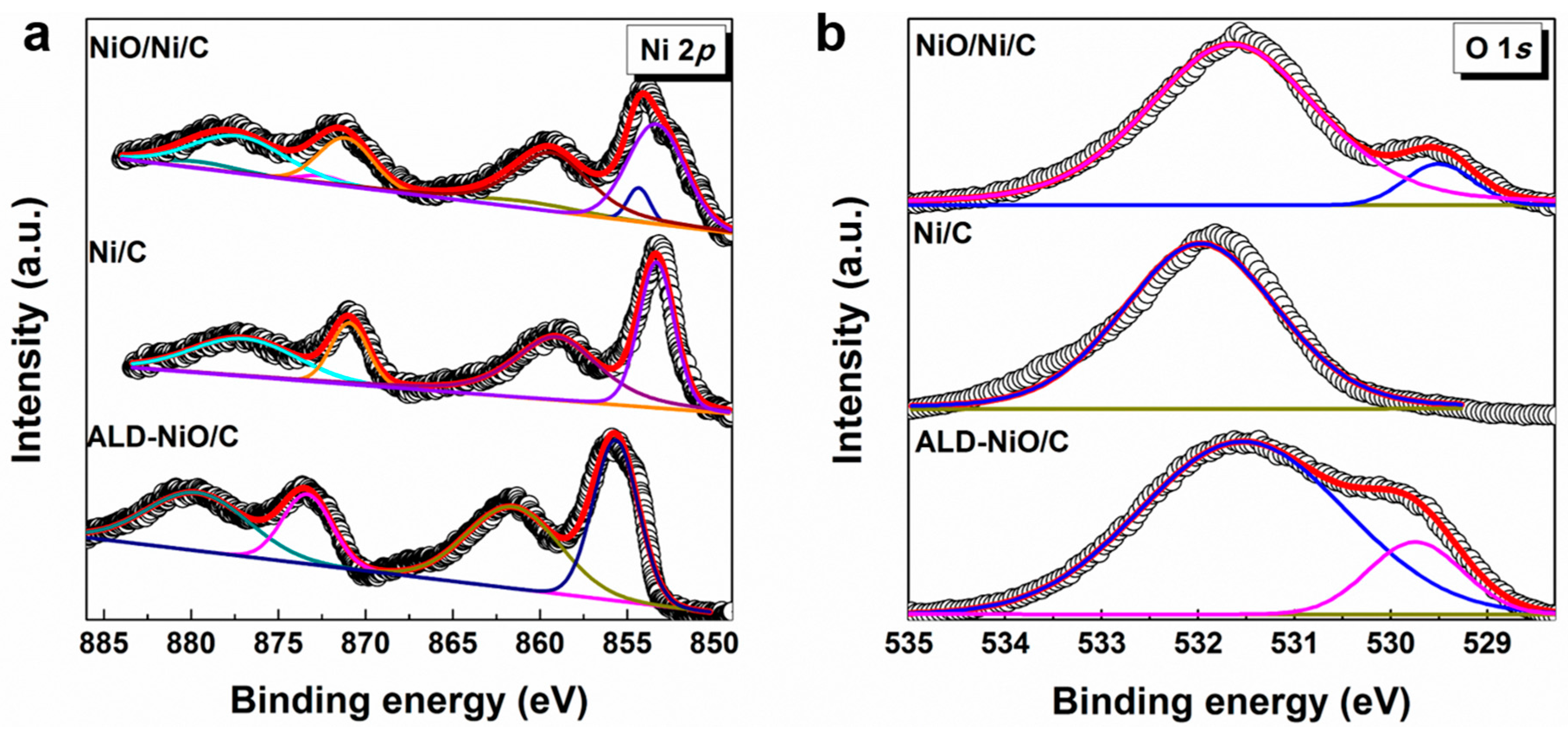
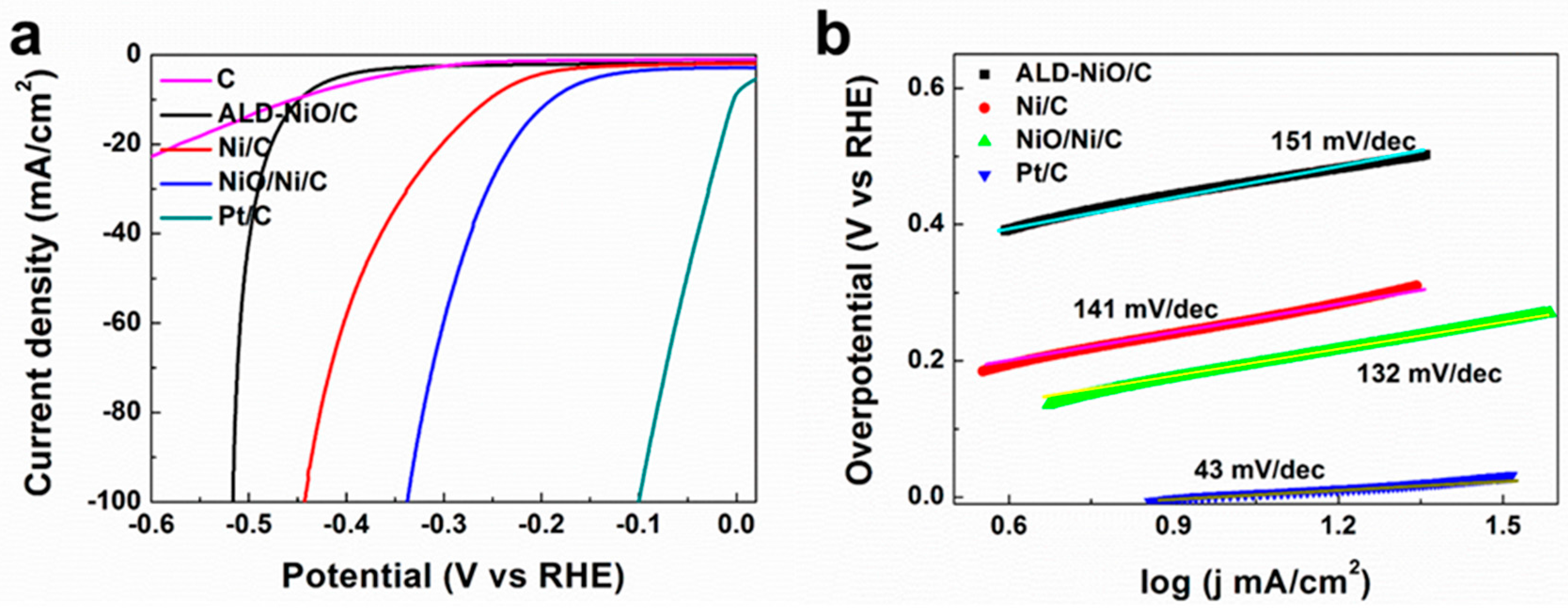
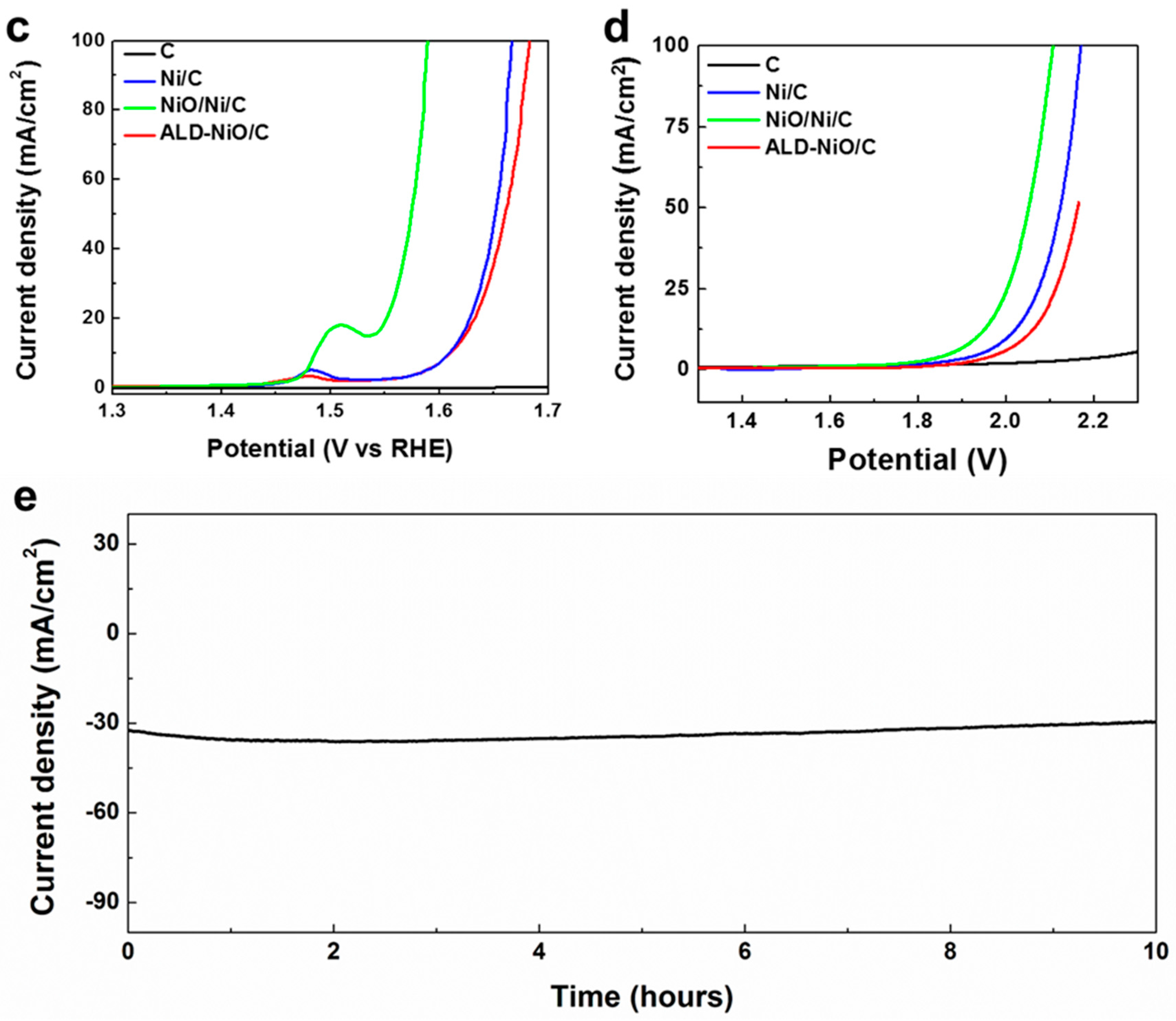
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.J.; Loh, K.P.; Zhang, H. Photoanode current of large-area MoS2 ultrathin nanosheets with vertically mesh-shaped structure on indium tin oxide. Nat. Chem. 2016, 5, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Kibsgaard, J.; Chen, Z.B.; Reinecke, B.N.; Jaramillo, T.F. Engineering the surface structure of MoS2 to preferentially expose active edge sites for electrocatalysis. Nat. Mater. 2012, 11, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Lukowski, M.A.; Daniel, A.S.; Meng, F.; Forticaux, A.; Li, L.S.; Jin, S. Enhanced hydrogen evolution catalysis from chemically exfoliated metallic MoS2 nanosheets. J. Am. Chem. Soc. 2013, 135, 10274–10277. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.F.; Zhang, H.; Li, S.; Wang, R.X.; Sun, X.; Zhou, M.; Zhou, J.F.; Lou, X.W.; Xie, Y. Defect-rich MoS2 ultrathin nanosheets with additional active edge sites for enhanced electrocatalytic hydrogen evolution. Adv. Mater. 2013, 25, 5807–5813. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Wang, H.T.; Lu, Z.Y.; Cui, Y. CoSe2 nanoparticles grown on carbon fiber paper: An efficient and stable electrocatalyst for hydrogen evolution reaction. J. Am. Chem. Soc. 2014, 136, 4897–4900. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Dong, X.L.; Wang, Y.G.; Xia, Y.Y. Separating hydrogen and oxygen evolution in alkaline water electrolysis using nickel hydroxide. Nat. Commun. 2016, 7, 11741. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Wang, H.T.; Zhou, J.G.; Wang, J.; Duchesne, P.N.; Muir, D.; Zhang, P.; Han, N.; Zhao, F.P.; Zeng, M.; et al. Highly active and durable methanol oxidation electrocatalyst based on the synergy of platinum-nickel hydroxide-graphene. Nat. Commun. 2015, 6, 10035. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Zhou, W.; Tsai, M.C.; Zhou, J.G.; Guan, M.Y.; Lin, M.C.; Zhang, B.; Hu, Y.F.; Wang, D.Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.T.; Lee, H.W.; Deng, Y.; Lu, Z.Y.; Hsu, P.C.; Liu, Y.Y.; Lin, D.C.; Cui, Y. Bifunctional non-noble metal oxide nanoparticle electrocatalysts through lithium-induced conversion for overall water splitting. Nat. Commun. 2015, 6, 7261. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Wang, D.Y.; Chen, C.C.; Hwang, B.J.; Dai, H.J. A mini review on nickel-based electrocatalysts for alkaline hydrogen evolution reaction. Nano Res. 2016, 9, 28–46. [Google Scholar] [CrossRef]
- You, B.; Sun, Y.J. Hierarchically porous nickel sulfide multifunctional superstructures. Adv. Energy Mater. 2016, 6, 1502333. [Google Scholar] [CrossRef]
- Fan, L.L.; Liu, P.F.; Yan, X.C.; Gu, L.; Yang, Z.Z.; Yang, H.G.; Qiu, S.L.; Yao, X.D. Nickel nanoparticles coated with graphene layers as efficient co-catalyst for photocatalytic hydrogen evolution. Nat. Commun. 2016, 7, 10667. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Feng, G.; Li, P.S.; Bi, Y.M.; Li, Y.P.; Sun, M.X. Single-crystalline ultrathin nickel nanosheets array from in situ topotactic reduction for active and stable electrocatalysis. Angew. Chew. Int. Ed. 2016, 55, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Nardi, K.L.; Yang, N.Y.; Dickens, C.F.; Strickler, A.L.; Bent, S.F. Creating highly active atomic layer deposited NiO electrocatalysts for the oxygen evolution reaction. Adv. Energy Mater. 2015, 5, 1500412. [Google Scholar] [CrossRef]
- Lin, T.D.; Li, X.L.; Jang, J. High performance p-type NiOx thin-film transistor by Sn doping. Appl. Phys. Lett. 2016, 108, 233503. [Google Scholar] [CrossRef]
- Kim, S.I.; Lee, J.S.; Ahn, H.J.; Song, J.K.; Jang, J.H. Facile route to an efficient NiO supercapacitor with a three-dimensional nanonetwork morphology. ACS Appl. Mater. Interfaces 2013, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ke, X.K.; Bao, J.H.; Wang, C.L.; Dong, L.; Chen, Y.W.; Chen, H.L. Synthesis of flower-like NiO and effects of morphology on its catalytic properties. J. Phys. Chem. C 2009, 113, 14440–14447. [Google Scholar] [CrossRef]
- Moralesguio, C.G.; Thorwarth, K.; Niesen, B.; Liardet, L.; Patscheider, J.; Ballif, C.; Hu, X.L. Solar hydrogen production by amorphous silicon photocathodes coated with a magnetron sputter deposited Mo2C catalyst. J. Am. Chem. Soc. 2015, 137, 7035–7038. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.W.; Bruller1, S.; Dong, R.H.; Zhang, J.; Feng, X.L.; Mullen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun. 2015, 6, 7992. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.B.; Xia, B.Y.; Yu, L.; Yu, X.Y.; Lou, X.W. Porous molybdenum carbide nano-octahedrons synthesized via confined carburization in metal-organic frameworks for efficient hydrogen production. Nat. Commun. 2015, 6, 6512. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.S.; Im, J.H.; Mayer, M.T.; Schreier, M.; Nazeeruddin, M.K.; Park, N.G.; Tilley, S.D.; Fan, H.J.; Grätzel, M. Water photolysis at 12.3% efficiency via perovskite photovoltaics and earth-abundant catalysts. Science 2014, 345, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.L.; Yu, G.T.; Wu, Y.Y.; Li, G.D.; LI, H.; Sun, Y.H.; Asefa, T.; Chen, W.; Zou, X.X. High-index faceted Ni3S2 nanosheet arrays as highly active and ultrastable electrocatalysts for water splitting. J. Am. Chem. Soc. 2015, 137, 14023–14026. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Cheng, N.Y.; Pu, Z.H.; Xing, W.; Sun, X.P. NiSe nanowire film supported on nickel foam: An efficient and stable 3D bifunctional electrode for full water splitting. Angew. Chem. Int. Ed. 2015, 54, 9351–9355. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.Q.; Liu, Q.; Asiri, A.M.; Sun, X.P. Self-supported nanoporous cobalt phosphide nanowire arrays: An efficient 3D hydrogen-evolving cathode over the wide range of pH 0–14. J. Am. Chem. Soc. 2014, 136, 7587–7590. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.X.; Huang, X.X.; Goswami, A.; Silva, R.; Sathe, B.R.; Mikmekov, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. Int. Ed. 2014, 53, 4372–4376. [Google Scholar] [CrossRef] [PubMed]
- Fei, H.L.; Yang, Y.; Peng, Z.W.; Ruan, G.D.; Zhong, Q.F.; Li, L.; Samuel, E.L.G.; Tour, J.M. Cobalt nanoparticles embedded in nitrogen-doped carbon for the hydrogen evolution reaction. ACS Appl. Mater. Interfaces 2015, 7, 8083–8087. [Google Scholar] [CrossRef] [PubMed]
- Juodkazis, K.; Juodkazytė, J.; Vilkauskaitė, R.; Jasulaitienė, V. Nickel surface anodic oxidation and electrocatalysis of oxygen evolution. J. Solid State Electrochem. 2008, 12, 1469–1479. [Google Scholar] [CrossRef]
- Xun, K.; Chen, P.Z.; Li, X.L.; Tong, Y.; Ding, H.; Wu, X.J.; Chu, W.S.; Peng, Z.M.; Wu, C.Z.; Xie, Y. Metallic nickel nitride nanosheets realizing enhanced electrochemical water oxidation. J. Am. Chem. Soc. 2015, 137, 4119–4125. [Google Scholar]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, R.; Wei, H.; Si, W.; Ou, G.; Zhao, C.; Song, M.; Zhang, C.; Wu, H. Enhanced Electrocatalytic Activity for Water Splitting on NiO/Ni/Carbon Fiber Paper. Materials 2017, 10, 15. https://doi.org/10.3390/ma10010015
Zhang R, Wei H, Si W, Ou G, Zhao C, Song M, Zhang C, Wu H. Enhanced Electrocatalytic Activity for Water Splitting on NiO/Ni/Carbon Fiber Paper. Materials. 2017; 10(1):15. https://doi.org/10.3390/ma10010015
Chicago/Turabian StyleZhang, Ruoyu, Hehe Wei, Wenjie Si, Gang Ou, Chunsong Zhao, Mingjun Song, Cheng Zhang, and Hui Wu. 2017. "Enhanced Electrocatalytic Activity for Water Splitting on NiO/Ni/Carbon Fiber Paper" Materials 10, no. 1: 15. https://doi.org/10.3390/ma10010015