Dysprosium Acetylacetonato Single-Molecule Magnet Encapsulated in Carbon Nanotubes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
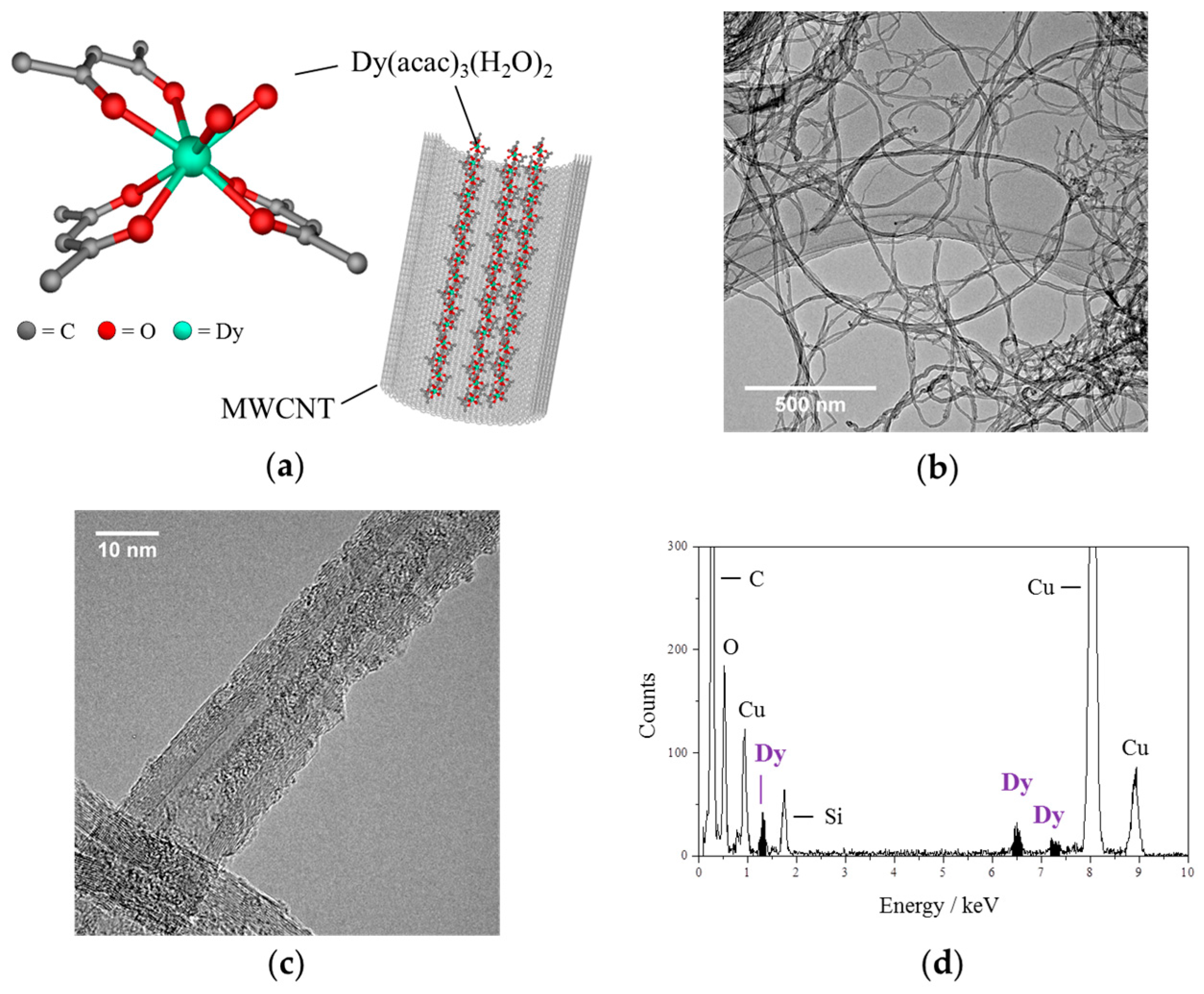
2.2. Transmission Electron Microscopy, Elemental Analysis and Thermogravimetry
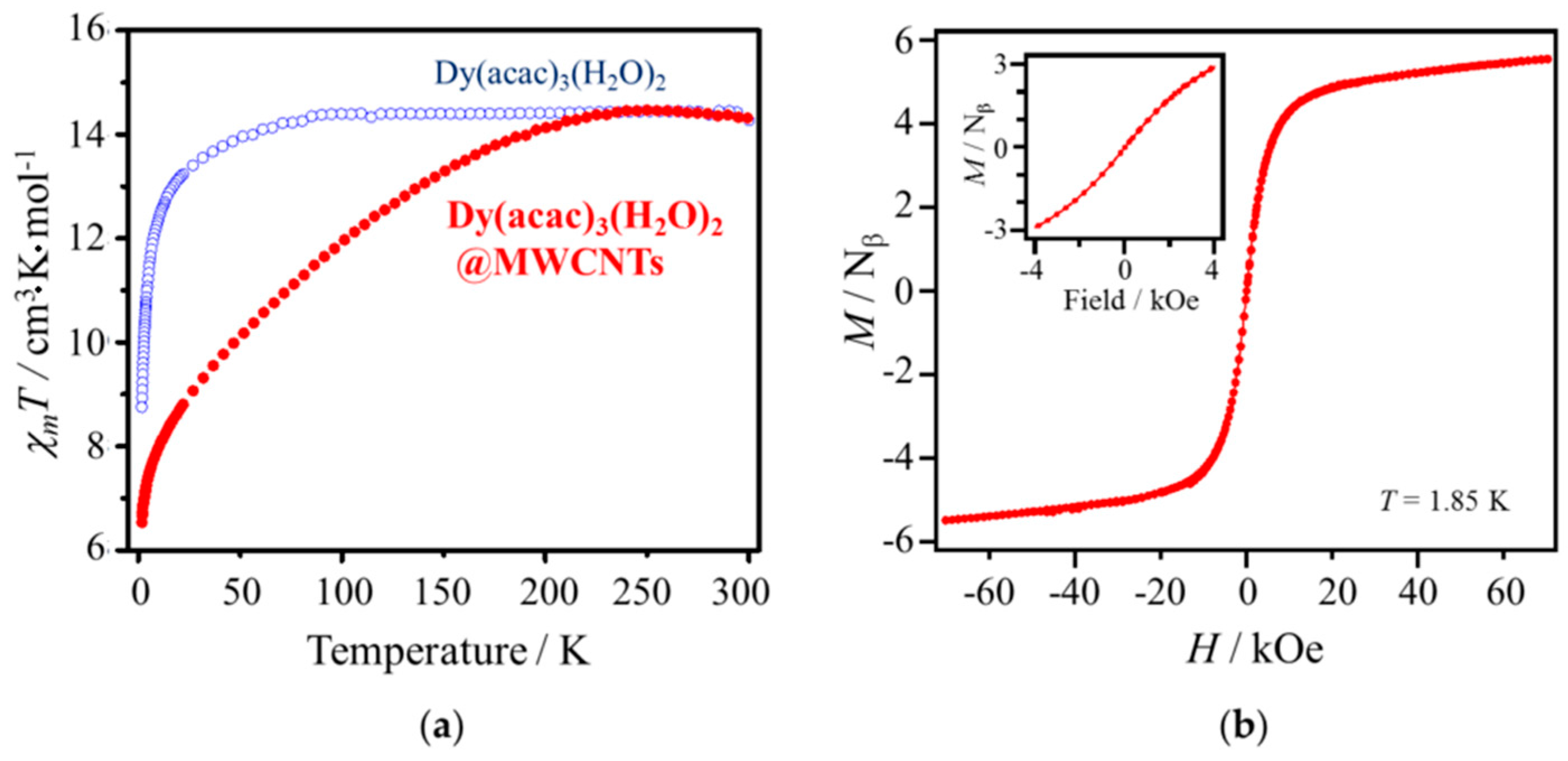
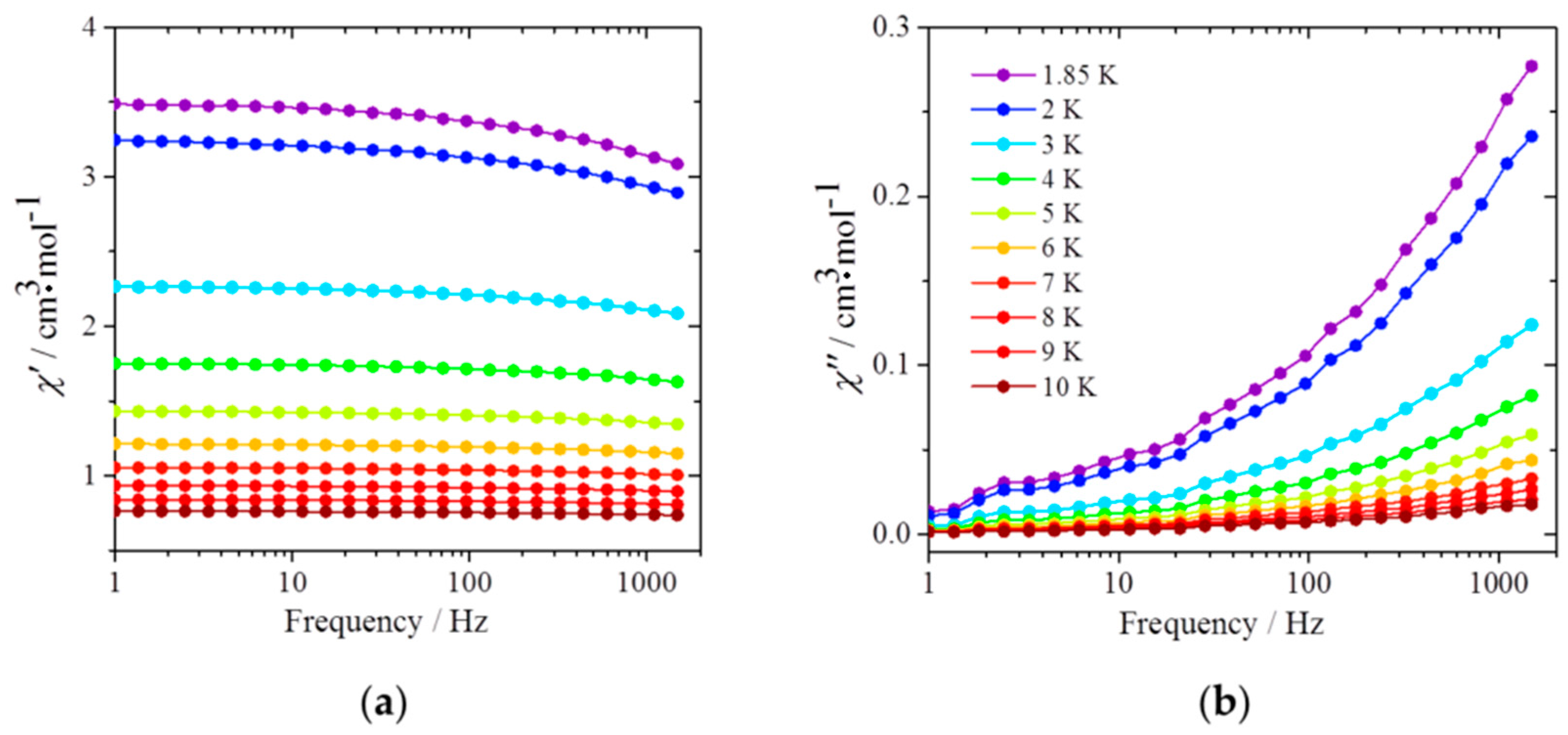
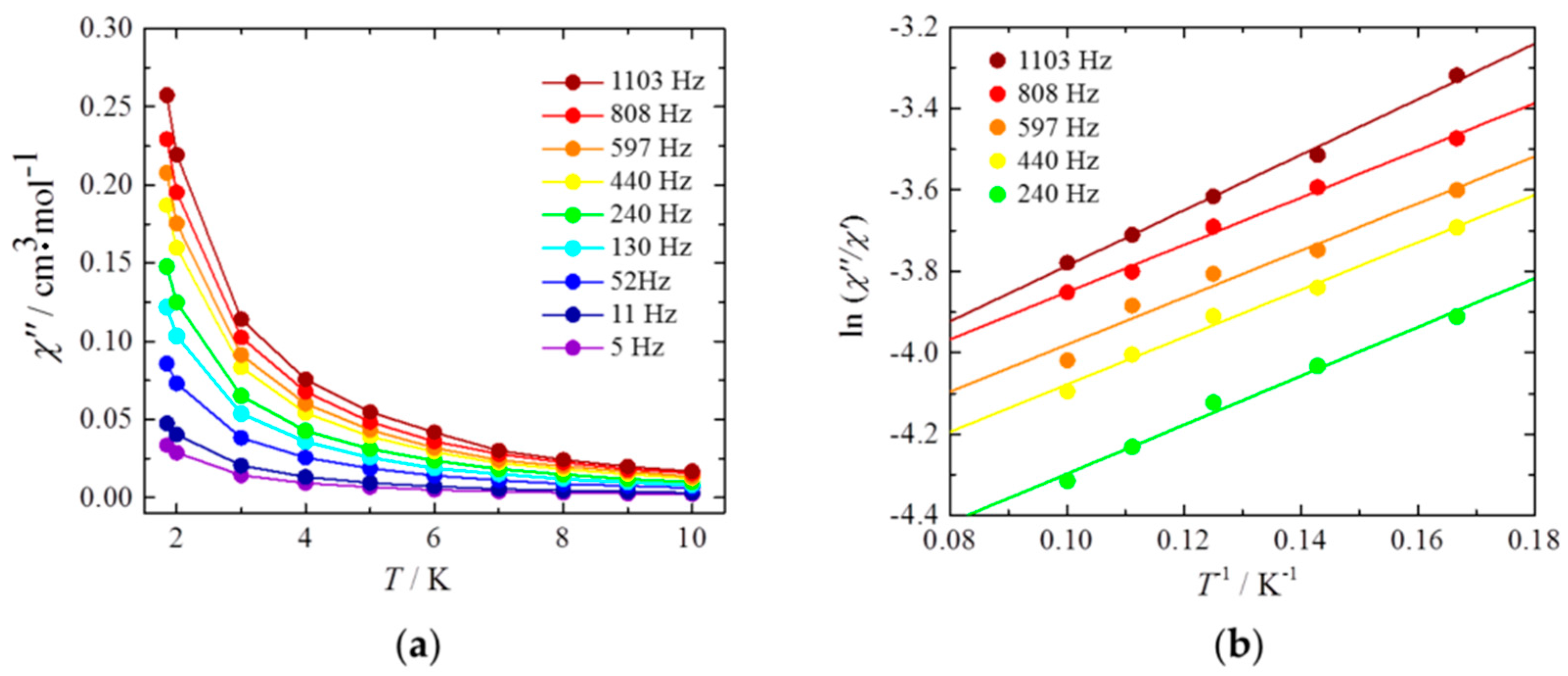
2.3. Magnetic Properties
3. Materials and Methods
3.1. General
3.2. Synthesis
3.3. TEM Observation
3.4. Thermogravimetric Analysis
3.5. Magnetic Susceptibility Measurement
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sessoli, R.; Gatteschi, D.; Caneschi, A.; Novak, M.A. Magnetic bistability in a metal-ion cluster. Nature 1993, 365, 141–143. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.-Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, D.N.; Winpenny, R.E.P.; Layfield, R.A. Lanthanide single-molecule magnets. Chem. Rev. 2013, 113, 5110–5148. [Google Scholar] [CrossRef] [PubMed]
- Horii, Y.; Katoh, K.; Yasuda, N.; Breedlove, B.K.; Yamashita, M. Effects of f–f interactions on the single-molecule magnet properties of terbium(III)–phthalocyaninato quintuple-decker complexes. Inorg. Chem. 2015, 54, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Lionti, F.; Ballou, R.; Gatteschi, D.; Sessoli, R.; Barbara, B. Macroscopic quantum tunnelling of magnetization in a single crystal of nanomagnets. Nature 1996, 383, 145–147. [Google Scholar] [CrossRef]
- Friedman, J.R.; Sarachik, M.P.; Tejada, J.; Ziolo, R. Macroscopic measurement of resonant magnetization tunneling in high-spin molecules. Phys. Rev. Lett. 1996, 76, 3830–3833. [Google Scholar] [CrossRef] [PubMed]
- Gatteschi, D.; Sessoli, R. Quantum tunneling of magnetization and related phenomena in molecular materials. Angew. Chem. Int. Ed. 2003, 42, 268–297. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Pineider, F.; Danieli, C.; Totti, F.; Sorace, L.; Sainctavit, P.; Arrio, M.A.; Otero, E.; Joly, L.; Cezar, J.C.; et al. Quantum tunnelling of the magnetization in a monolayer of oriented single-molecule magnets. Nature 2010, 468, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Ardavan, A.; Rival, O.; Morton, J.J.L.; Blundell, S.J.; Tyryshkin, A.M.; Timco, G.A.; Winpenny, R.E.P. Will spin-relaxation times in molecular magnets permit quantum information processing? Phys. Rev. Lett. 2007, 98, 057201. [Google Scholar] [CrossRef] [PubMed]
- Mannini, M.; Pineider, F.; Sainctavit, P.; Danieli, C.; Otero, E.; Sciancalepore, C.; Talarico, A.M.; Arrio, M.-A.; Cornia, A.; Gatteschi, D.; et al. Magnetic memory of a single-molecule quantum magnet wired to a gold surface. Nat. Mater. 2009, 8, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Komeda, T.; Isshiki, H.; Liu, J.; Zhang, Y.-F.; Lorente, N.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Observation and electric current control of a local spin in a single-molecule magnet. Nat. Commun. 2011, 2, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogani, L.; Wernsdorfer, W. Molecular spintronics using single-molecule magnets. Nat. Mater. 2008, 7, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Leuenberger, M.N.; Loss, D. Quantum computing in molecular magnets. Nature 2001, 410, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Urdampilleta, M.; Klyatskaya, S.; Cleuziou, J.P.; Ruben, M.; Wernsdorfer, W. Supramolecular spin valves. Nat. Mater. 2011, 10, 502–506. [Google Scholar] [CrossRef] [PubMed]
- Candini, A.; Klyatskaya, S.; Ruben, M.; Wernsdorfer, W.; Affronte, M. Graphene spintronic devices with molecular nanomagnets. Nano Lett. 2011, 11, 2634–2639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Carmen Giménez-López, M.; Moro, F.; La Torre, A.; Gómez-García, C.J.; Brown, P.D.; van Slageren, J.; Khlobystov, A.N. Encapsulation of single-molecule magnets in carbon nanotubes. Nat. Commun. 2011, 2, 407. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, D.; Pyser, J.B.; Zhang, X.; Yakovenko, A.A.; Dunbar, K.R.; Wriedt, M. Metal–organic frameworks as platforms for the controlled nanostructuring of single-molecule magnets. J. Am. Chem. Soc. 2015, 137, 9254–9257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.-D.; Wang, B.-W.; Su, G.; Wang, Z.-M.; Gao, S. A Mononuclear dysprosium complex featuring single-molecule-magnet behavior. Angew. Chem. Int. Ed. 2010, 49, 7448–7451. [Google Scholar] [CrossRef] [PubMed]
- Ajayan, P.M.; Iijima, S. Capillarity-induced filling of carbon nanotubes. Nature 1993, 361, 333–334. [Google Scholar] [CrossRef]
- Yudasaka, M.; Ajima, K.; Suenaga, K.; Ichihashi, T.; Hashimoto, A.; Iijima, S. Nano-extraction and nano-condensation for C60 incorporation into single-wall carbon nanotubes in liquid phases. Chem. Phys. Lett. 2003, 380, 42–46. [Google Scholar] [CrossRef]
- Yu, A.; Bekyarova, E.; Itkis, M.E.; Fakhrutdinov, D.; Webster, R.; Haddon, R.C. Application of centrifugation to the large-scale purification of electric arc-produced single-walled carbon nanotubes. J. Am. Chem. Soc. 2006, 128, 9902–9908. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.; Guo, Y.N.; Zhao, L.; Guo, Y.; Lin, S.Y.; Jiang, S.D.; Tang, J.; Wang, B.W.; Gao, S. Capping ligand perturbed slow magnetic relaxation in dysprosium single-ion magnets. Chem. Eur. J. 2011, 17, 12476–12481. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Breedlove, B.K.; Yamashita, M. Symmetry of octa-coordination environment has a substantial influence on dinuclear TbIII triple-decker single-molecule magnets. Chem. Sci. 2016, 7, 4329–4340. [Google Scholar] [CrossRef]
- Luis, F.; Bartolomé, J.; Fernández, J.F.; Tejada, J.; Hernández, J.M.; Zhang, X.X.; Ziolo, R. Thermally activated and field-tuned tunneling in Mn12Ac studied by ac magnetic susceptibility. Phys. Rev. B 1997, 55, 11448–11456. [Google Scholar] [CrossRef] [Green Version]
- Bartolomé, J.; Filoti, G.; Kuncser, V.; Schinteie, G.; Mereacre, V.; Anson, C.E.; Powell, A.K.; Prodius, D.; Turta, C. Magnetostructural correlations in the tetranuclear series of {Fe3LnO2} butterfly core clusters: Magnetic and Mössbauer spectroscopic study. Phys. Rev. B 2009, 80, 014430. [Google Scholar] [CrossRef]
- Ferrando-Soria, J.; Cangussu, D.; Eslava, M.; Journaux, Y.; Lescouëzec, R.; Julve, M.; Lloret, F.; Pasán, J.; Ruiz-Pérez, C.; Lhotel, E.; et al. Rational enantioselective design of chiral heterobimetallic single-chain magnets: Synthesis, crystal structures and magnetic properties of oxamato-bridged MIICuII chains (M=Mn, Co). Chem. Eur. J. 2011, 17, 12482–12494. [Google Scholar] [CrossRef] [PubMed]
- Gass, I.A.; Moubaraki, B.; Langley, S.K.; Batten, S.R.; Murray, K.S. A π-π 3D network of tetranuclear μ2/μ3-carbonato Dy(III) bis-pyrazolylpyridine clusters showing single molecule magnetism features. Chem. Commun. 2012, 48, 2089–2091. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, R.; Yatoo, M.A.; Katoh, K.; Breedlove, B.K.; Yamashita, M. Dysprosium Acetylacetonato Single-Molecule Magnet Encapsulated in Carbon Nanotubes. Materials 2017, 10, 7. https://doi.org/10.3390/ma10010007
Nakanishi R, Yatoo MA, Katoh K, Breedlove BK, Yamashita M. Dysprosium Acetylacetonato Single-Molecule Magnet Encapsulated in Carbon Nanotubes. Materials. 2017; 10(1):7. https://doi.org/10.3390/ma10010007
Chicago/Turabian StyleNakanishi, Ryo, Mudasir Ahmad Yatoo, Keiichi Katoh, Brian K. Breedlove, and Masahiro Yamashita. 2017. "Dysprosium Acetylacetonato Single-Molecule Magnet Encapsulated in Carbon Nanotubes" Materials 10, no. 1: 7. https://doi.org/10.3390/ma10010007
APA StyleNakanishi, R., Yatoo, M. A., Katoh, K., Breedlove, B. K., & Yamashita, M. (2017). Dysprosium Acetylacetonato Single-Molecule Magnet Encapsulated in Carbon Nanotubes. Materials, 10(1), 7. https://doi.org/10.3390/ma10010007





