Electrochemical Detection of Hydrogen Peroxide by Inhibiting the p-Benzenediboronic Acid-Triggered Assembly of Citrate-Capped Au/Ag Nanoparticles on Electrode Surface
Abstract
:1. Introduction
2. Results and Discussion
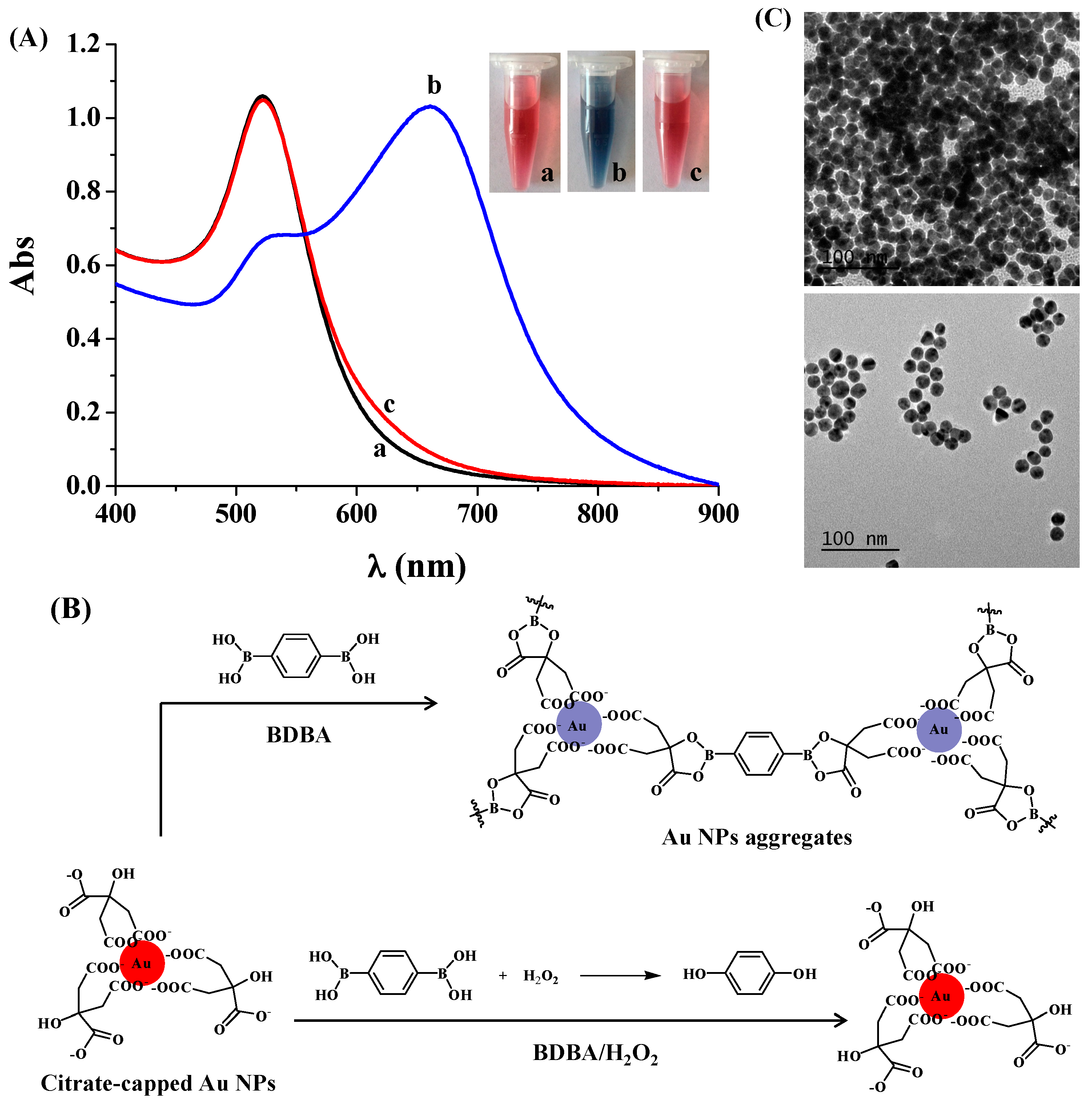
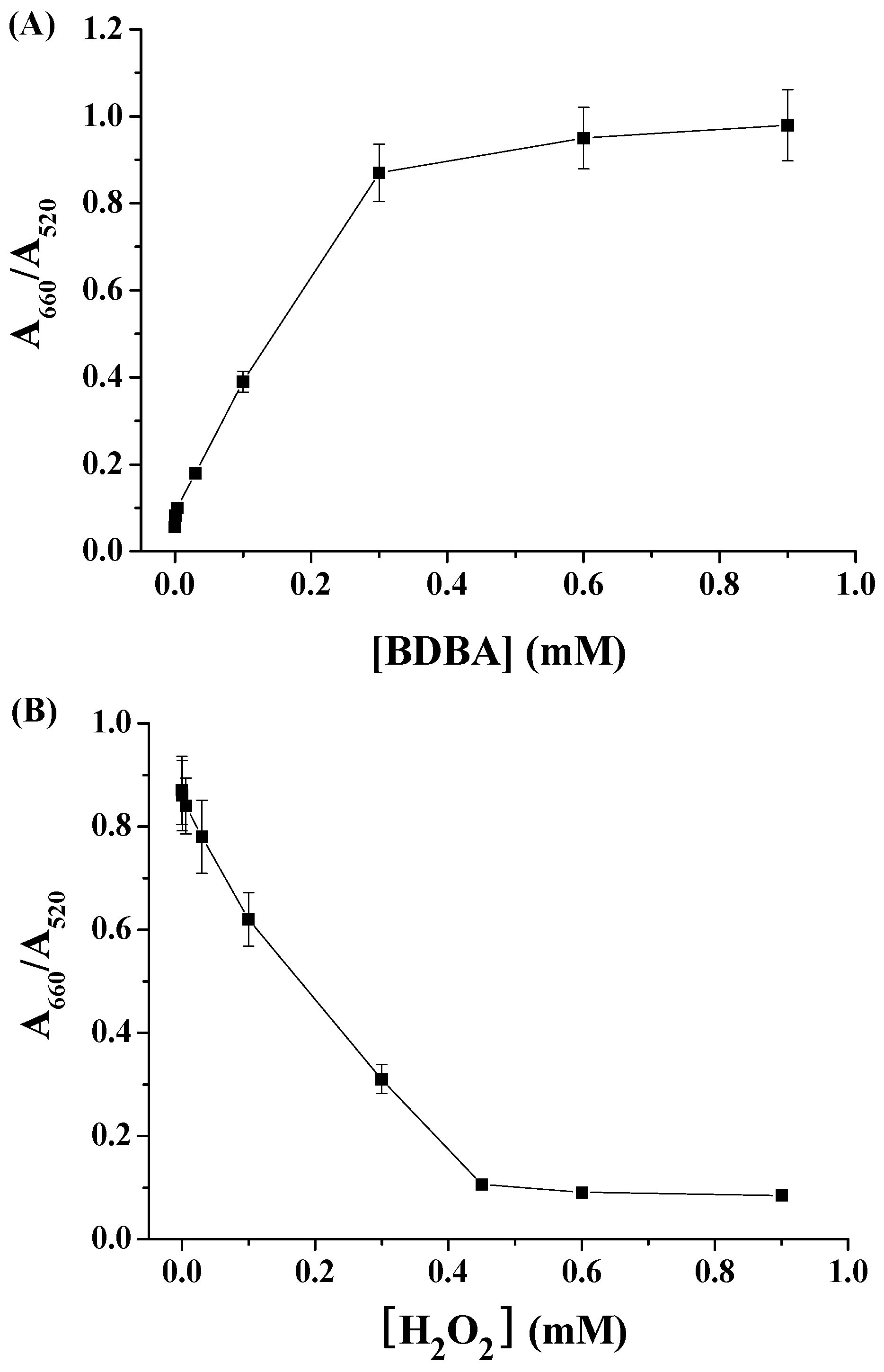
2.1. Colorimetric Assay of H2O2
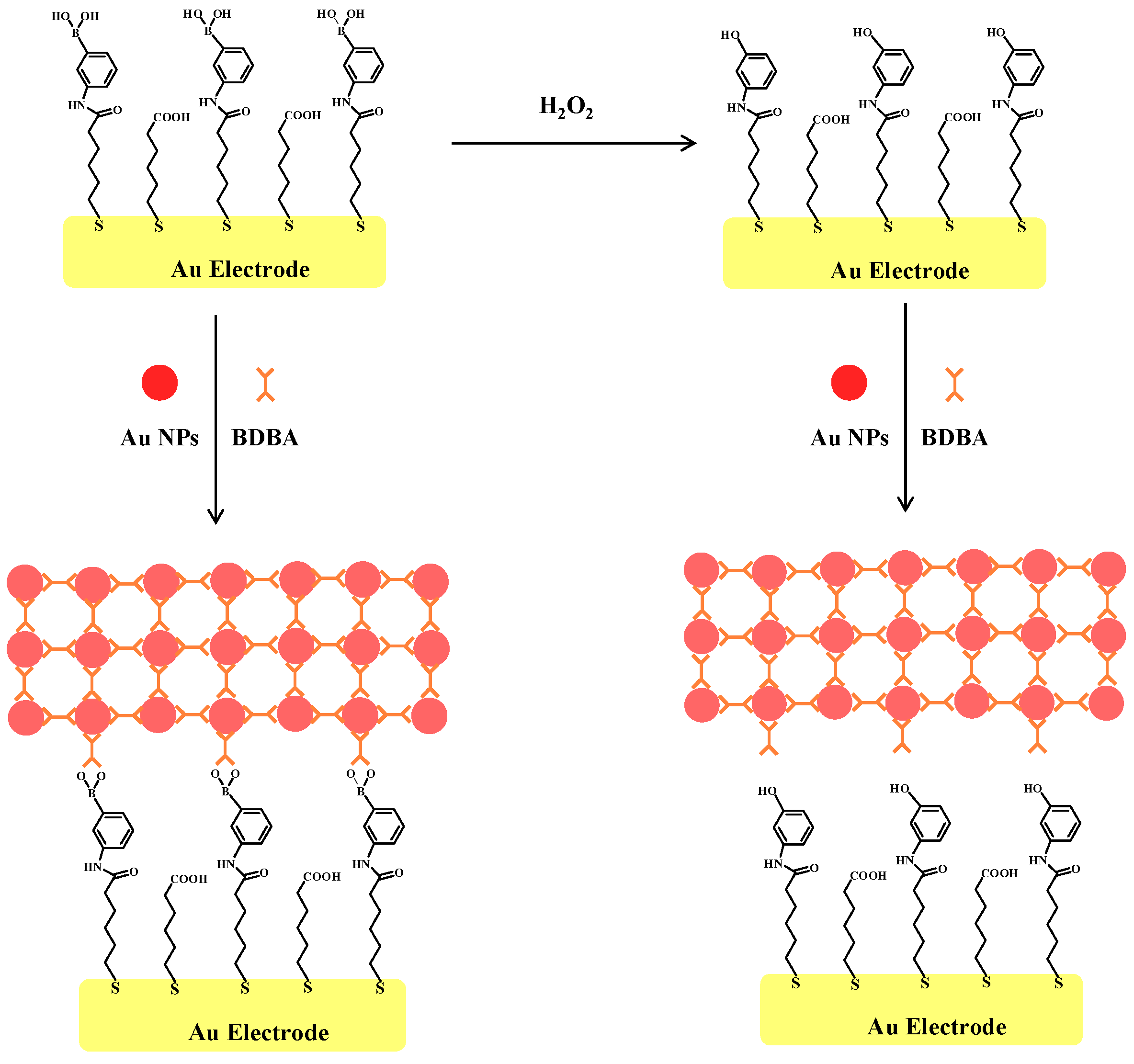
2.2. Principle of Electrochemical Assay of H2O2 by Au NPs
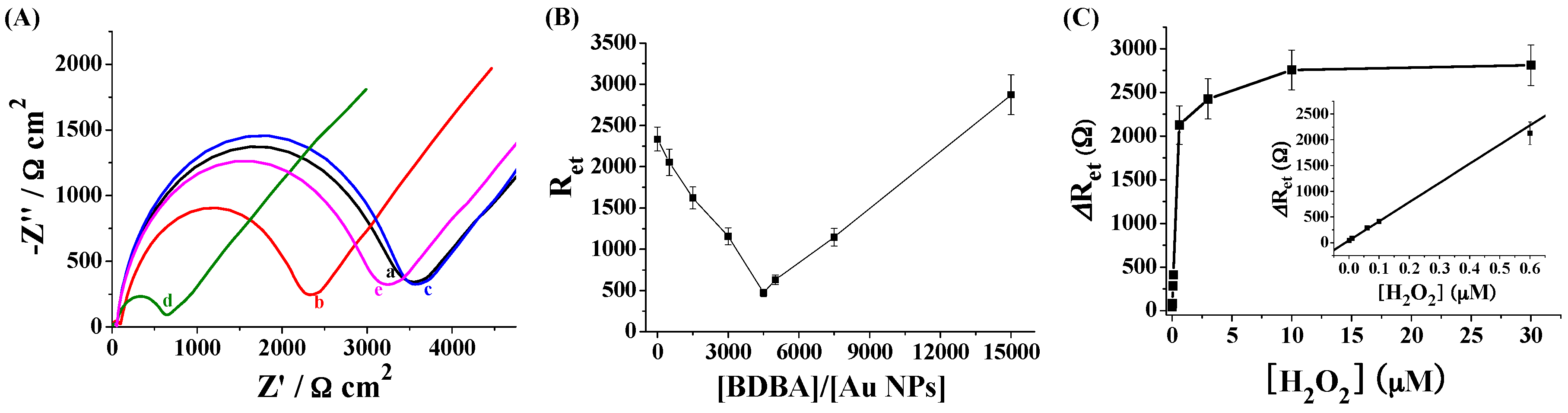
2.3. Electrochemical Detection of H2O2 Based on the Signal Amplification of Au NPs
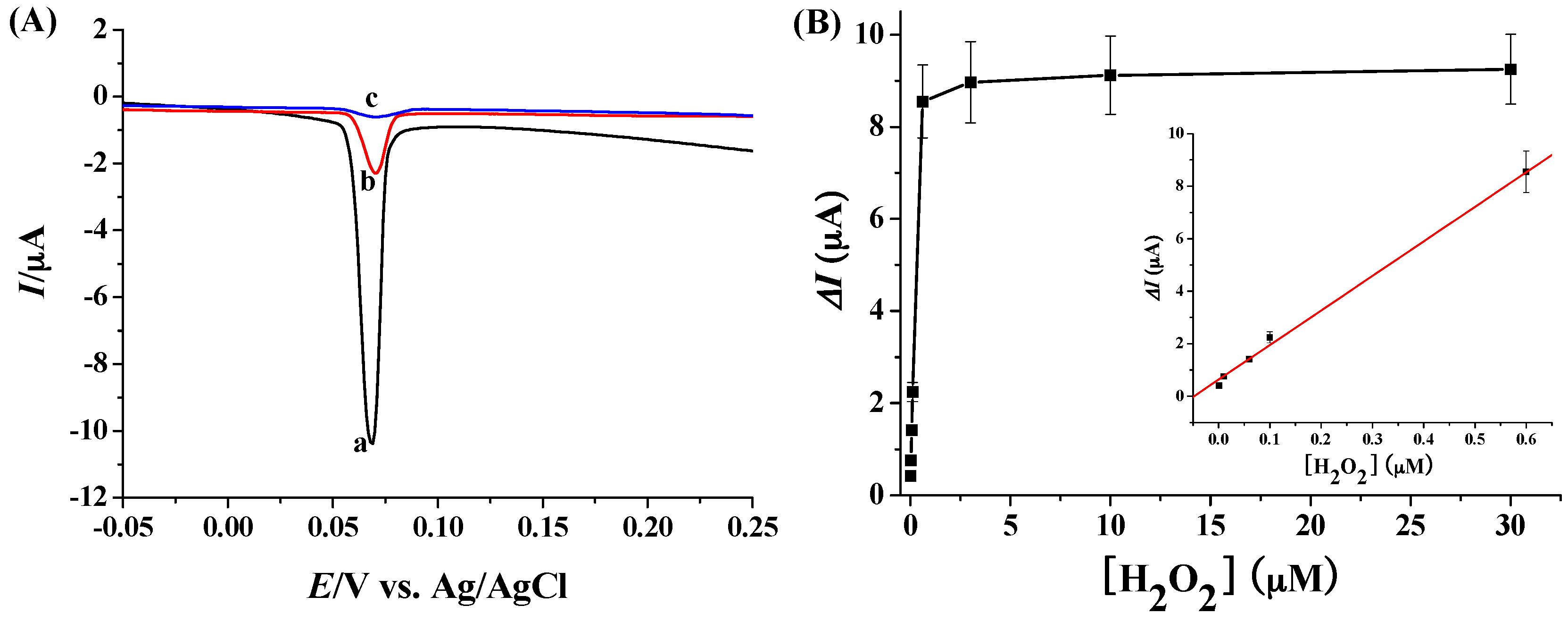
2.4. Electrochemical Detection of H2O2 with Ag NPs as the Redox Reporters
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instruments
3.3. Colorimetric Detection of H2O2
3.4. Electrochemical Detection of H2O2
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Ronkainen, N.J.; Okon, S.L. Nanomaterial-based electrochemical immunosensors for clinically significant biomarkers. Materials 2014, 7, 4669–4709. [Google Scholar] [CrossRef]
- Hayat, A.; Catanante, G.; Marty, J.L. Current trends in nanomaterial-based amperometric biosensors. Sensors 2014, 14, 23439–23461. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R. The use of gold nanoparticles in diagnostics and detection. Chem. Soc. Rev. 2008, 37, 2028–2045. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: Developing chemistries that facilitate nanotechnology. Chem. Rev. 2006, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene-gold nanoparticles hybrid-synthesis, functionalization, and application in a electrochemical and surface-enhanced Raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef]
- Bülbül, G.; Hayat, A.; Andreescu, S. Portable nanoparticle-based sensors for food safety assessment. Sensors 2015, 15, 30736–30758. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, F.; Ma, F.; Zhang, L.; Yang, S.; Xia, N. Electrochemical detection of β-amyloid peptides on electrode covered with N-terminus-specific antibody based on electrocatalytic O2 reduction by Aβ(1–16)-heme-modified gold nanoparticles. Biosens. Bioelectron. 2013, 49, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.D.; Jin, H.Z.; Guo, Z.R.; Ma, J.; Zhao, J.; Li, D.D.; Wu, H.; Gu, N. Silver nanoparticles outperform gold nanoparticles in radiosensitizing U251 cells in vitro and in an intracranial mouse model of glioma. Int. J. Nanomed. 2016, 11, 5003–5013. [Google Scholar] [CrossRef] [PubMed]
- McFarland, A.D.; Van Duyne, R.P. Single silver nanoparticles as real-time optical sensors with zeptomole sensitivity. Nano Lett. 2003, 3, 1057–1062. [Google Scholar] [CrossRef]
- Nam, J.-M.; Thaxton, C.S.; Mirkin, C.A. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science 2003, 301, 1884–1886. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, X.; Zhou, B.; Wu, Y.; Mao, W.; Liu, L. Electrochemical detection of amyloid-β oligomers based on the signal amplification of a network of silver nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 19303–19311. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Zhou, B.; Huang, N.; Jiang, M.; Zhang, J.; Liu, L. Visual and fluorescent assays for selective detection of beta-amyloid oligomers based on the inner filter effect of gold nanoparticles on the fluorescence of CdTe quantum dots. Biosens. Bioelectron. 2016, 85, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.C.; Ho, J.A.A. Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal. Chem. 2015, 87, 10362–10367. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Yu, H.; Hu, Y.; Chen, M.Z.; Shao, S.J. A gold nanoparticle-based fluorescence sensor for high sensitive and selective detection of thiols in living cells. Biosens. Bioelectron. 2016, 75, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Wang, X.; Wang, X.; Zhou, B. Gold nanoparticle-based colorimetric and electrochemical methods for dipeptidyl peptidase-IV activity assay and inhibitor screening. Materials 2016, 9, 857. [Google Scholar] [CrossRef]
- Wei, T.; Dong, T.; Wang, Z.; Bao, J.; Tu, W.; Dai, Z. Aggregation of individual sensing units for signal accumulation: Conversion of liquid-phase colorimetric assay into enhanced surface-tethered electrochemical analysis. J. Am. Chem. Soc. 2015, 137, 8880–8883. [Google Scholar] [CrossRef] [PubMed]
- Sobotta, M.C.; Liou, W.; Stocker, S.; Talwar, D.; Oehler, M.; Ruppert, T.; Scharf, A.N.; Dick, T.P. Peroxiredoxin-2 and STAT3 form a redox relay for H2O2 signaling. Nat. Chem. Biol. 2015, 11, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lippert, A.R.; Van de Bittner, G.C.; Chang, C. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc. Chem. Res. 2011, 44, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.; Kwon, B.; Han, E.; Park, M.; Yang, W.; Cho, W.; Yoo, W.; Khang, G.; Lee, D. Amplification of oxidative stress by a dual stimuli-responsive hybrid drug enhances cancer cell death. Nat. Commun. 2015, 6, 6907. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, X.; Qiu, W.; Zhang, M. In vivo monitoring of H2O2 with polydopamine and prussian blue-coated microelectrode. Anal. Chem. 2016, 88, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Wang, H.; Schultz, Z.D.; Camden, J.P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on surface-enhanced Raman spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hasebe, Y. Carbon felt-based bioelectrocatalytic flow-through detectors: 2,6-Dichlorophenol indophenol and peroxidase coadsorbed carbon-felt for flow-amperometric determination of hydrogen peroxide. Materials 2014, 7, 1142–1154. [Google Scholar] [CrossRef]
- De Gracia Lux, C.; Joshi-Barr, S.; Nguyen, T.; Mahmoud, E.; Schopf, E.; Fomina, N.; Almutairi, A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J. Am. Chem. Soc. 2012, 134, 15758–15764. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Zielonka, J.; Gapys, E.; Marcinek, A.; Joseph, J.; Kalyanaraman, B. Real-time measurements of amino acid and protein hydroperoxides using coumarin boronic acid. J. Biol. Chem. 2014, 289, 22536–22553. [Google Scholar] [CrossRef] [PubMed]
- Van de Bittner, G.C.; Dubikovskaya, E.A.; Bertozzi, C.R.; Chang, C.J. In vivo imaging of hydrogen peroxide production in a murine tumor model with a chemoselective bioluminescent reporter. Proc. Natl. Acad. Sci. USA 2010, 107, 21316–21321. [Google Scholar] [CrossRef] [PubMed]
- Webb, K.S.; Levy, D. A facile oxidation of boronic acids and boronic esters. Tetrahedron Lett. 1995, 36, 5117–5118. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, T.; Ji, Y.; Han, L.; Wu, Y.; Song, H.; Bai, L.; Ba, X. Selective modification of halloysite nanotubes with 1-pyrenylboronic acid: A novel fluorescence probe with highly selective and sensitive response to hyperoxide. ACS Appl. Mater. Interfaces 2015, 7, 23805–23811. [Google Scholar] [CrossRef] [PubMed]
- Miller, E.W.; Albers, A.E.; Pralle, A.; Isacoff, E.Y.; Chang, C.J. Boronate-based fluorescent probes for imaging cellular hydrogen peroxide. J. Am. Chem. Soc. 2005, 127, 16652–16659. [Google Scholar] [CrossRef] [PubMed]
- Manimala, J.C.; Wiskur, S.L.; Ellington, A.D.; Anslyn, E.V. Tuning the specificity of a synthetic receptor using a selected nucleic acid receptor. J. Am. Chem. Soc. 2004, 126, 16515–16519. [Google Scholar] [CrossRef] [PubMed]
- Bosch, L.I.; Fyles, T.M.; James, T.D. Binary and ternary phenylboronic acid complexes with saccharides and Lewis bases. Tetrahedron 2004, 60, 11175–11190. [Google Scholar] [CrossRef]
- Wiskur, S.L.; Lavigne, J.J.; Metzger, A.; Tobey, S.L.; Lynch, V.; Anslyn, E.V. Thermodynamic analysis of receptors based on guanidinium/boronic acid groups for the complexation of carboxylates, a-hydroxycarboxylates, and diols: Driving force for binding and cooperativity. Chem. Eur. J. 2004, 10, 3792–3804. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Tseng, W.-L. 1,4-Benzenediboronic-acid-induced aggregation of gold nanoparticles: Application to hydrogen peroxide detection and biotin-avidin-mediated immunoassay with naked-eye detection. Anal. Chem. 2016, 88, 5355–5362. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Wang, B.; Han, K.; Tang, Y. Electrochemical impedance spectroscopy study of proteolysis using unmodified gold nanoparticles. Electrochem. Commun. 2014, 47, 21–24. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.; Yin, L.; Liu, M.; Wang, Z.; Shu, Y.; Li, G. Enhanced charge transfer by gold nanoparticle at DNA modified electrode and its application to label-free DNA detection. ACS Appl. Mater. Interfaces 2014, 6, 7579–7584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, X.; Li, T.; Li, G. Self-assembled multilayer of gold nanoparticles for amplified electrochemical detection of cytochrome c. Analyst 2008, 133, 1242–1245. [Google Scholar] [CrossRef] [PubMed]
- Anzai, J. Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives. Mater. Sci. Eng. C-Mater. 2016, 67, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials 2014, 7, 1201–1220. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Liu, H.P.; Kang, X.J.; Liu, X.S.; Xue, C.; He, X.L. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Wu, J.; Wang, M.; Yan, F.; Ju, H. Triple signal amplification of graphene film, polybead carried gold nanoparticles as tracing tag and silver deposition for ultrasensitive electrochemical immunosensing. Anal. Chem. 2012, 84, 3662–3668. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Parent, K.L.; Buttry, D.A. Electrochemical solid-state phase transformations of silver nanoparticles. J. Am. Chem. Soc. 2012, 134, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, D.; Xing, Y.; Li, S.; Yuan, B.; Chen, J.; Xia, N. Activity analysis of the carbodiimide-mediated amine coupling reaction on self-assembled monolayers by cyclic voltammetry. Electrochim. Acta 2013, 89, 616–622. [Google Scholar] [CrossRef]
- Liu, S.; Wollenberger, U.; Halámek, J.; Leupold, E.; Stçcklein, W.; Warsinke, A.; Scheller, F.W. Affinity Interactions between phenylboronic acid-carrying self-assembled monolayers and flavin adenine dinucleotide or horseradish peroxidase. Chem. Eur. J. 2005, 11, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Yildiz, H.B.; Freeman, R.; Gill, R.; Willner, I. Electrochemical, photoelectrochemical, and piezoelectric analysis of tyrosinase activity by functionalized nanoparticles. Anal. Chem. 2008, 80, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Sun, T.; Ren, H. Electrochemical Detection of Hydrogen Peroxide by Inhibiting the p-Benzenediboronic Acid-Triggered Assembly of Citrate-Capped Au/Ag Nanoparticles on Electrode Surface. Materials 2017, 10, 40. https://doi.org/10.3390/ma10010040
Liu L, Sun T, Ren H. Electrochemical Detection of Hydrogen Peroxide by Inhibiting the p-Benzenediboronic Acid-Triggered Assembly of Citrate-Capped Au/Ag Nanoparticles on Electrode Surface. Materials. 2017; 10(1):40. https://doi.org/10.3390/ma10010040
Chicago/Turabian StyleLiu, Lin, Ting Sun, and Huizhu Ren. 2017. "Electrochemical Detection of Hydrogen Peroxide by Inhibiting the p-Benzenediboronic Acid-Triggered Assembly of Citrate-Capped Au/Ag Nanoparticles on Electrode Surface" Materials 10, no. 1: 40. https://doi.org/10.3390/ma10010040
APA StyleLiu, L., Sun, T., & Ren, H. (2017). Electrochemical Detection of Hydrogen Peroxide by Inhibiting the p-Benzenediboronic Acid-Triggered Assembly of Citrate-Capped Au/Ag Nanoparticles on Electrode Surface. Materials, 10(1), 40. https://doi.org/10.3390/ma10010040





