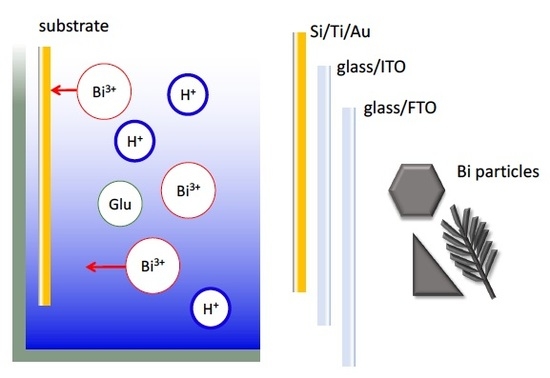
Electrochemical Synthesis of Bismuth Particles: Tuning Particle Shape through Substrate Type within a Narrow Potential Window
Abstract
:1. Introduction
2. Results and Discussion
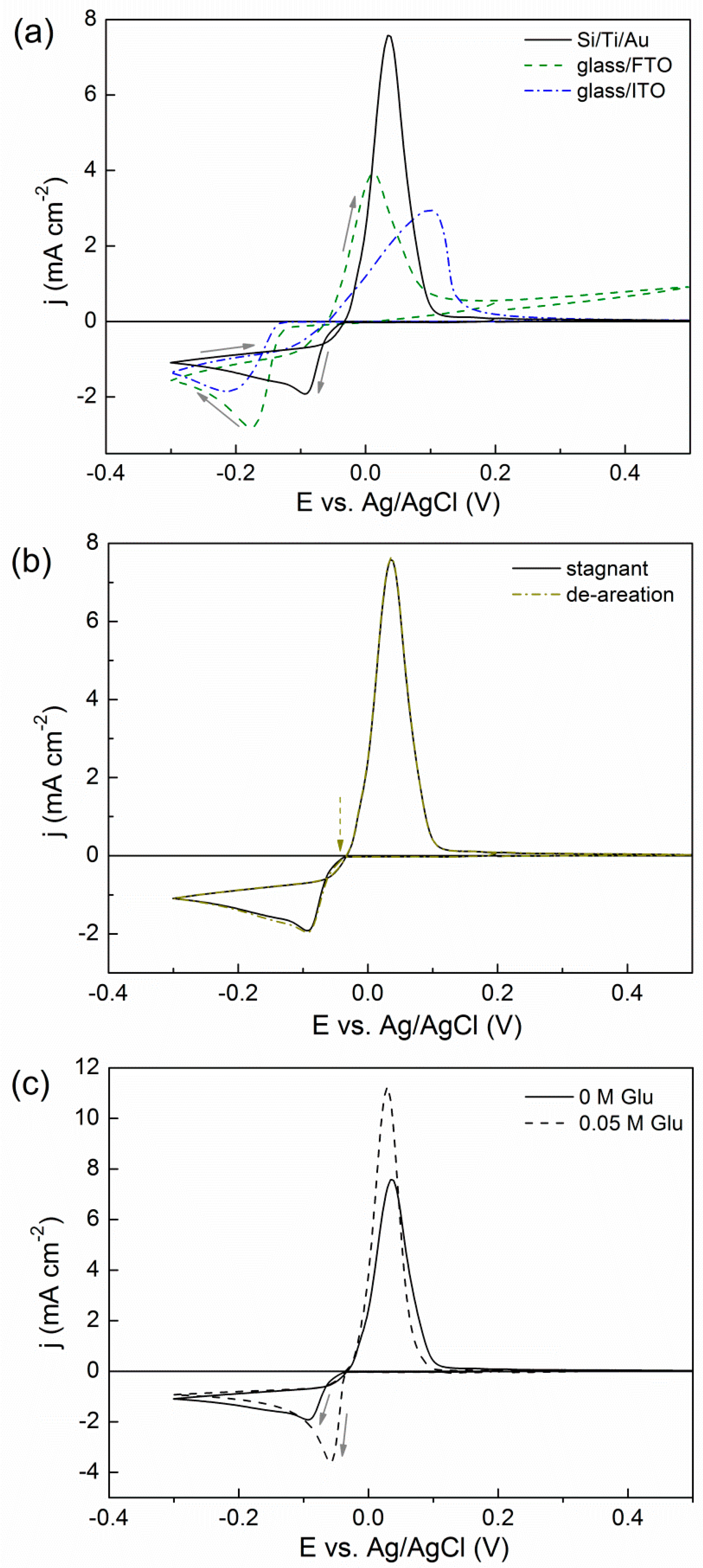
2.1. Cyclic Voltammetry (CV) Studies
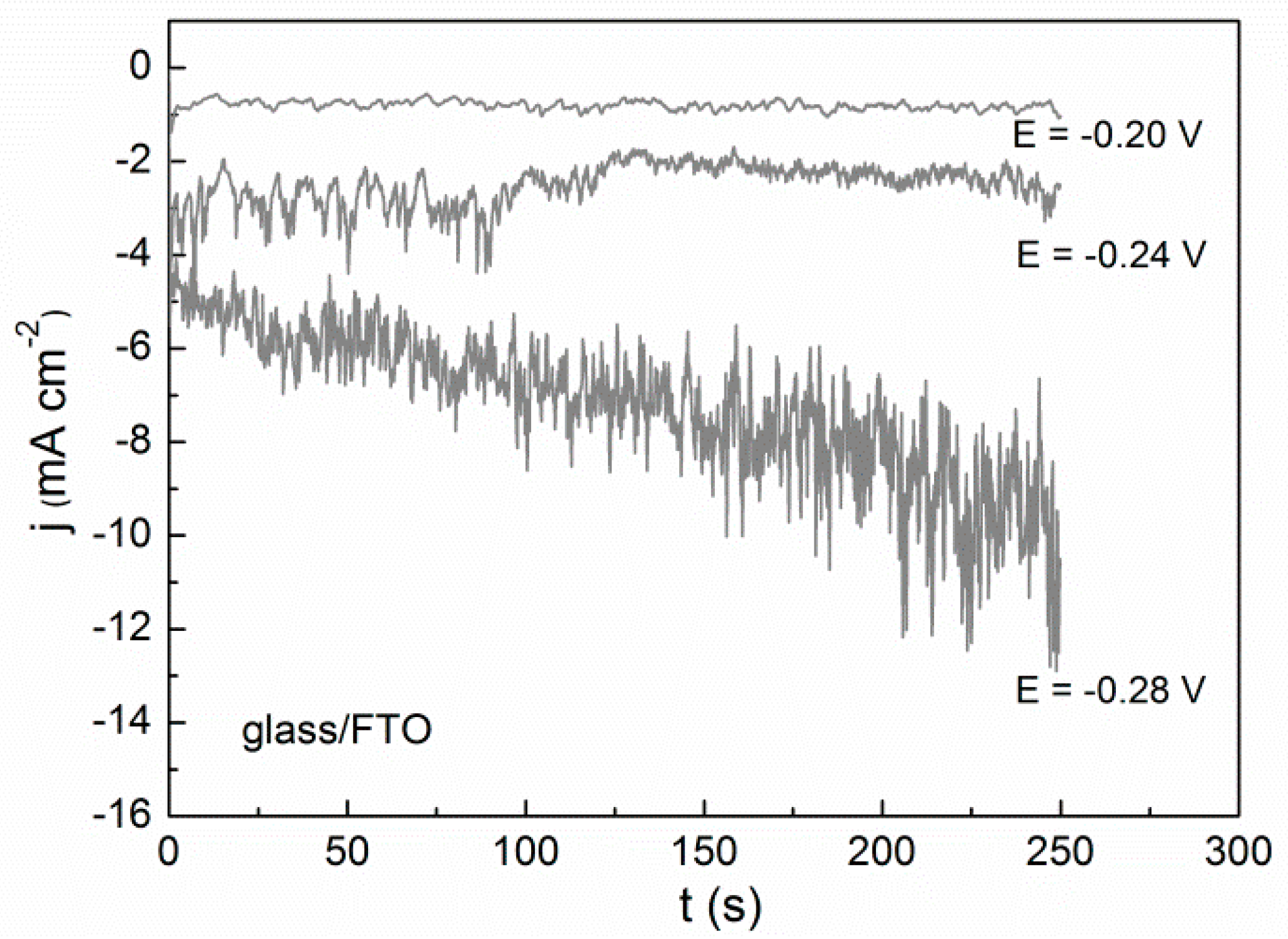
2.2. Potentiostatic Deposition and Morphological Characterization of Deposits
2.3. X-ray Diffraction Analysis
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kai, L.; Chien, C.L.; Searson, P.C.; Yu-Zhang, K. Giant positive magnetoresistance in arrays of semi-metallic bismuth nanowires. IEEE Trans. Magn. 1998, 34, 1093–1095. [Google Scholar]
- Yang, F.Y.; Liu, K.; Hong, K.; Reich, D.H.; Searson, P.C.; Chien, C.L. Large magnetoresistance of electrodeposited single-crystal bismuth thin films. Science 1999, 284, 1335–1337. [Google Scholar] [CrossRef] [PubMed]
- Agapescu, C.; Cojocaru, A.; Cotarta, A.; Visan, T. Electrodeposition of bismuth, tellurium, and bismuth telluride from choline chloride-oxalic acid ionic liquid. J. Appl. Electrochem. 2013, 43, 309–321. [Google Scholar] [CrossRef]
- Su, C.; Lu, Z.; Zhao, H.; Yang, H.; Chen, R. Photoinduced switchable wettability of bismuth coating with hierarchical dendritic structure between superhydrophobicity and superhydrophilicity. Appl. Surf. Sci. 2015, 353, 735–743. [Google Scholar] [CrossRef]
- Wang, J. Stripping analysis at bismuth electrodes. A Review. Electroanalysis 2005, 17, 1341–1346. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, H.; Dai, L.; Li, Y.; Zhu, J.; Wang, L. Preparation of dendritic bismuth film electrodes and their application for detection of trace Pb (II) and Cd (II). Chin. J. Chem. Eng. 2016, 24, 410–414. [Google Scholar] [CrossRef]
- Ananthi, A.; Kumar, S.S.; Phani, K.L. Facile one-step direct electrodeposition of bismuth nanowires on glassy carbon electrode for selective determination of folic acid. Electrochim. Acta 2015, 151, 584–590. [Google Scholar] [CrossRef]
- Tsai, Y.-D.; Lien, C.-H.; Hu, C.-C. Effects of polyethylene glycol and gelatin on the crystal size, morphology, and Sn2+-sensing ability of bismuth deposits. Elecrochim. Acta 2011, 56, 7615–7621. [Google Scholar] [CrossRef]
- Som, T.; Simo, A.; Fenger, R.; Troppenz, G.V.; Bansen, R.; Pfänder, N.; Emmerling, F.; Rappich, J.; Boeck, T.; Rademann, K. Bismuth hexagons: Facile mass synthesis, stability and applications. ChemPhysChem 2012, 13, 2162–2169. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, Y.-H.; Luo, F.; Du, N.; Yan, C.H. Synthesis of bismuth with various morphologies by electrodeposition. Inorg. Chem. Commun. 2003, 6, 781–785. [Google Scholar] [CrossRef]
- Yang, M.; Hu, Z. Electrodeposition of bismuth onto glassy carbon electrodes from nitrate solutions. J. Electroanal. Chem. 2005, 583, 46–55. [Google Scholar] [CrossRef]
- Sandnes, E.; Williams, M.E.; Bertocci, U.; Vaudin, M.D.; Stafford, G.R. Electrodeposition of bismuth from nitric acid electrolyte. Electrochim. Acta 2007, 52, 6221–6228. [Google Scholar] [CrossRef]
- Yang, M. Fern-shaped bismuth dendrites electrodeposited at hydrogen evolution potentials. J. Mater. Chem. 2011, 21, 3119–3124. [Google Scholar] [CrossRef]
- Frutos, A.A.; Sala, L.F.; Escandar, G.M.; Devillers, M.; Salas Peregrı́n, J.M.; González Sierra, M. Bismuth(III) complexes of d-gluconic acid. Studies in aqueous solution and in the solid phase. Polyhedron 1999, 18, 989–994. [Google Scholar] [CrossRef]
- Zhang, Z.; Gibson, P.; Clark, S.B.; Tian, G.; Zanonato, P.L.; Rao, L. Lactonization and protonation of gluconic acid: A thermodynamic and kinetic study by potentiometry, NMR and ESI-MS. J. Solut. Chem. 2007, 36, 1187–1200. [Google Scholar] [CrossRef]
- Senthilkumar, M.; Mathiyarasu, J.; Joseph, J.; Phani, K.L.N.; Yegnaraman, V. Electrochemical instability of indium tin oxide (ITO) glass in acidic pH range during cathodic polarization. Mater. Chem. Phys. 2008, 108, 403–407. [Google Scholar] [CrossRef]
- Takeno, N. Atlas of Eh-pH Diagrams; Geological Survey of Japan Open File Report No. 419; Intercomparison of Thermodynamic Databases: Tsukuba, Japan, 2005. [Google Scholar]
- Bretti, C.; Cigala, R.M.; de Stefano, C.; Lando, G.; Sammartano, S. Acid-Base and Thermodynamic Properties of D-Gluconic Acid and Its Interaction with Sn2+ and Zn2+. J. Chem. Eng. Data 2016, 61, 2040–2051. [Google Scholar] [CrossRef]
- Das, A.; Sangaranarayanan, M.V. Shape-controlled synthesis of three-dimensional triangular bismuth microstructures and sensing of H2O2. CrystEngComm 2016, 18, 1147–1155. [Google Scholar] [CrossRef]
- Guo, L.; Oskam, G.; Radisic, A.; Hoffmann, P.M.; Searson, P.C. Island growth in electrodeposition. J. Phys. D Appl. Phys. 2011, 44, 443001. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD: A friendly Java program for material analysis using diffraction. Newsl. CPD 1999, 21, 14–15. [Google Scholar]




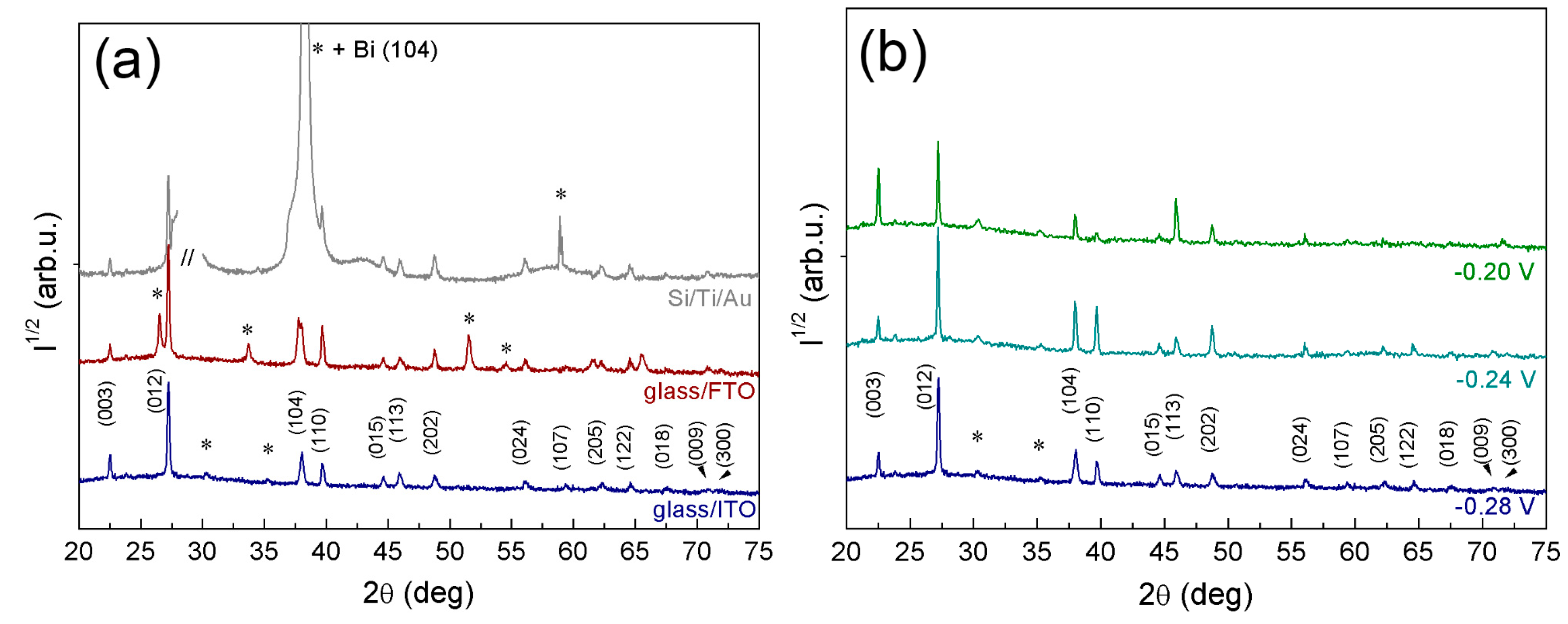
| Plane | I(hkl)/I(012) (%) | |||||
|---|---|---|---|---|---|---|
| Theoretical | Glass/FTO (−0.28 V) | Si/Ti/Au (−0.28 V) | Glass/ITO (−0.28 V) | Glass/ITO (−0.24 V) | Glass/ITO (−0.20 V) | |
| (003) | 5 | 9 | 7 | 16 | 17 | 62 |
| (012) | 100 | 100 | 100 | 100 | 100 | 100 |
| (104) | 33 | 24 | – | 18 | 26 | 17 |
| (110) | 34 | 19 | 30 | 11 | 23 | 7 |
| (202) | 16 | 7 | 6 | 5 | 12 | 10 |
| Substrate | Potential (V) | Cell Parameter (Å) | Crystallite Size (Å) | |
|---|---|---|---|---|
| a | c | |||
| glass/FTO | –0.28 | 4.54256 ± 2.9 × 10−4 | 11.84982 ± 1.7 × 10−4 | 486 ± 59 |
| Si/Ti/Au | –0.28 | 4.54525 ± 2.1 × 10−4 | 11.84953 ± 1.5 × 10−4 | 369 ± 45 |
| glass/ITO | –0.28 | 4.54161 ± 1.1 × 10−4 | 11.85492 ± 3.3 × 10−4 | 456 ± 52 |
| glass/ITO | –0.24 | 4.54647 ± 1.7 × 10−4 | 11.86142 ± 8.8 × 10−4 | 770 ± 96 |
| glass/ITO | –0.20 | 4.54818 ± 2.4 × 10−4 | 11.86089 ± 6.6 × 10−4 | 154 ± 62 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilican, D.; Fornell, J.; Sort, J.; Pellicer, E. Electrochemical Synthesis of Bismuth Particles: Tuning Particle Shape through Substrate Type within a Narrow Potential Window. Materials 2017, 10, 43. https://doi.org/10.3390/ma10010043
Bilican D, Fornell J, Sort J, Pellicer E. Electrochemical Synthesis of Bismuth Particles: Tuning Particle Shape through Substrate Type within a Narrow Potential Window. Materials. 2017; 10(1):43. https://doi.org/10.3390/ma10010043
Chicago/Turabian StyleBilican, Doga, Jordina Fornell, Jordi Sort, and Eva Pellicer. 2017. "Electrochemical Synthesis of Bismuth Particles: Tuning Particle Shape through Substrate Type within a Narrow Potential Window" Materials 10, no. 1: 43. https://doi.org/10.3390/ma10010043