Facile Synthesis of V2O5 Hollow Spheres as Advanced Cathodes for High-Performance Lithium-Ion Batteries
Abstract
:1. Introduction
2. Discussion and Results
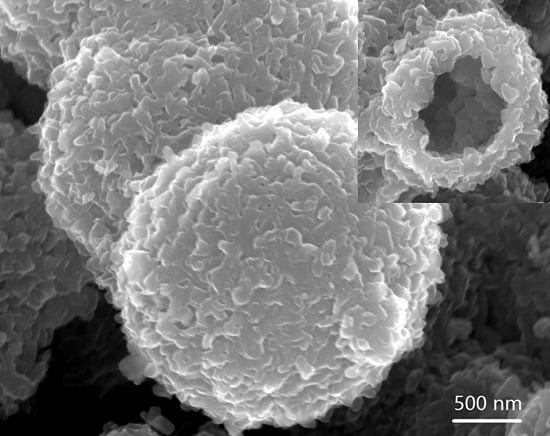
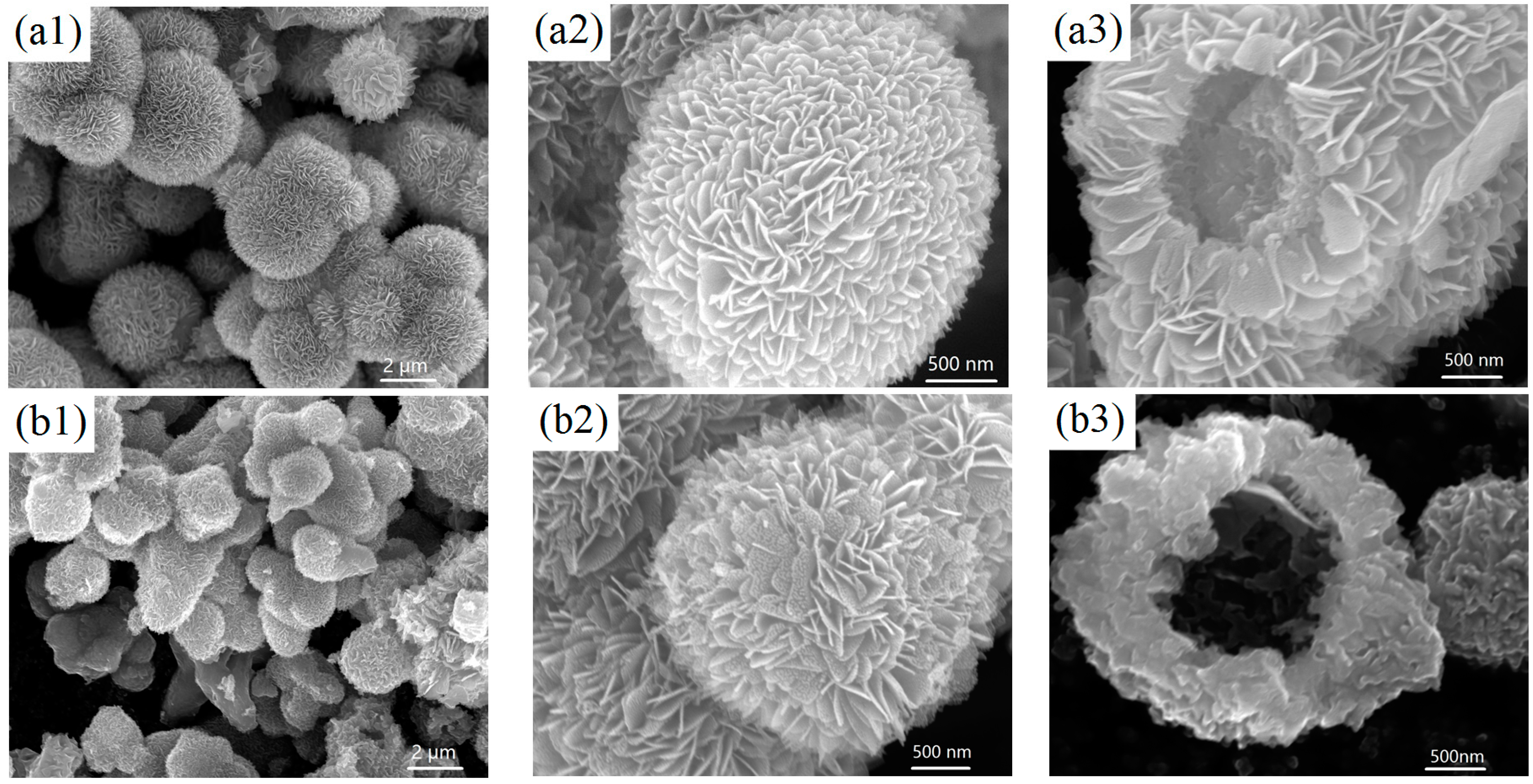
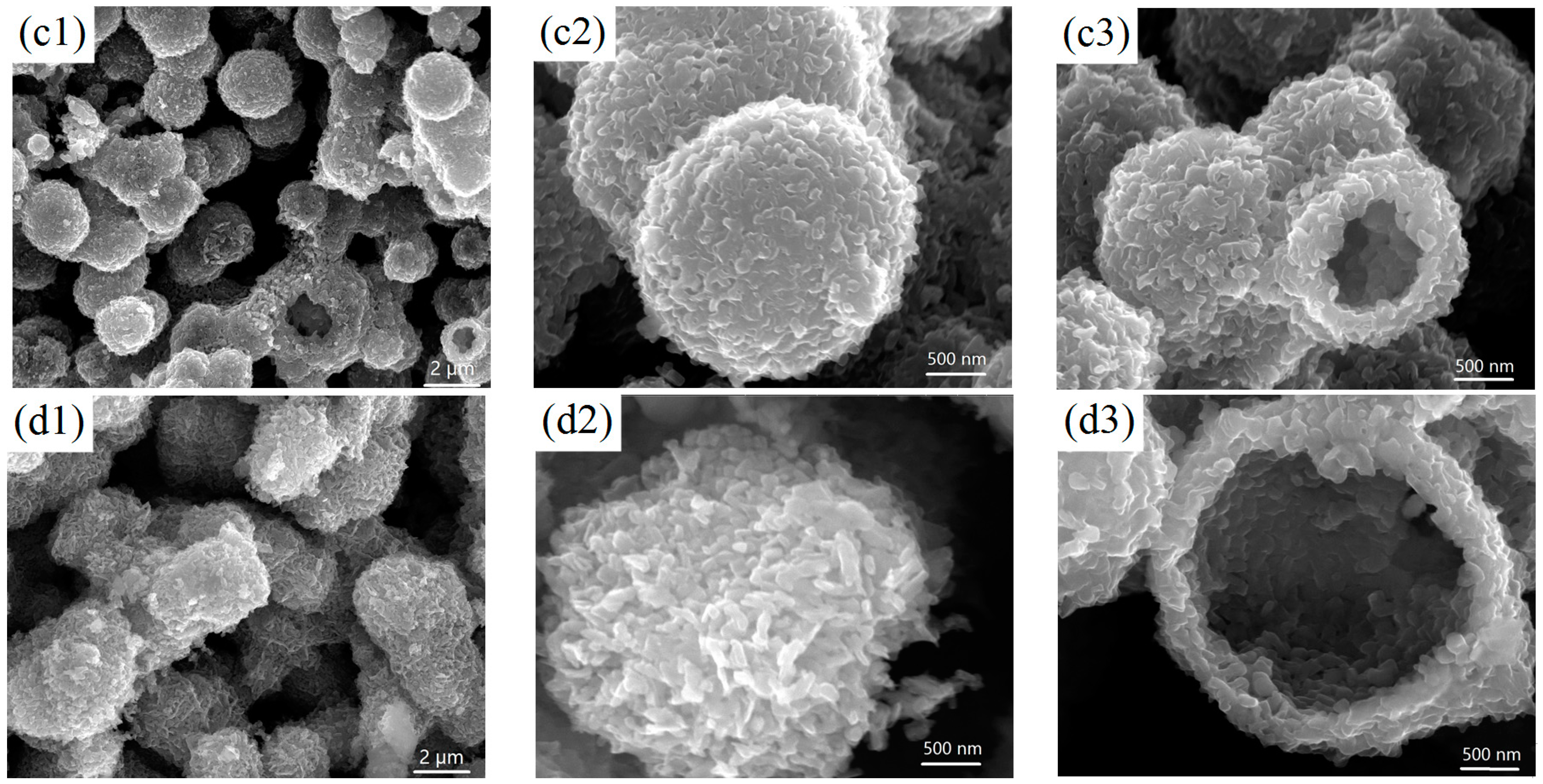
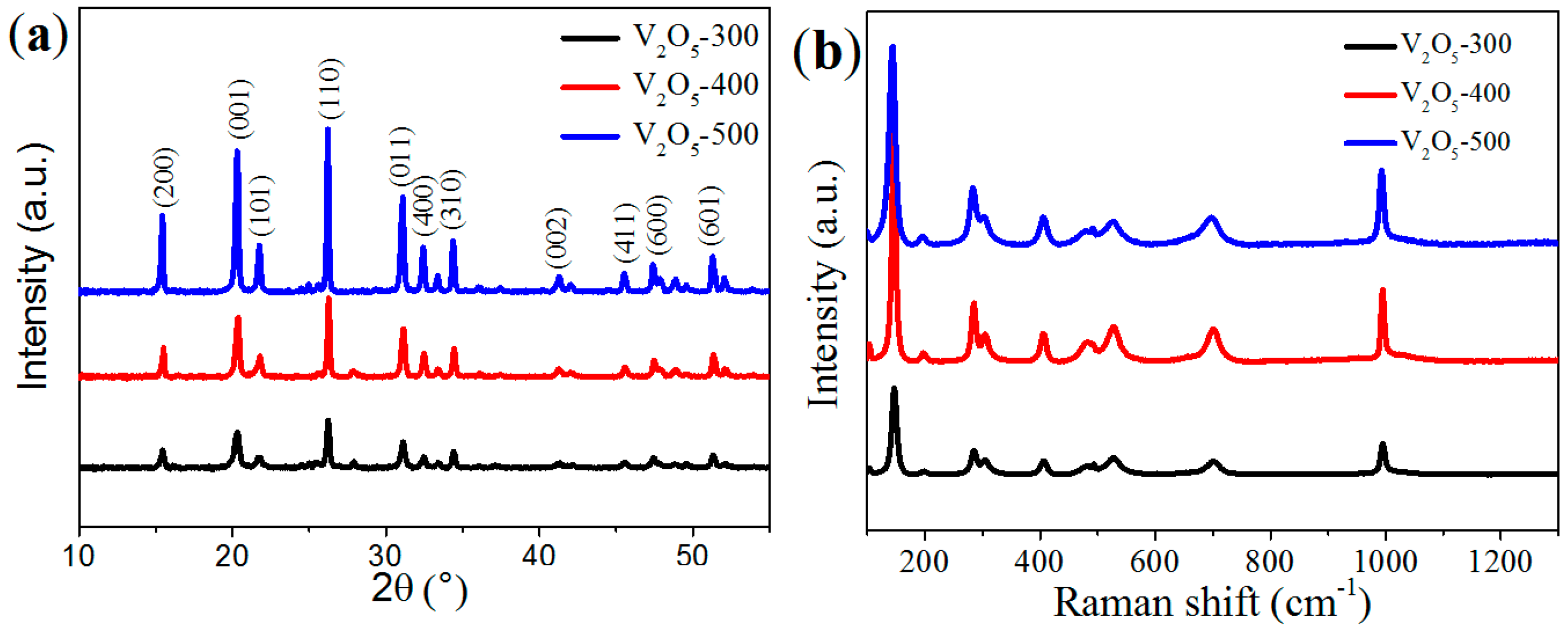
2.1. Structure and Morphology of the V2O5 Microspheres
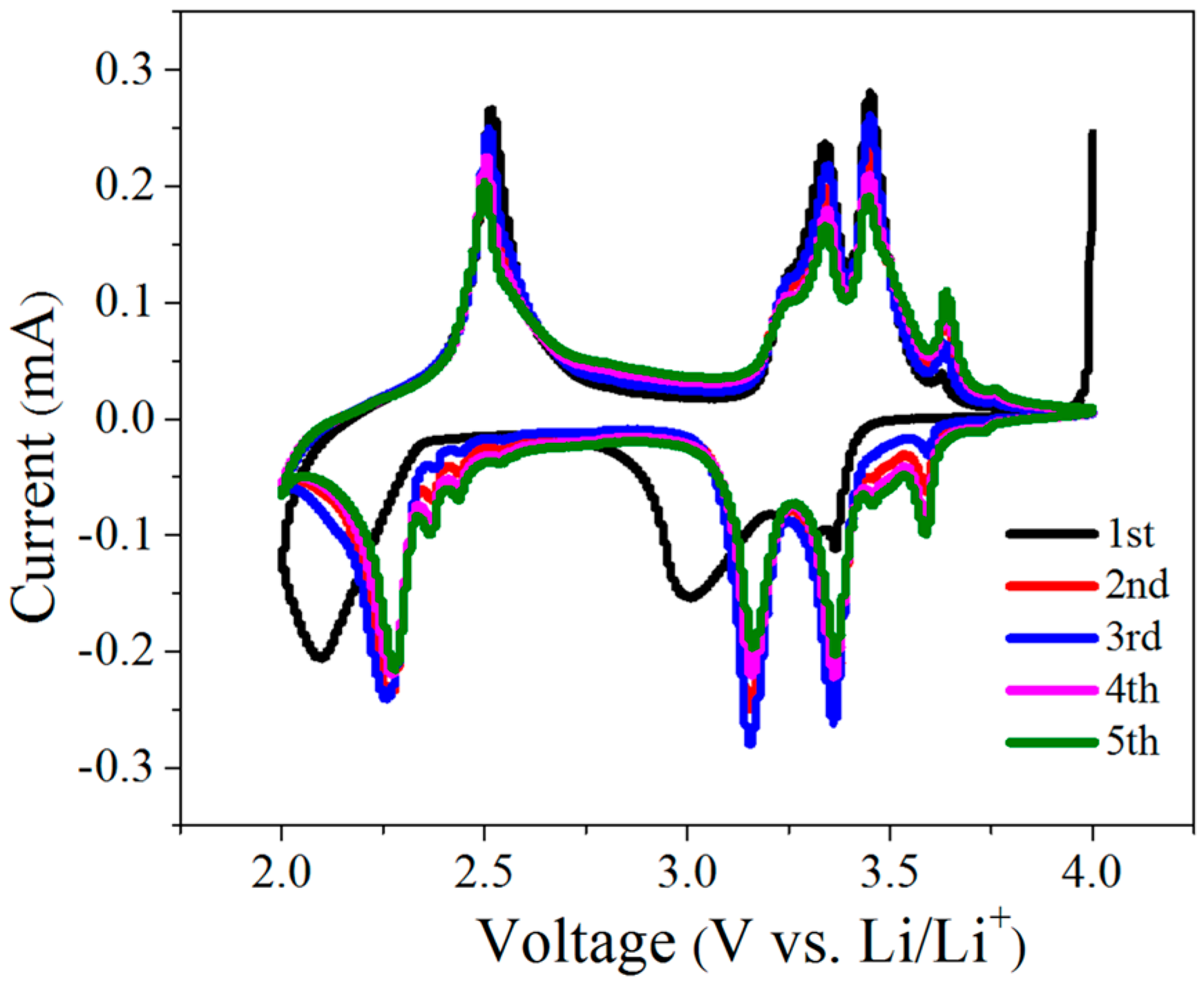
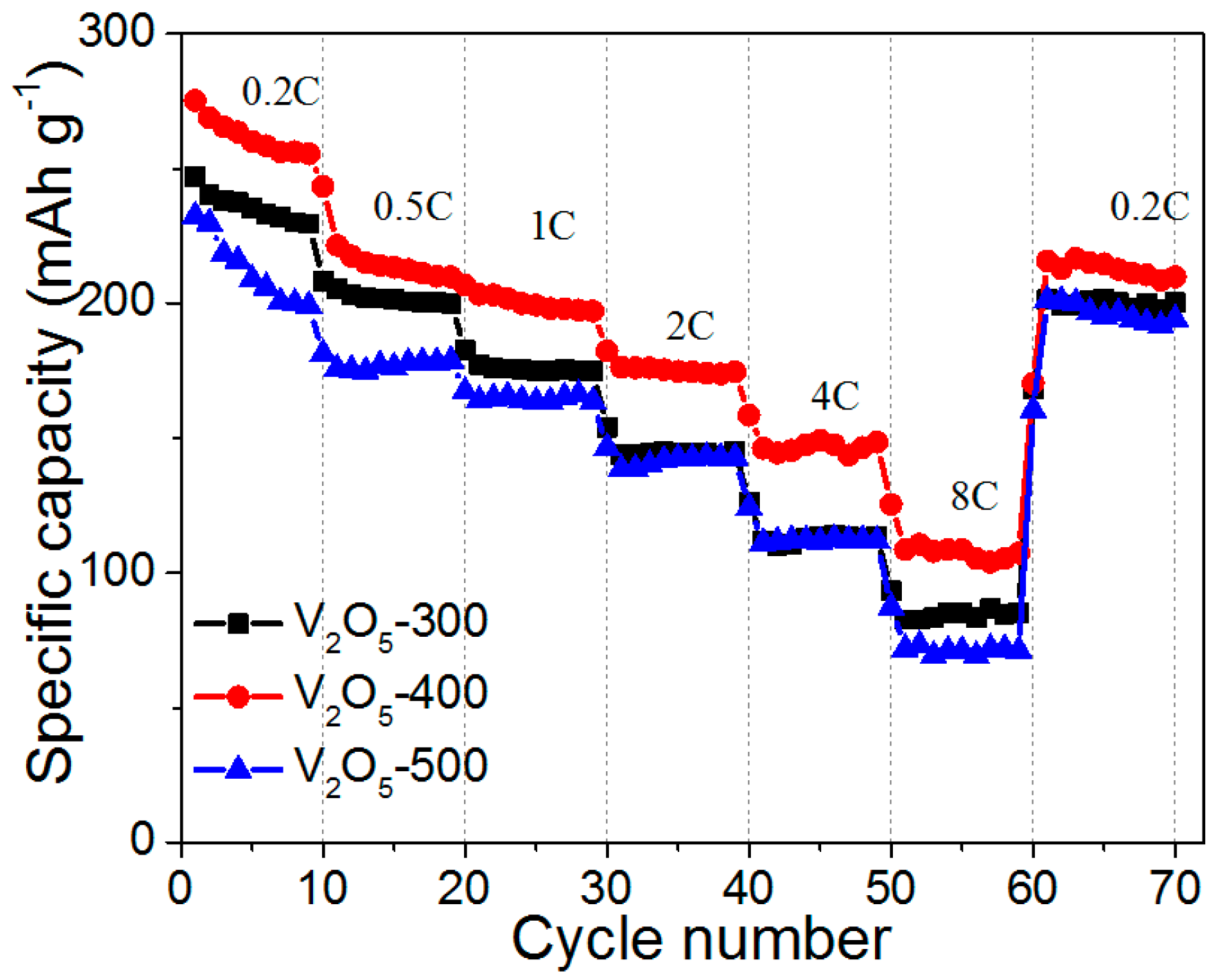
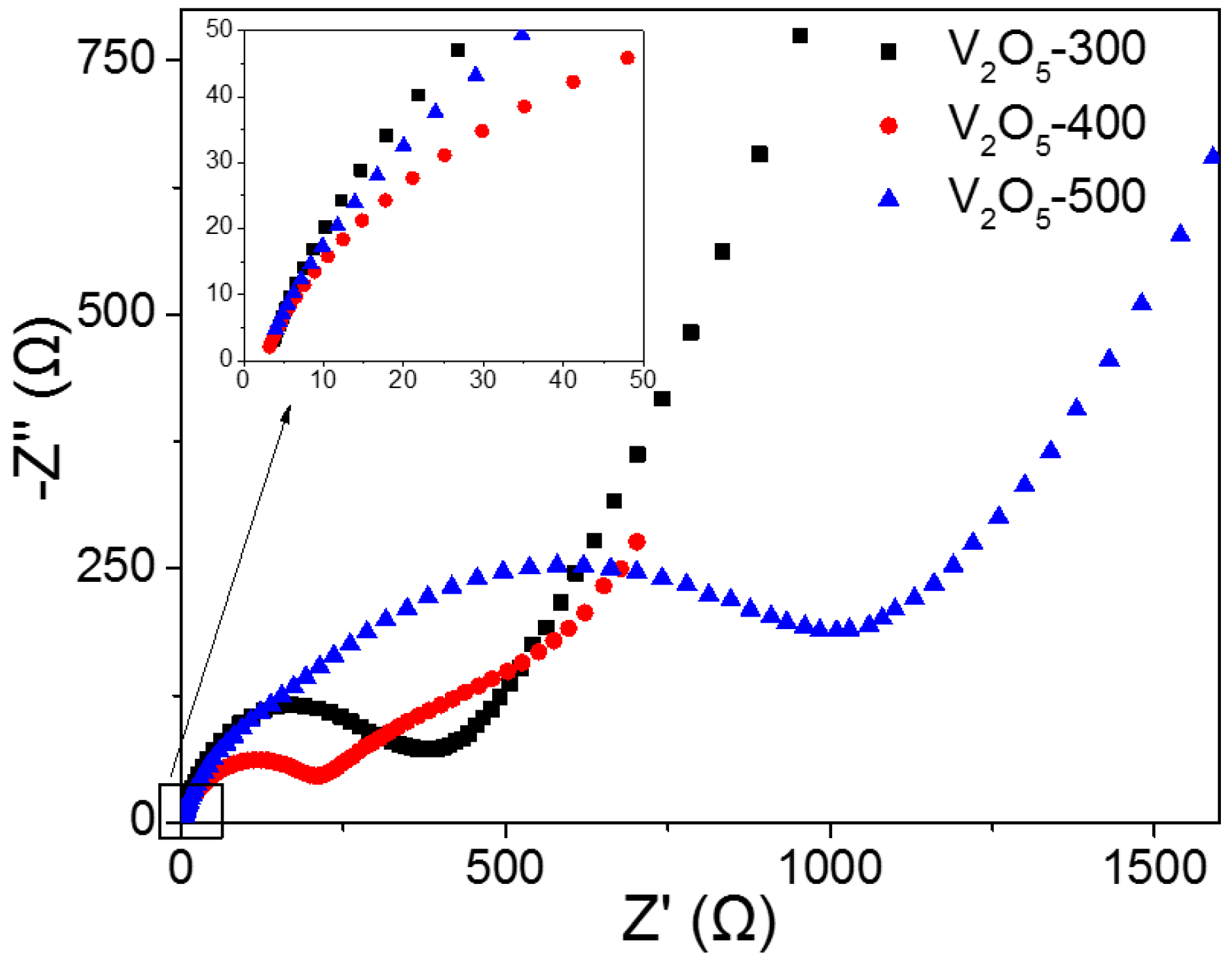
2.2. Electrochemical Performance
3. Materials and Methods
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Functional materials for rechargeable batteries. Adv. Mater. 2011, 23, 1695–1715. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Han, X.; Li, J.; Hua, J.; Ouyang, M. A review on the key issues for lithium-ion battery management in electric vehicles. J. Power Sources 2013, 226, 272–288. [Google Scholar] [CrossRef]
- Wang, J.-G.; Kang, F.; Wei, B. Engineerging of MnO2-based nanocomposites for high performance supercapacitors. Prog. Mater. Sci. 2015, 74, 51–124. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Kong, L.; Taniguchi, I. Correlation between porous structure and electrochemical properties of porous nanostructured vanadium pentoxide synthesized by novel spray pyrolysis. J. Power Sources 2016, 312, 36–44. [Google Scholar] [CrossRef]
- Wang, J.-G.; Jin, D.; Zhou, R.; Li, X.; Liu, X.-R.; Shen, C.; Xie, K.; Li, B.; Kang, F.; Wei, B. Highly Flexible Graphene/Mn3O4 Nanocomposite Membrane as Advanced Anodes for Li-Ion Batteries. ACS Nano 2016, 10, 6227–6234. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Wei, Q.; Zhang, P.; Sheng, J.; Hercule, K.M.; Lv, F.; Wang, Q.; Wei, X.; Mai, L. Three-dimensional interconnected vanadium pentoxide nanonetwork cathode for high-rate long-life lithium batteries. Small 2015, 11, 2654–2660. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-G.; Jin, D.; Liu, H.; Zhang, C.; Zhou, R.; Shen, C.; Xie, K.; Wei, B. All-manganese-based Li-ion batteries with high rate capability and ultralong cycle life. Nano Energy 2016, 22, 524–532. [Google Scholar] [CrossRef]
- Wu, H.B.; Pan, A.; Hng, H.H.; Lou, X.W.D. Template-assisted formation of rattle-type V2O5 hollow microspheres with enhanced lithium storage properties. Adv. Funct. Mater. 2013, 23, 5669–5674. [Google Scholar] [CrossRef]
- Zhou, X.; Cui, C.; Wu, G.; Yang, H.; Wu, J.; Wang, J.; Gao, G. A novel and facile way to synthesize vanadium pentoxide nanospike as cathode materials for high performance lithium ion batteries. J. Power Sources 2013, 238, 95–102. [Google Scholar] [CrossRef]
- McNulty, D.; Buckley, D.N.; O’Dwyer, C. Synthesis and electrochemical properties of vanadium oxide materials and structures as li-ion battery positive electrodes. J. Power Sources 2014, 267, 831–873. [Google Scholar] [CrossRef]
- Su, Y.; Pan, A.; Wang, Y.; Huang, J.; Nie, Z.; An, X.; Liang, S. Template-assisted formation of porous vanadium oxide as high performance cathode materials for lithium ion batteries. J. Power Sources 2015, 295, 254–258. [Google Scholar] [CrossRef]
- Wu, H.; Qin, M.; Li, X.; Cao, Z.; Jia, B.; Zhang, Z.; Zhang, D.; Qu, X.; Volinsky, A.A. One step synthesis of vanadium pentoxide sheets as cathodes for lithium ion batteries. Electrochim. Acta 2016, 206, 301–306. [Google Scholar] [CrossRef]
- An, Q.; Zhang, P.; Xiong, F.; Wei, Q.; Sheng, J.; Wang, Q.; Mai, L. Three-dimensional porous V2O5 hierarchical octahedrons with adjustable pore architectures for long-life lithium batteries. Nano Res. 2015, 8, 481–490. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. Engl. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Z.; Cai, Y.; Jin, J.; Li, Y.; Zheng, X.-F.; Wang, H.-E.; Wu, M.; Chen, L.-H.; Su, B.-L. Annealed vanadium oxide nanowires and nanotubes as high performance cathode materials for lithium ion batteries. J. Mater. Chem. A 2014, 2, 14099. [Google Scholar] [CrossRef]
- Wang, J.; Cui, C.; Gao, G.; Zhou, X.; Wu, J.; Yang, H.; Li, Q.; Wu, G. A new method to prepare vanadium oxide nano-urchins as a cathode for lithium ion batteries. RSC Adv. 2015, 5, 47522–47528. [Google Scholar] [CrossRef]
- Li, Z.; Liu, G.; Guo, M.; Ding, L.-X.; Wang, S.; Wang, H. Electrospun porous vanadium pentoxide nanotubes as a high-performance cathode material for lithium-ion batteries. Electrochim. Acta 2015, 173, 131–138. [Google Scholar] [CrossRef]
- Tang, W.; Gao, X.; Zhu, Y.; Yue, Y.; Shi, Y.; Wu, Y.; Zhu, K. A hybrid of V2O5 nanowires and mwcnts coated with polypyrrole as an anode material for aqueous rechargeable lithium batteries with excellent cycling performance. J. Mater. Chem. 2012, 22, 20143–20145. [Google Scholar] [CrossRef]
- Liu, H.; Yang, W. Ultralong single crystalline V2O5 nanowire/graphene composite fabricated by a facile green approach and its lithium storage behavior. Energy Environ. Sci. 2011, 4, 4000–4008. [Google Scholar] [CrossRef]
- Mai, L.; Dong, F.; Xu, X.; Luo, Y.; An, Q.; Zhao, Y.; Pan, J.; Yang, J. Cucumber-like V2O5/poly(3,4-ethylenedioxythiophene)&MnO2 nanowires with enhanced electrochemical cyclability. Nano Lett. 2013, 13, 740–745. [Google Scholar] [PubMed]
- Lee, M.; Balasingam, S.K.; Jeong, H.Y.; Hong, W.G.; Lee, H.B.; Kim, B.H.; Jun, Y. One-step hydrothermal synthesis of graphene decorated V2O5 nanobelts for enhanced electrochemical energy storage. Sci. Rep. 2015, 5, 8151. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Li, J.; Jin, H.; Shi, H.; Zhu, Y.; Wang, W.; Cao, M. Self-template processed hierarchical V2O5 nanobelts as cathode for high performance lithium ion battery. Electrochim. Acta 2015, 182, 621–628. [Google Scholar] [CrossRef]
- Qin, M.; Liang, Q.; Pan, A.; Liang, S.; Zhang, Q.; Tang, Y.; Tan, X. Template-free synthesis of vanadium oxides nanobelt arrays as high-rate cathode materials for lithium ion batteries. J. Power Sources 2014, 268, 700–705. [Google Scholar] [CrossRef]
- Chao, D.; Xia, X.; Liu, J.; Fan, Z.; Ng, C.F.; Lin, J.; Zhang, H.; Shen, Z.X.; Fan, H.J. A V2O5/conductive-polymer core/shell nanobelt array on three-dimensional graphite foam: A high-rate, ultrastable, and freestanding cathode for lithium-ion batteries. Adv. Mater. 2014, 26, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xia, X.; Yuan, J.; Yin, J.; Chen, Q. Free-standing three-dimensional continuous multilayer V2O5 hollow sphere arrays as high-performance cathode for lithium batteries. J. Power Sources 2015, 288, 145–149. [Google Scholar] [CrossRef]
- Ren, X.C.; Zhai, Y.J.; Zhu, L.; He, Y.Y.; Li, A.H.; Guo, C.L.; Xu, L.Q. Fabrication of various V2O5 hollow microspheres as excellent cathode for lithium storage and the application in full cells. ACS Appl. Mater. Interfaces 2016, 8, 17205–17211. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Yu, L.; Lou, X.W.D. Metal sulfide hollow nanostructures for electrochemical energy storage. Adv. Energy. Mater. 2016, 6, 1501333. [Google Scholar] [CrossRef]
- Kim, T.; Shin, J.; You, T.-S.; Lee, H.; Kim, J. Thermally controlled V2O5 nanoparticles as cathode materials for lithium-ion batteries with enhanced rate capability. Electrochim. Acta 2015, 164, 227–234. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Chen, M.; Gao, G. Facile synthesis of vanadium pentoxide@carbon core–shell nanowires for high-performance supercapacitors. J. Power Sources 2015, 273, 804–809. [Google Scholar] [CrossRef]
- Cao, Z.; Wei, B. V2O5/single-walled carbon nanotube hybrid mesoporous films as cathodes with high-rate capacities for rechargeable lithium ion batteries. Nano Energy 2013, 2, 481–490. [Google Scholar] [CrossRef]
- Li, G.; Qiu, Y.; Hou, Y.; Li, H.; Zhou, L.; Deng, H.; Zhang, Y. Synthesis of V2O5 hierarchical structures for long cycle-life lithium-ion storage. J. Mater. Chem. A 2015, 3, 1103–1109. [Google Scholar] [CrossRef]
- Wang, J.-G.; Zhang, C.; Jin, D.; Xie, K.; Wei, B. Synthesis of ultralong MnO/C coaxial nanowires as freestanding anodes for high-performance lithium ion batteries. J. Mater. Chem. A 2015, 3, 13699–13705. [Google Scholar] [CrossRef]
- Sun, B.; Huang, K.; Qi, X.; Wei, X.; Zhong, J. Rational construction of a functionalized V2O5 nanosphere/mwcnt layer-by-layer nanoarchitecture as cathode for enhanced performance of lithium-ion batteries. Adv. Funct. Mater. 2015, 25, 5633–5639. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, J.-G.; Liu, H.; Liu, H.; Wei, B. Facile Synthesis of V2O5 Hollow Spheres as Advanced Cathodes for High-Performance Lithium-Ion Batteries. Materials 2017, 10, 77. https://doi.org/10.3390/ma10010077
Zhang X, Wang J-G, Liu H, Liu H, Wei B. Facile Synthesis of V2O5 Hollow Spheres as Advanced Cathodes for High-Performance Lithium-Ion Batteries. Materials. 2017; 10(1):77. https://doi.org/10.3390/ma10010077
Chicago/Turabian StyleZhang, Xingyuan, Jian-Gan Wang, Huanyan Liu, Hongzhen Liu, and Bingqing Wei. 2017. "Facile Synthesis of V2O5 Hollow Spheres as Advanced Cathodes for High-Performance Lithium-Ion Batteries" Materials 10, no. 1: 77. https://doi.org/10.3390/ma10010077
APA StyleZhang, X., Wang, J.-G., Liu, H., Liu, H., & Wei, B. (2017). Facile Synthesis of V2O5 Hollow Spheres as Advanced Cathodes for High-Performance Lithium-Ion Batteries. Materials, 10(1), 77. https://doi.org/10.3390/ma10010077