1. Introduction
Polymers have been used as starting materials for several different applications, and polymeric composites have formed a particularly interesting niche in biomedical engineering. Currently there are a variety of metallic and ceramic materials that are being pursued as micro or nanofillers in a polymer matrix [
1]. Incorporation of additives into the polymeric matrix are mostly used to enhance the existing properties, however, it can also be considered a cost effective method for value added products [
1,
2]. New hybrid polymeric materials are being continually developed by the addition of fillers that fine tune the physical, chemical, and mechanical properties of the composites [
1,
3]. The effect of different nanofillers can be observed from the improved properties of the polymeric nanocomposites, however, fine tuning the process parameters of injection molding itself is also another important factor that must be considered.
Polyethylene has been widely studied in combination with metallic nanoparticles for their combined advantage in hard tissue replacement and tissue engineering [
3,
4]. Specifically, high density polyethylene (HDPE), a thermoplastic material, has shown great promise as a matrix component of bone implants; its structure consists of long chains of carbon and hydrogen atoms bonded together with variable branching throughout which determines its mechanical properties [
5]. Given the versatility of HDPE, many research groups have studied the effect of incorporation of fillers into the polymer matrix, including titanium dioxide, aluminum oxide, hydroxyapatite, and zirconia etc. that resulted in biocomposites with improved physical properties and enhanced cellular responses in bone-like cells [
3,
6,
7,
8,
9,
10].
Metallic fillers such as titanium, zinc, and copper are known to improve the thermal and electrical conductivities of polymer matrices [
1,
11]. Among them, titanium has been used as implant material since the 20th century, and its alloys have many uses in orthopedic and dental research [
9]. The popularity of titanium is attributed to its excellent biocompatibility, corrosion resistance, and high strength; nevertheless, its high modulus of elasticity limits its use as sole material for implants and hence the incorporation of pure titanium or titanium dioxide in polymers has resulted in feasible polymeric composites [
9]. A high modulus causes bone resorption, and therefore, minimizing this effect results in successful integration of implants in the body [
3,
9]. Other types of fillers such as quasi-crystalline (QC), hydroxyapatite, polyimidazole fibers, and aluminum oxide have also been added to HDPE matrix to fabricate specialized composites [
1,
3,
12,
13].
Nanocomposites based on polymeric matrix are fabricated by various techniques including injection molding [
3,
14], compression molding [
15,
16], in situ polymerization [
17,
18], sol-gel [
19], and sintering [
9]. Among them, in situ polymerization involves the dispersion of inorganic nanoparticles in a monomer phase as a first step, followed by bulk phase polymerization [
20]. The resulting solution produced polymeric nanocomposites with well-dispersed nanoparticles and good flowing properties [
17]. However, a limitation of this technique shows that the mixture consists of unstable nanocomposites that may revert into a different morphology other than the expected [
20]. Moreover, this process is not very common in industry and is mainly used for thermosetting polymers. In addition, compression molding is another technique used for fabricating nanocomposites; it involves a mold cavity in which the polymer blend is poured and results in composites of various dimensions. The processing conditions include very high pressure and the fabricated nanocomposites may not always have a uniform consistency [
16]. Furthermore, most compression molding techniques require pre-treatment of the nanoparticles with curing.
Injection molding offers higher production cycles than compression molding thermoplastics [
21]. This technique has become one of the most significant industrialized techniques in the field of polymer composites processing. The process provides high production rates, repeatable high tolerances, and low labor cost, and is anticipated to hold greater potential in the fabrication of uniform, good quality polymer micro- and sub-micrometer structures [
22]. Injection molding can be used in a variety of applications in both commercial and research fields. A large number of polymer parts with complex geometry and good dimensional accuracy can be automatically manufactured by injection molding [
23].
Liu et al. fabricated Ti-HDPE composites based on a sintering method that was carried out between a temperature of 1000–3000 °C and time ranging between 1 h and 12 h [
9]. Sintering, powder compaction and sol-gel are all alternative techniques to produce polymeric composites, however, the operating conditions (temperature, pressure etc.) and time needed are far in excess of those of injection molding [
24]. As a polymer matrix, high density polyethylene (HDPE) is well-known for its semi-crystalline structure which is closely bound to the overall macroscopic properties of the polymer such as mechanical and thermal characteristics [
25]. In injection molding, HDPE undergoes an isothermal crystallization process which is a complex phenomenon occurring due to the varying shear and temperature gradients that occur while the polymer melts and fills the mold [
23]. The various gradients contribute to the formation of different layers with different levels of crystallinity that affect the mechanical properties of the composites. In addition, the residence time (i.e., the time duration that the polymer spends in the barrel) along with barrel temperature, and the crystallization process of the polymer, especially HDPE, become affected [
23]. In a study by Bociaga and Palutkiewicz [
24], it was found that mold temperature had the greatest impact on the mechanical properties and surface structure. They investigated the effect of different injection molding conditions and concluded that low injection temperature, or barrel temperature, resulted in polymeric molded parts that consisted of a solid skin layer and a fine cellular core. Also, low weight HDPE parts were obtained at low injection temperature due to the formation of numerous tiny pores and the resultant high viscosity of the melting plastic, since temperatures used were close to the melting point of HDPE [
26]. In his study, Mourad [
27] concluded that the modulus of elasticity, yield strength, and tensile toughness decreased with increasing polyethylene (PE) content in the PE/PP blends of different concentrations and different process parameters. In a similar study by Mourad et al. [
28] on the thermal characteristics of thermally treated and untreated VLDPE and isotactic PP blends (iPP), it was found through TGA and DSC analysis that addition of VLDPE resulted in a decrease in the melting temperature, heat of fusion, and percent crystallinity of the iPP based blends.
Since injection molding is a commonly used technique for mass production in industrial applications, it is imperative to study the different operating conditions so that the process is optimized [
29]. Therefore, the aim of the current study was to prepare HDPE-TiO
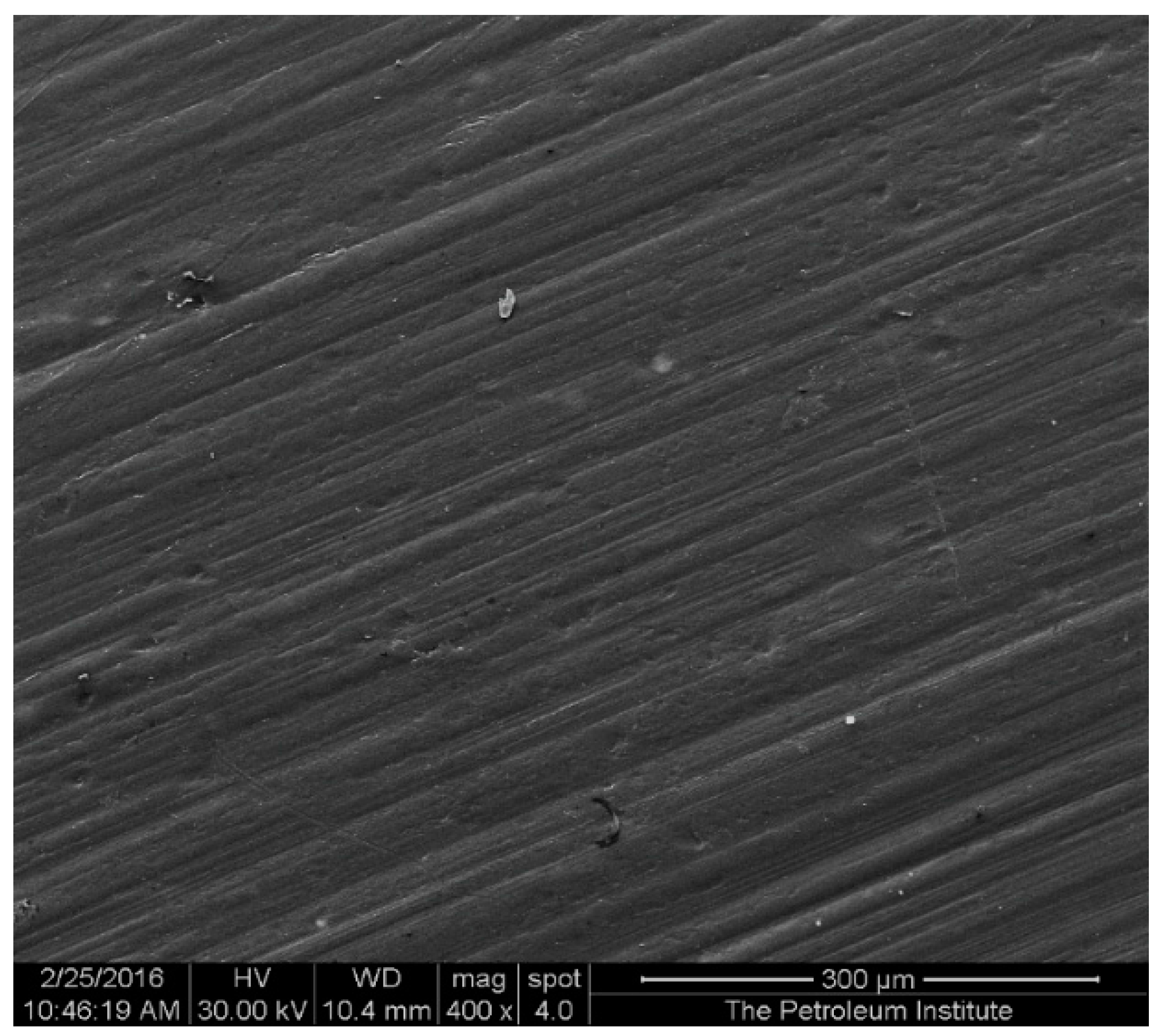
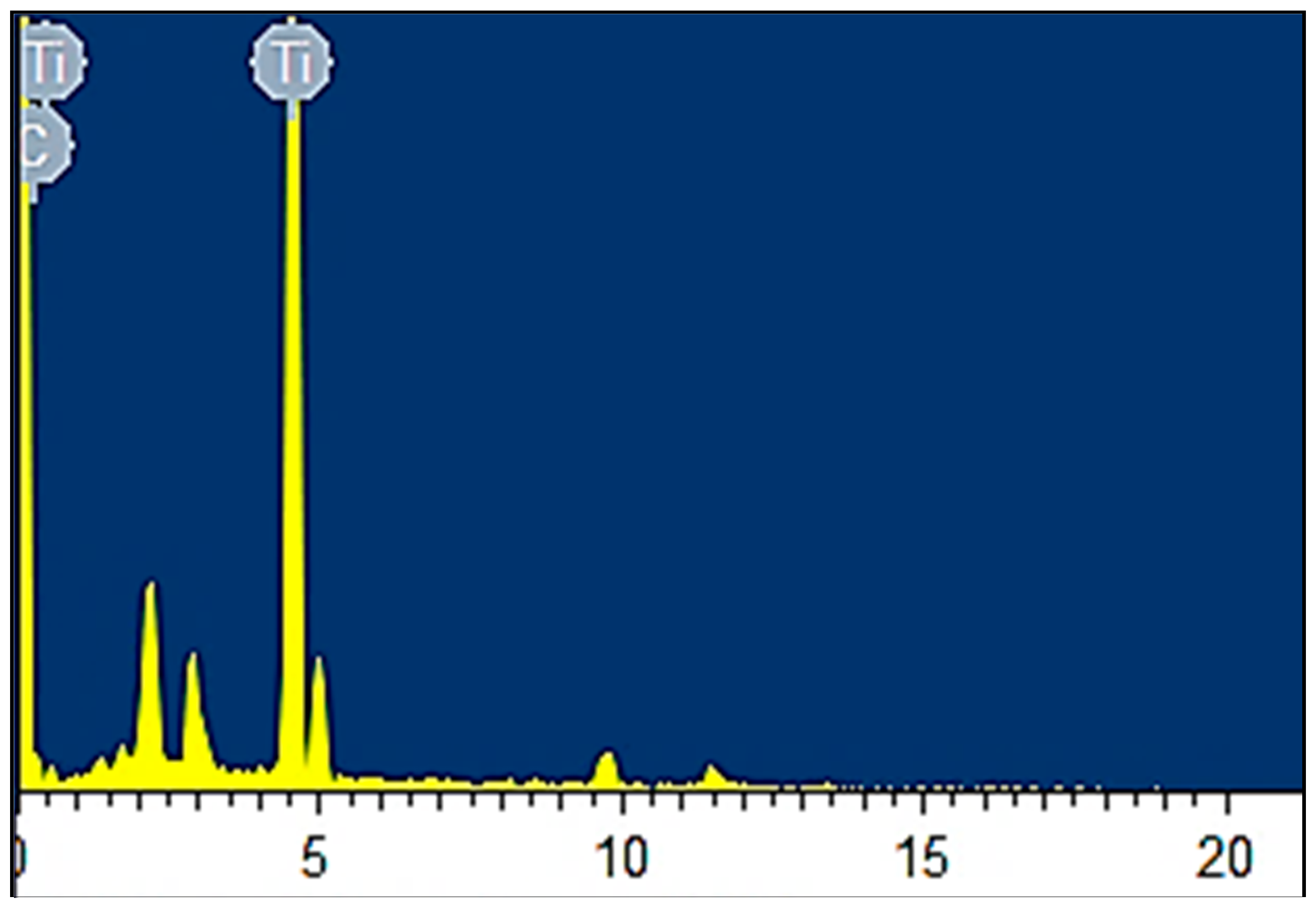
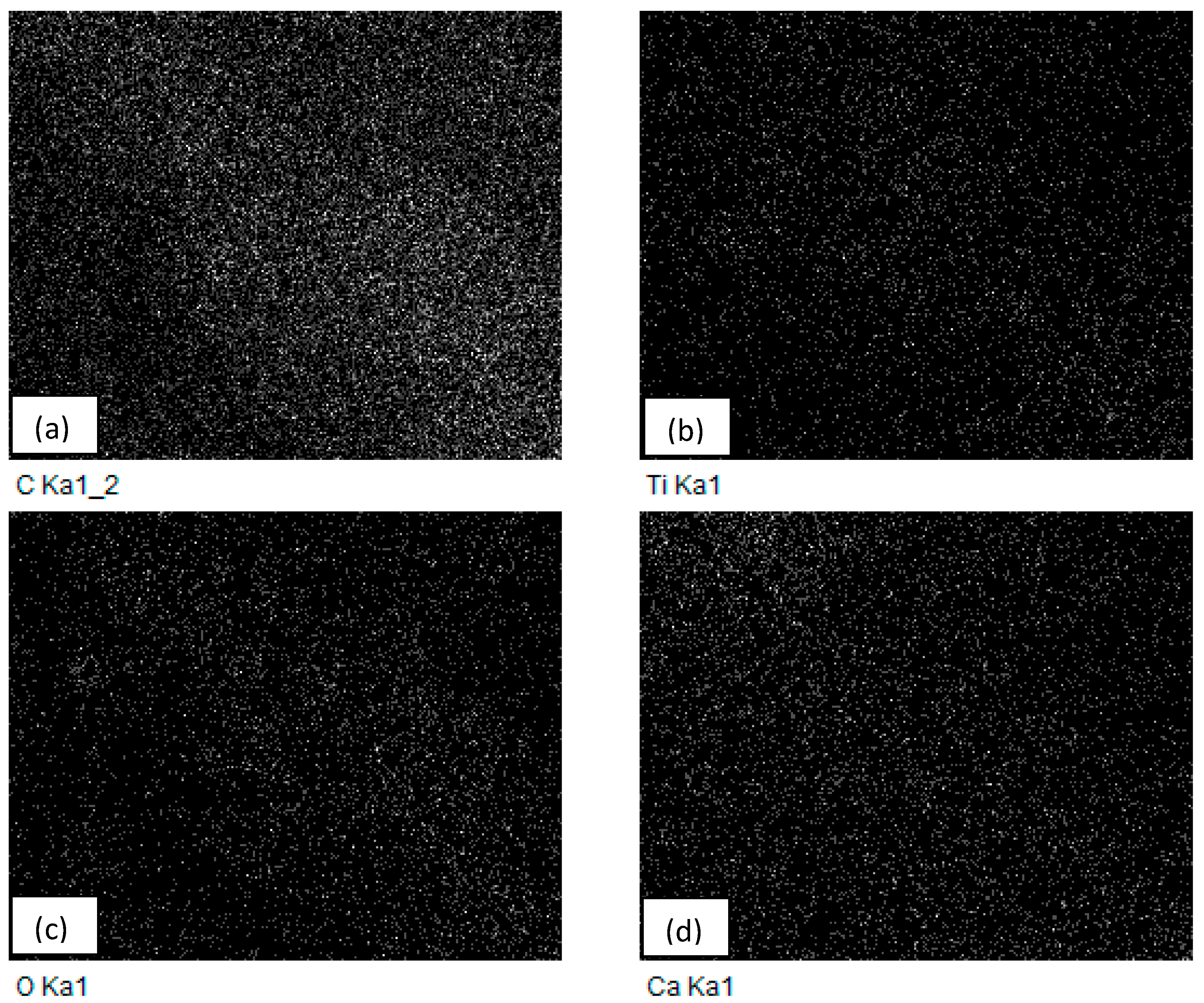
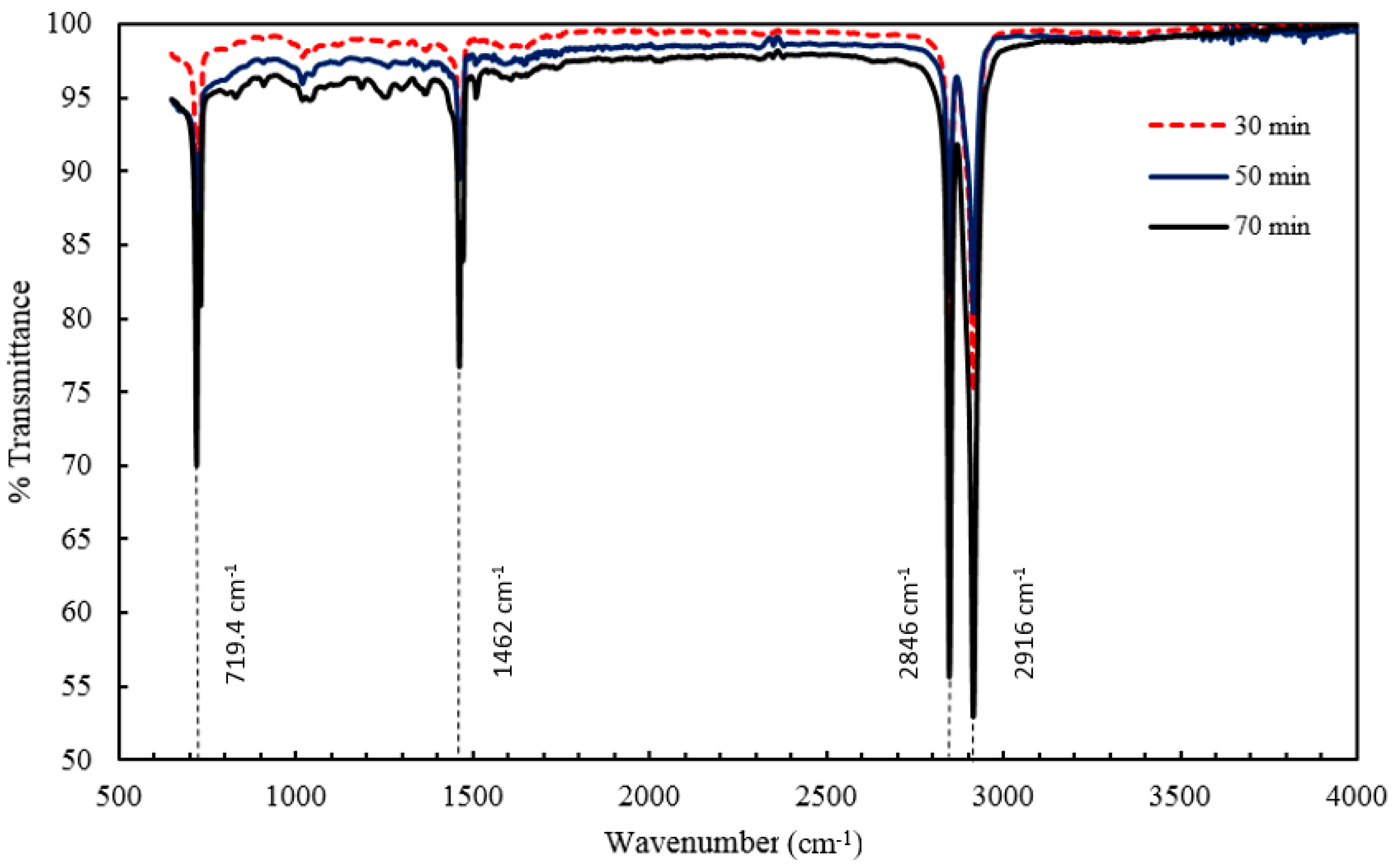
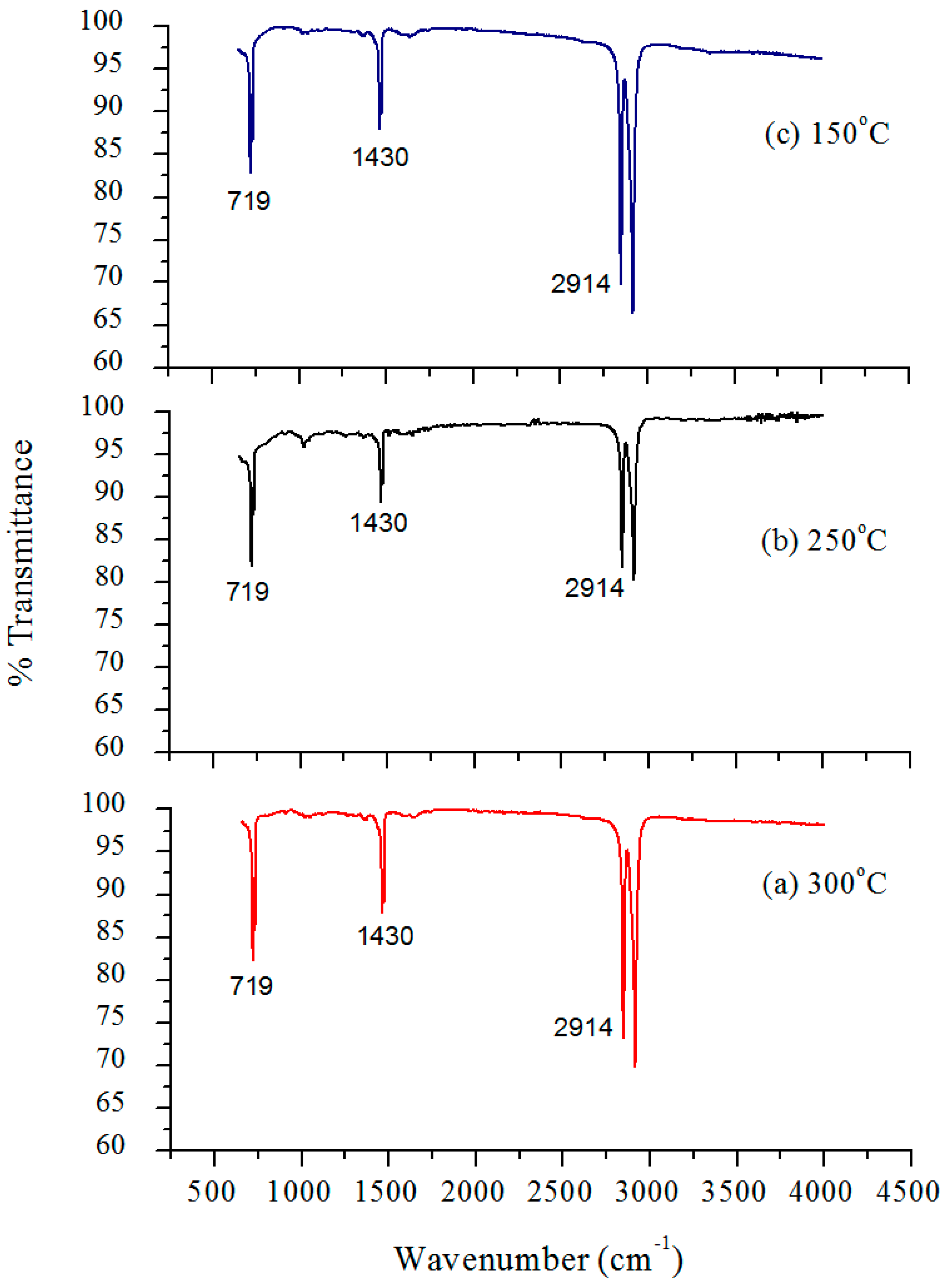
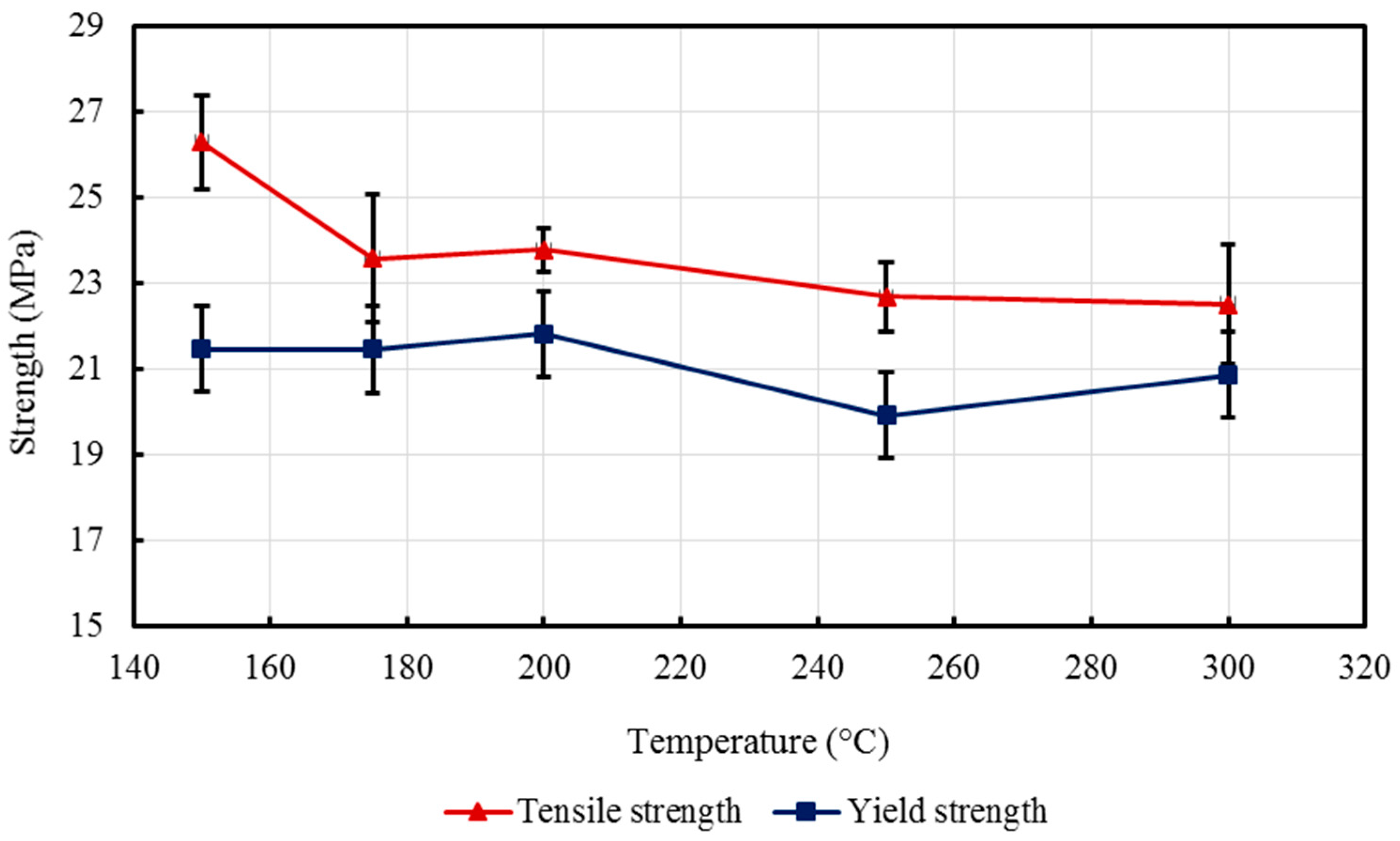
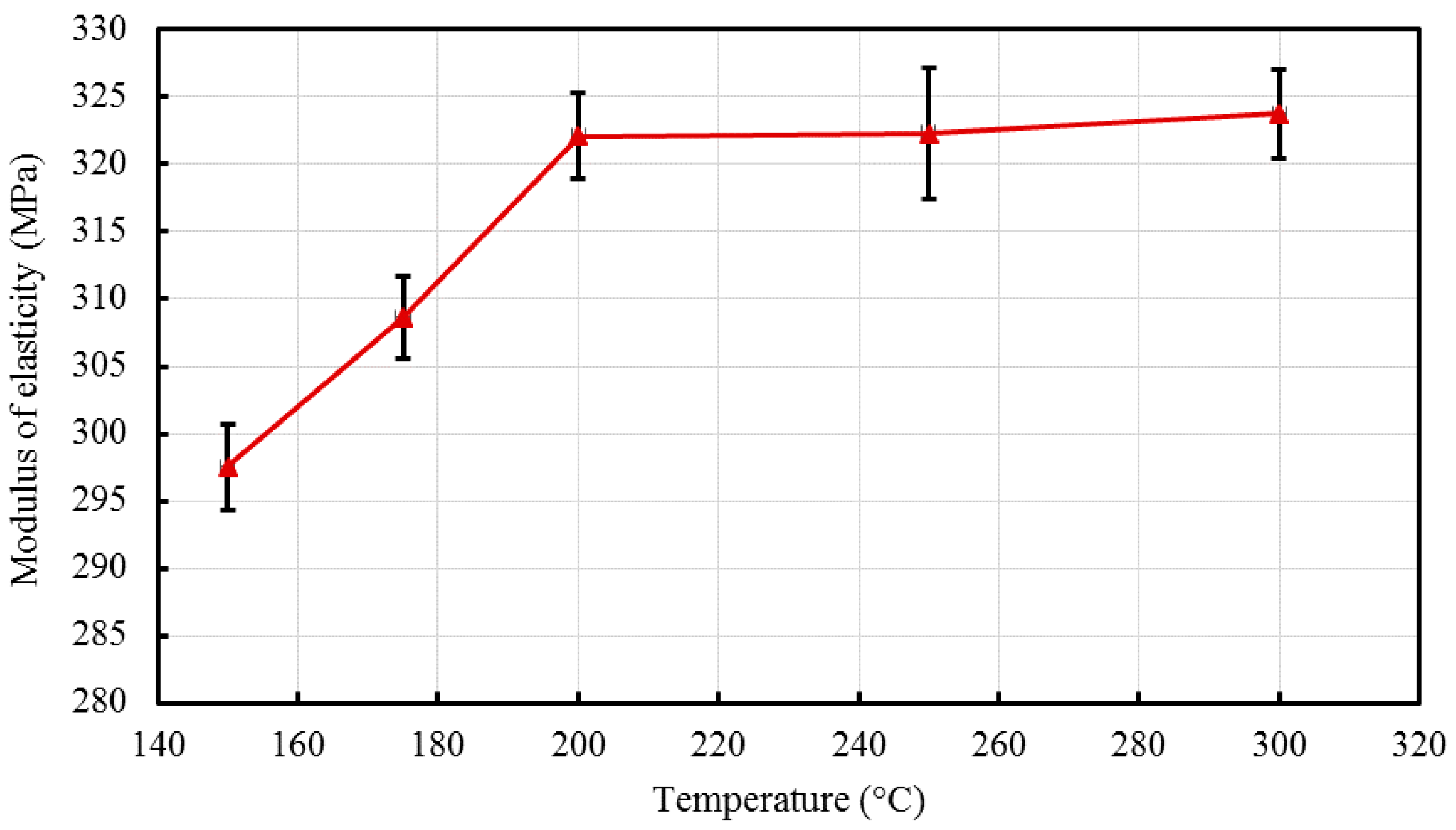
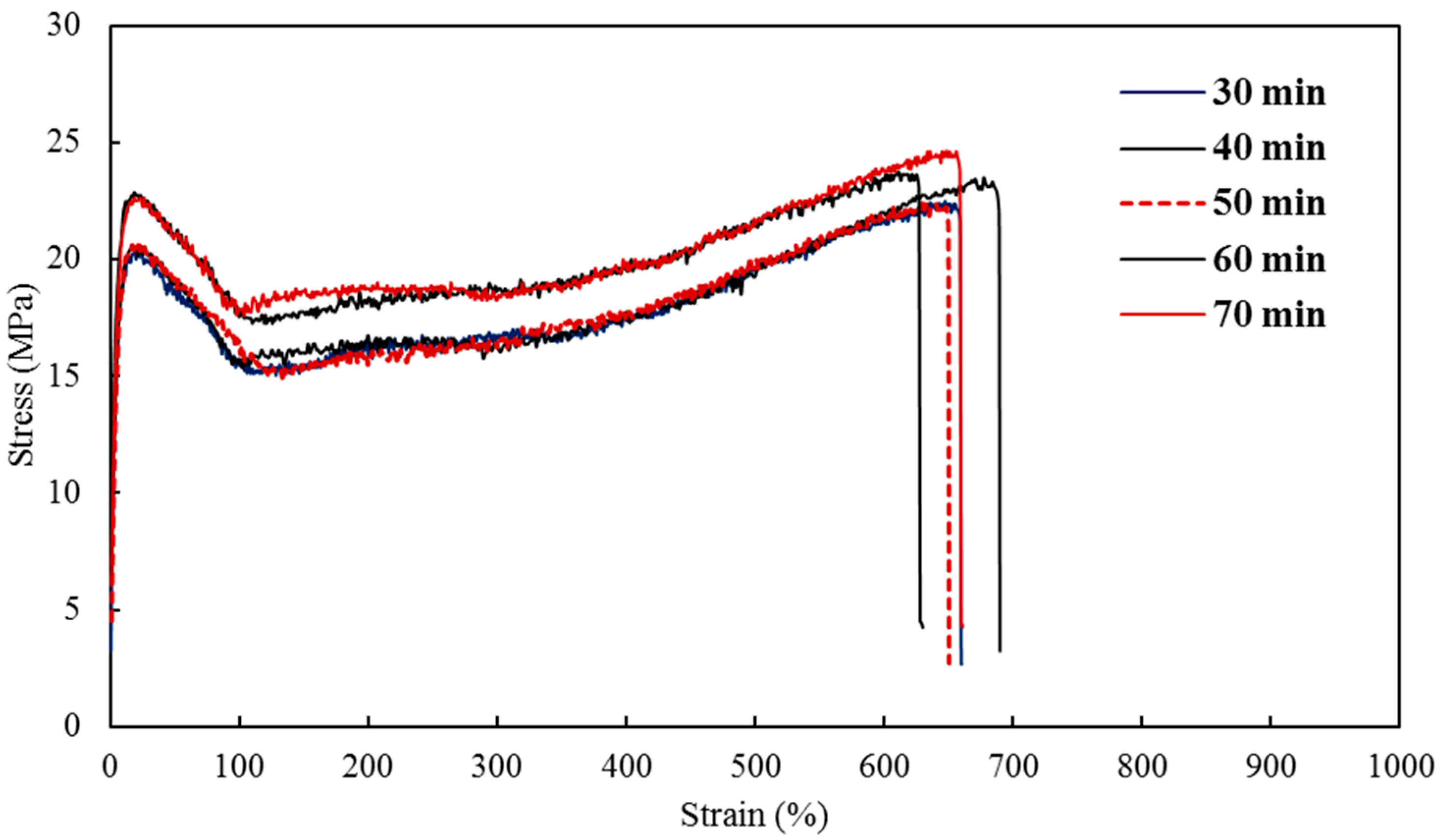
2 nanocomposites with a 5% constant nanofiller concentrations through injection molding. The two main parameters studied were the barrel temperature and the residence time fixed at 250 °C and 50 min respectively when either of the parameter was being studied. The effects of these process parameters were investigated based on the measured mechanical properties (i.e., Young’s modulus, tensile strength, and percent elongation) and thermal properties (e.g., crystallization and degradation temperatures) of the produced composites. Moreover, structural analyses such as X-ray Diffraction (XRD) and Fourier Transform Infrared (FTIR) spectroscopy were also used as tools to differentiate any structural changes of the polymer matrix. The surface morphology and titanium dioxide distribution was studied by means of scanning electron microscopy (SEM) along with energy dispersive spectroscopy (EDS) mapping of different elements along the surface of the injection molded substrates.
4. Conclusions
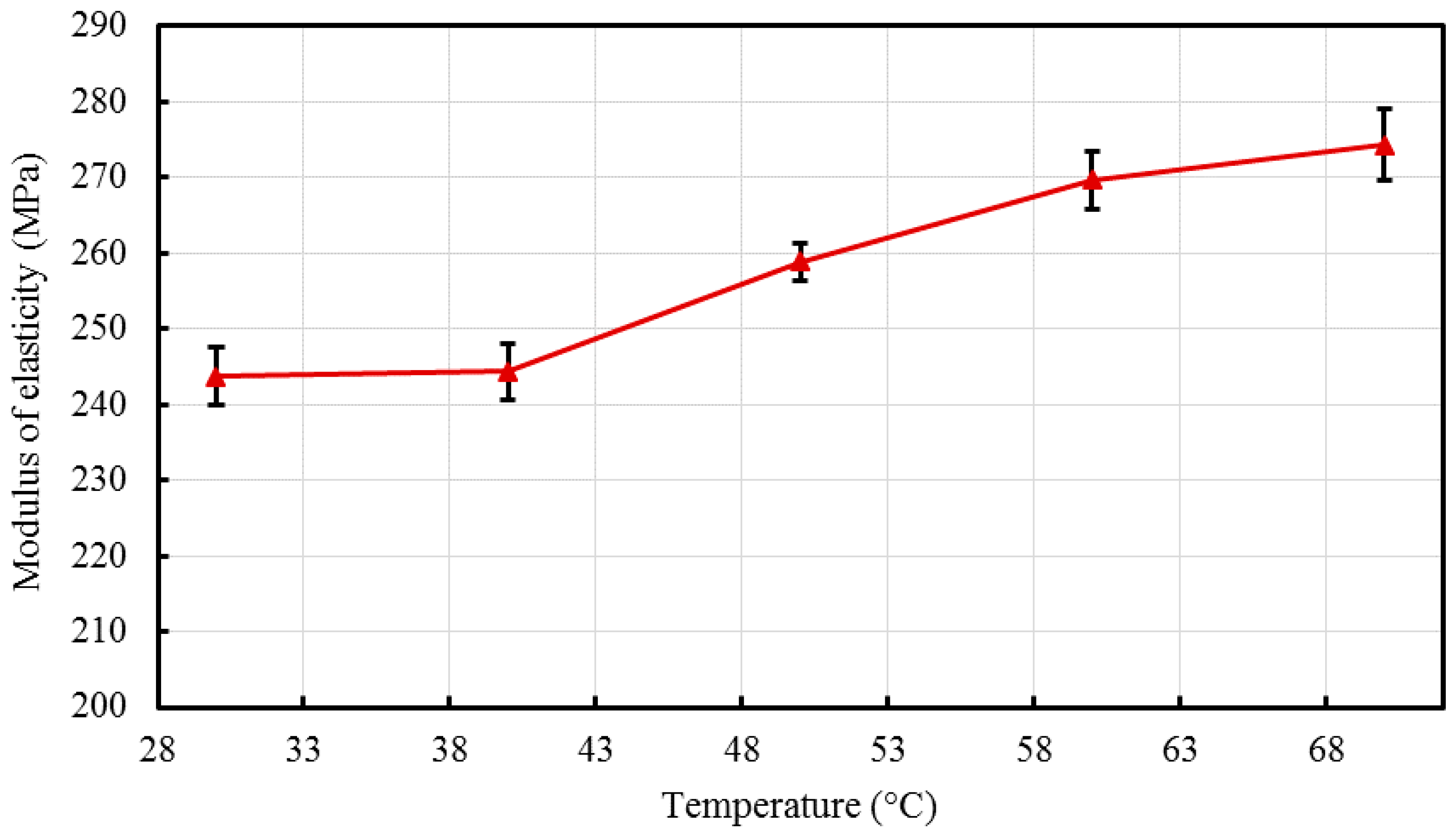
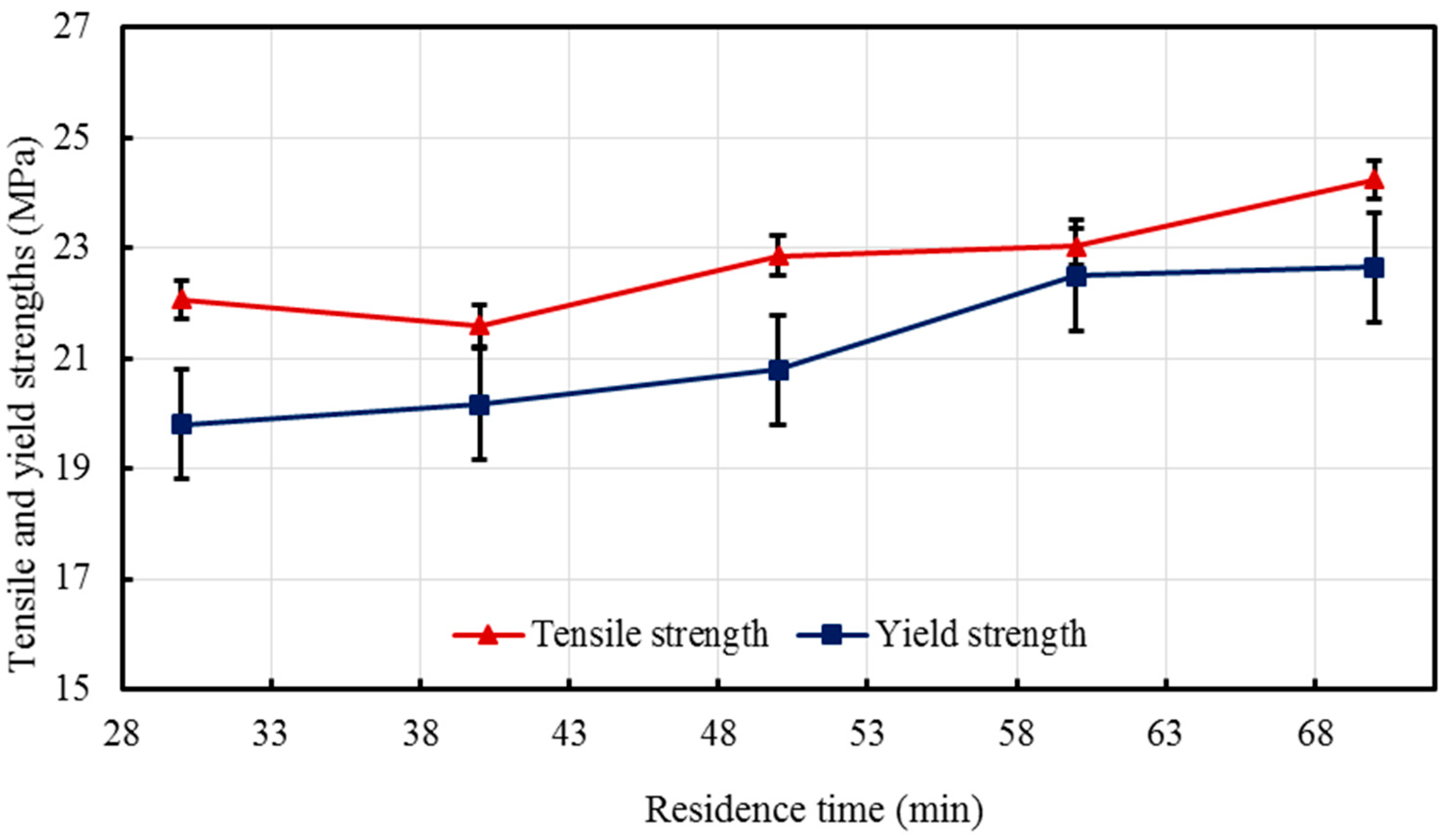
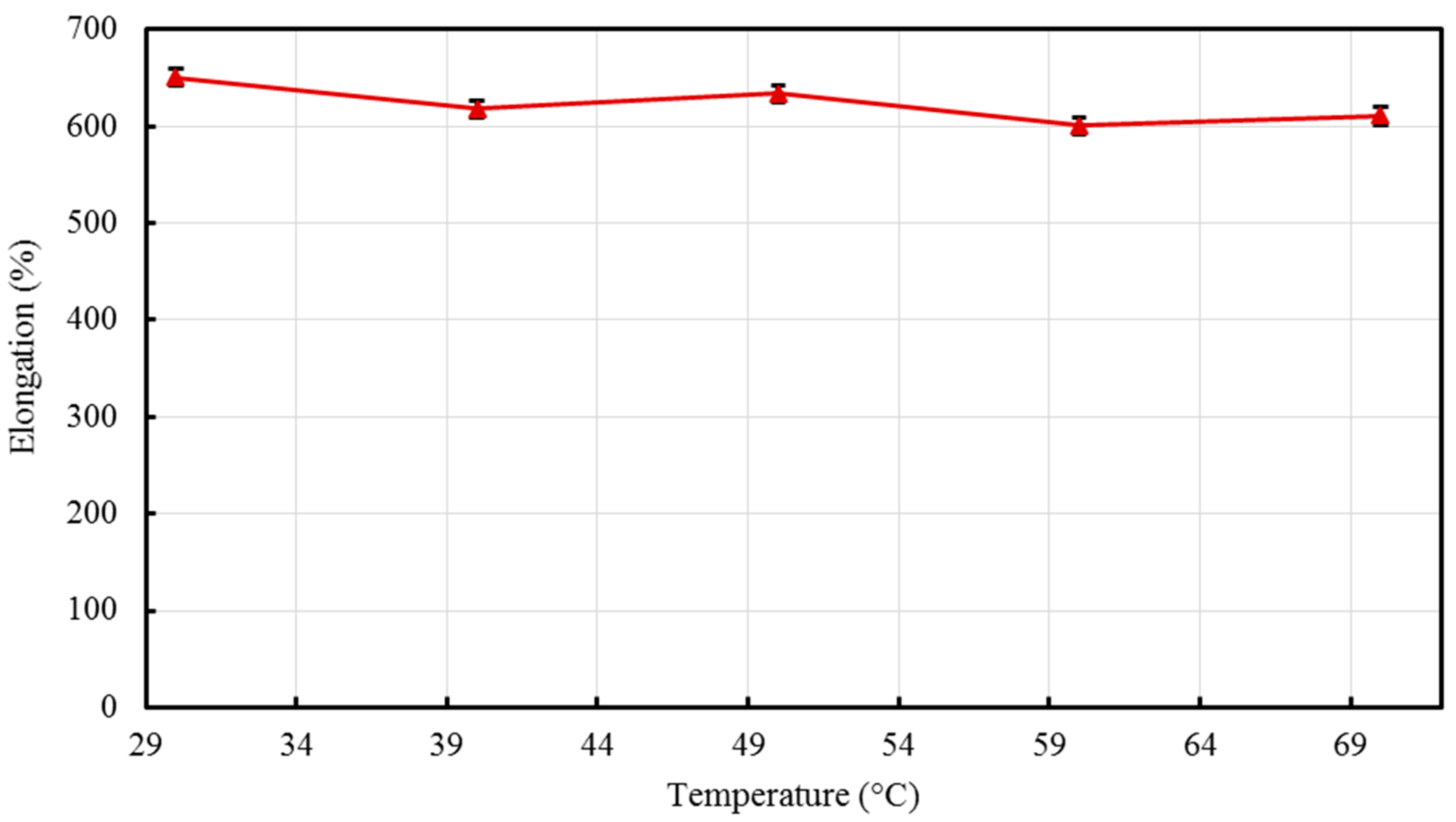
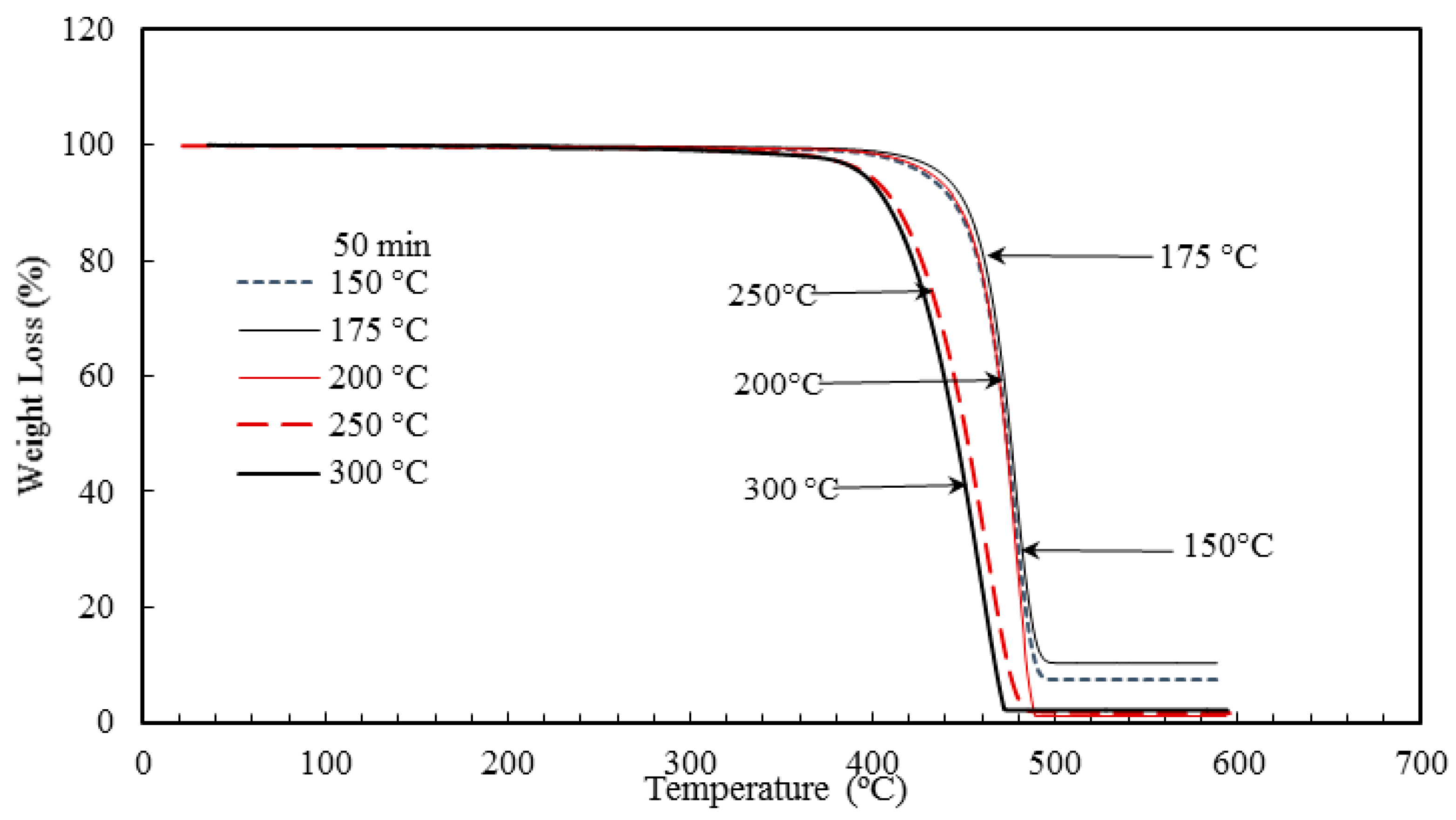
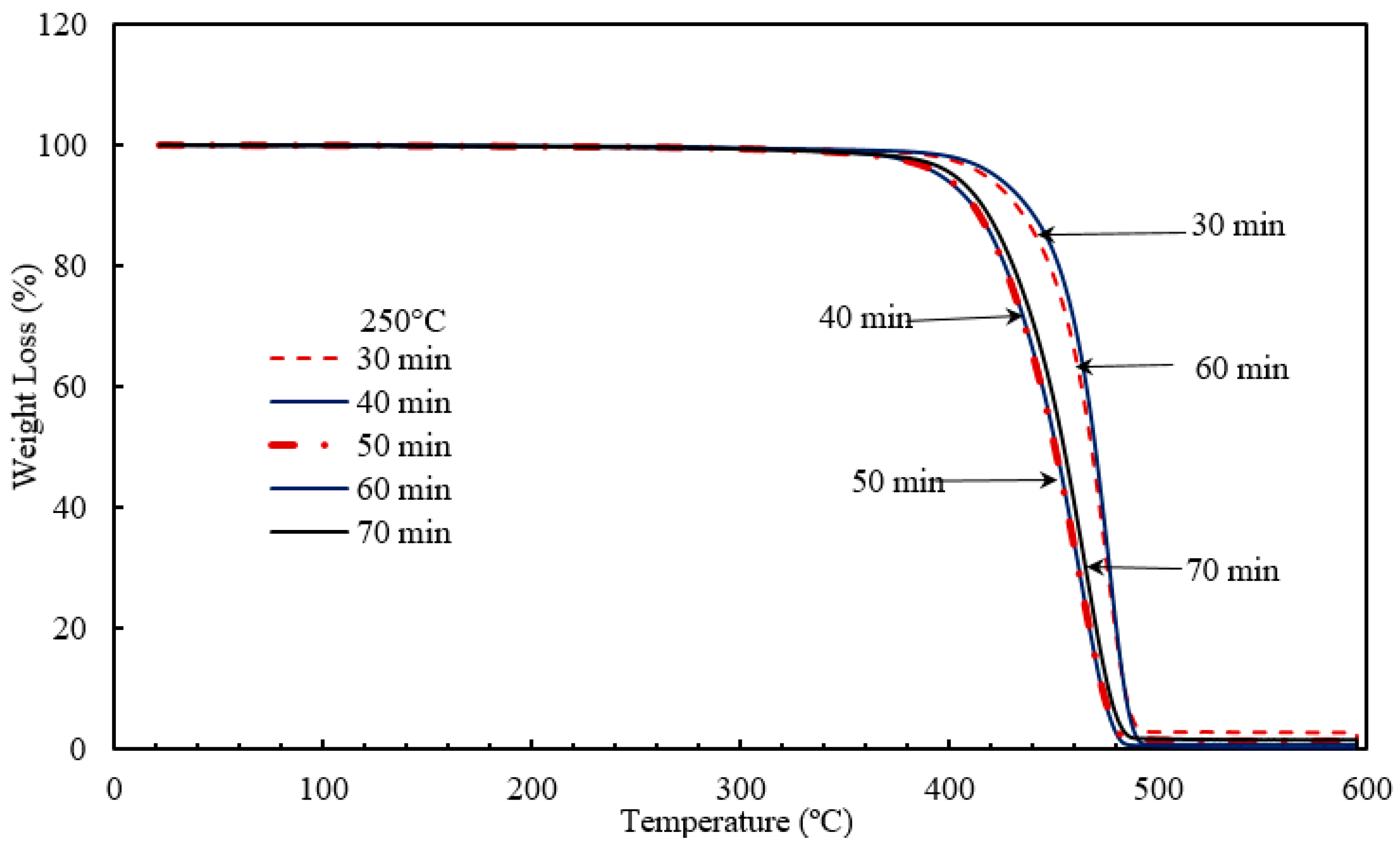
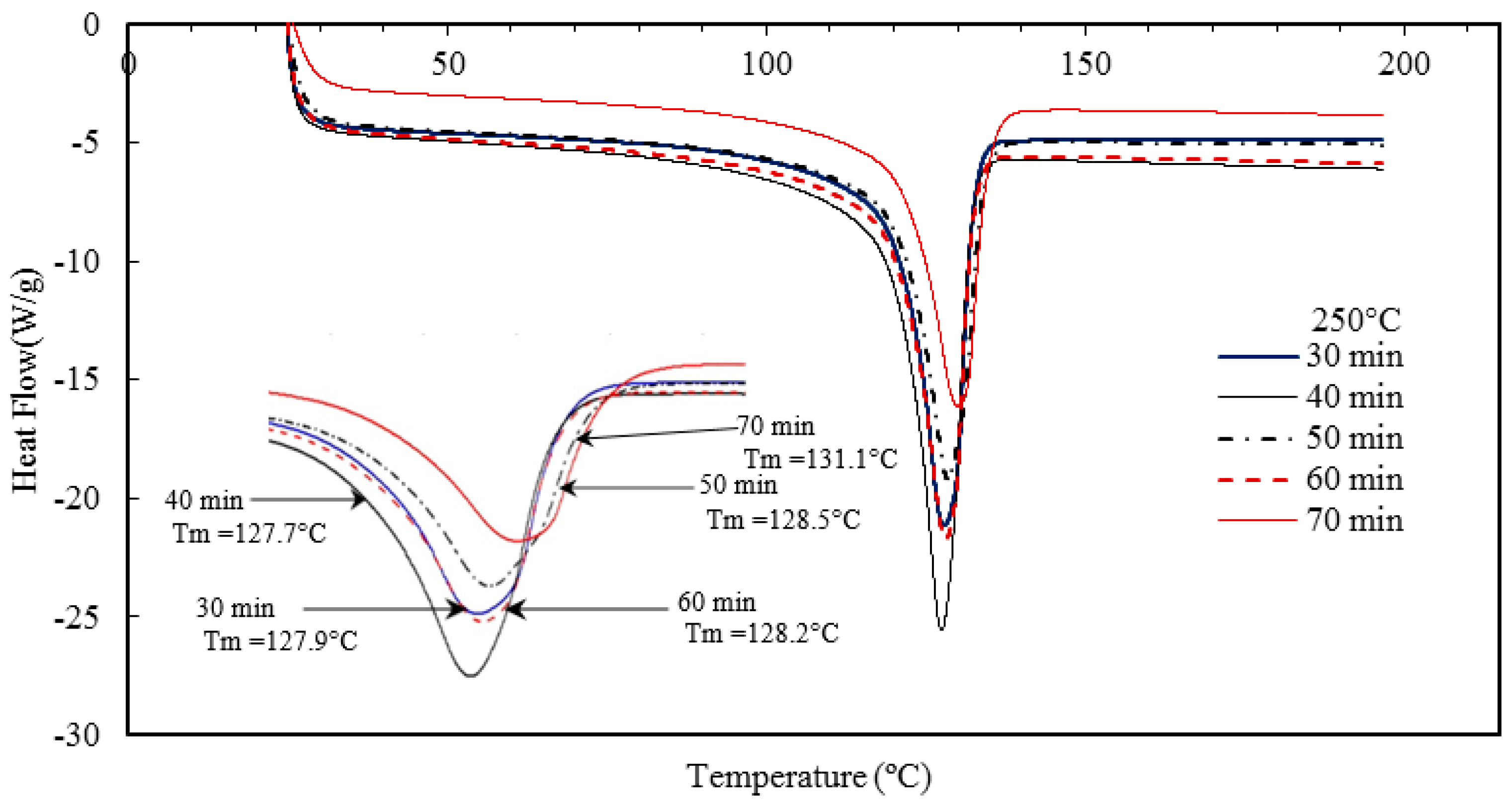
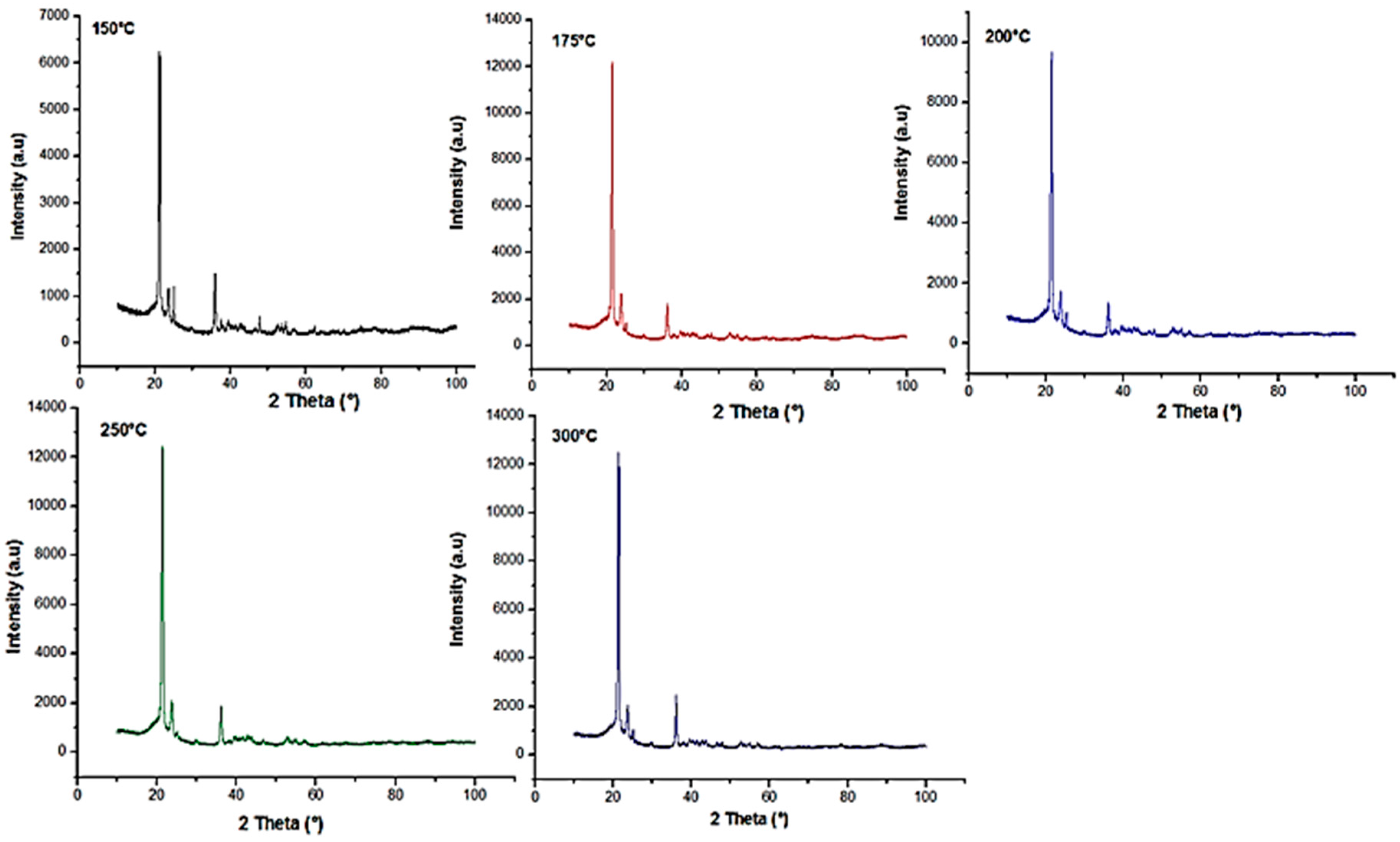
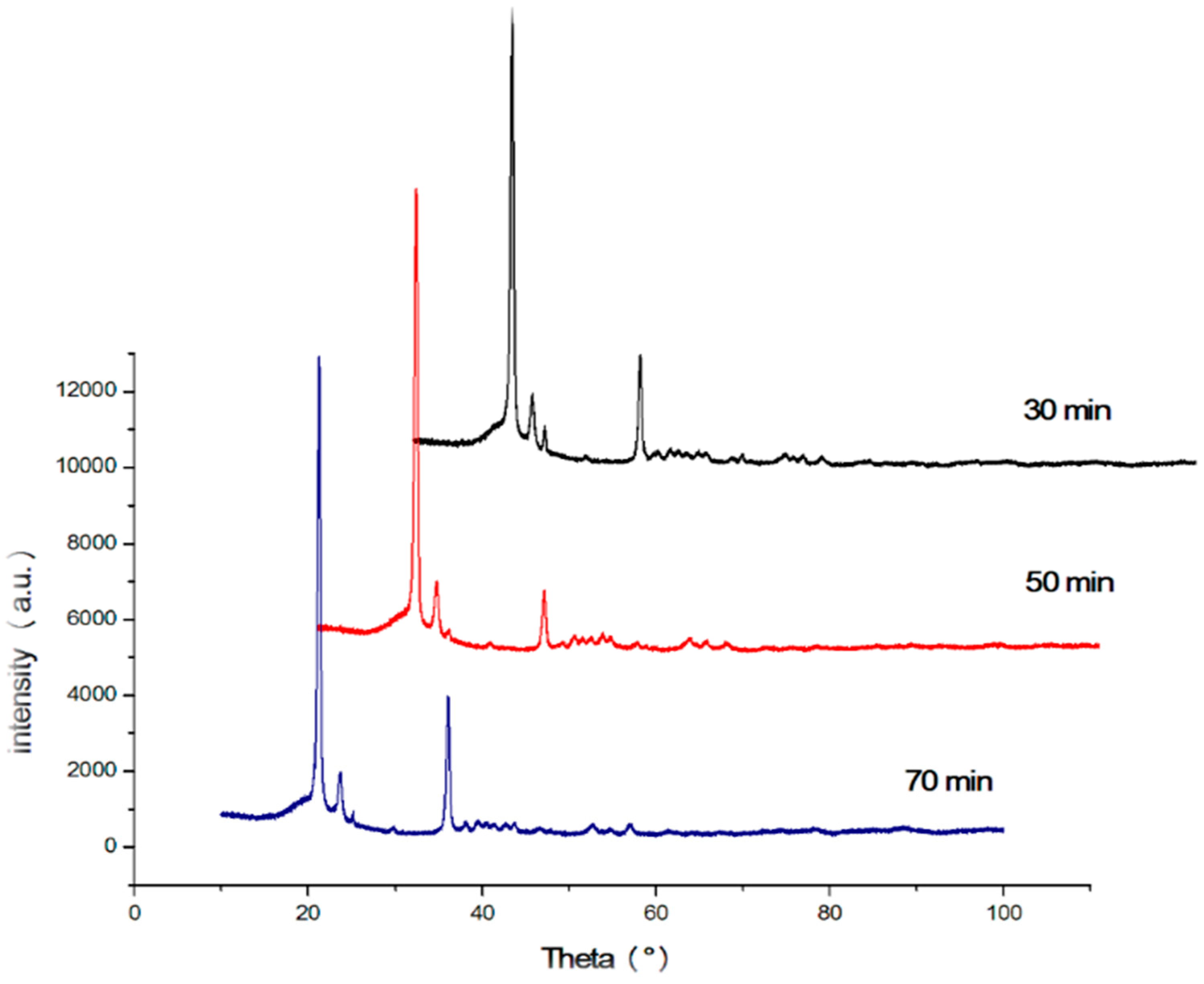
Nano-sized titanium dioxide enriched HDPE nanocomposites were fabricated through injection molding using dumbbell shaped inserts. In the injection molding process, the two main control variables are the barrel temperature and the residence time. Hence this study aimed to compare the nanocomposites’ mechanical, thermal, and structural properties obtained by changing these operating parameters. The SEM-EDS, FTIR, and XRD results confirmed the uniform distribution of TiO2 nanoparticles on the surface of the matrix. The degradation temperature decreased with an increase in the barrel temperature and residence time, however, the rate of crystallization showed a consistent rise in both cases; rate of crystallization was increased up to 75%.
The results revealed that there is no specific barrel temperature or residence time as an optimum value; for such nanomaterials, a range of temperature (150 °C–300 °C) can be considered for manufacturing these nanocomposites. However, the authors recommend researchers to use an operating barrel temperature lower than 300 °C since the polymer’s initial degradation temperature is about 424–460 °C. When samples were processed at temperatures around 300 °C, black spots were observed on the injection molded nanocomposites. Lastly, it is also recommended to use a temperature higher than 150 °C for easy material flow while injection molding. To conclude, the effect of varying the injection molding process parameters was studied and a wide range of barrel temperature and residence times can be used depending on the specific needs of the application.