Tensile Fracture Behavior and Failure Mechanism of Additively-Manufactured AISI 4140 Low Alloy Steel by Laser Engineered Net Shaping
Abstract
:1. Introduction
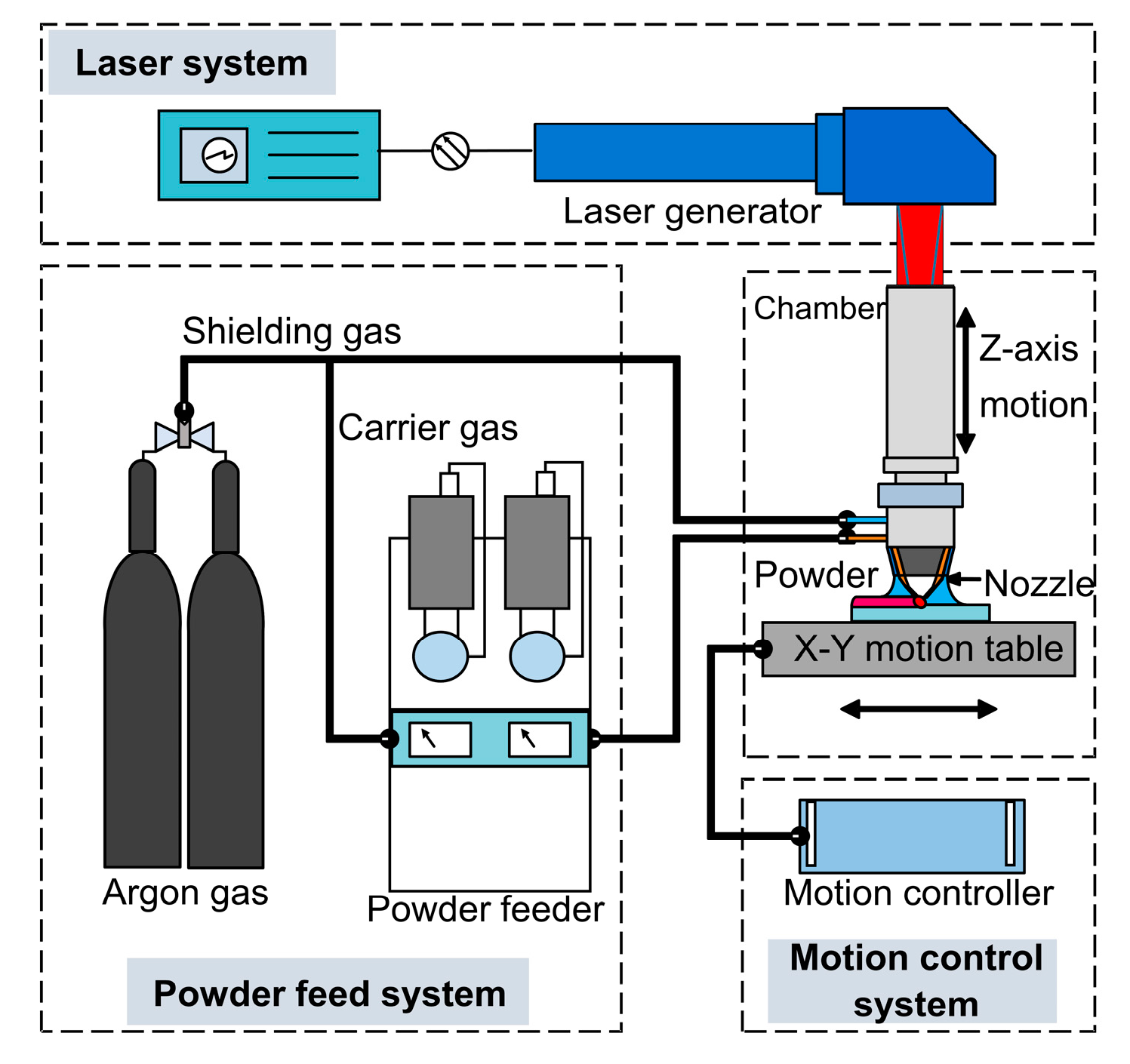
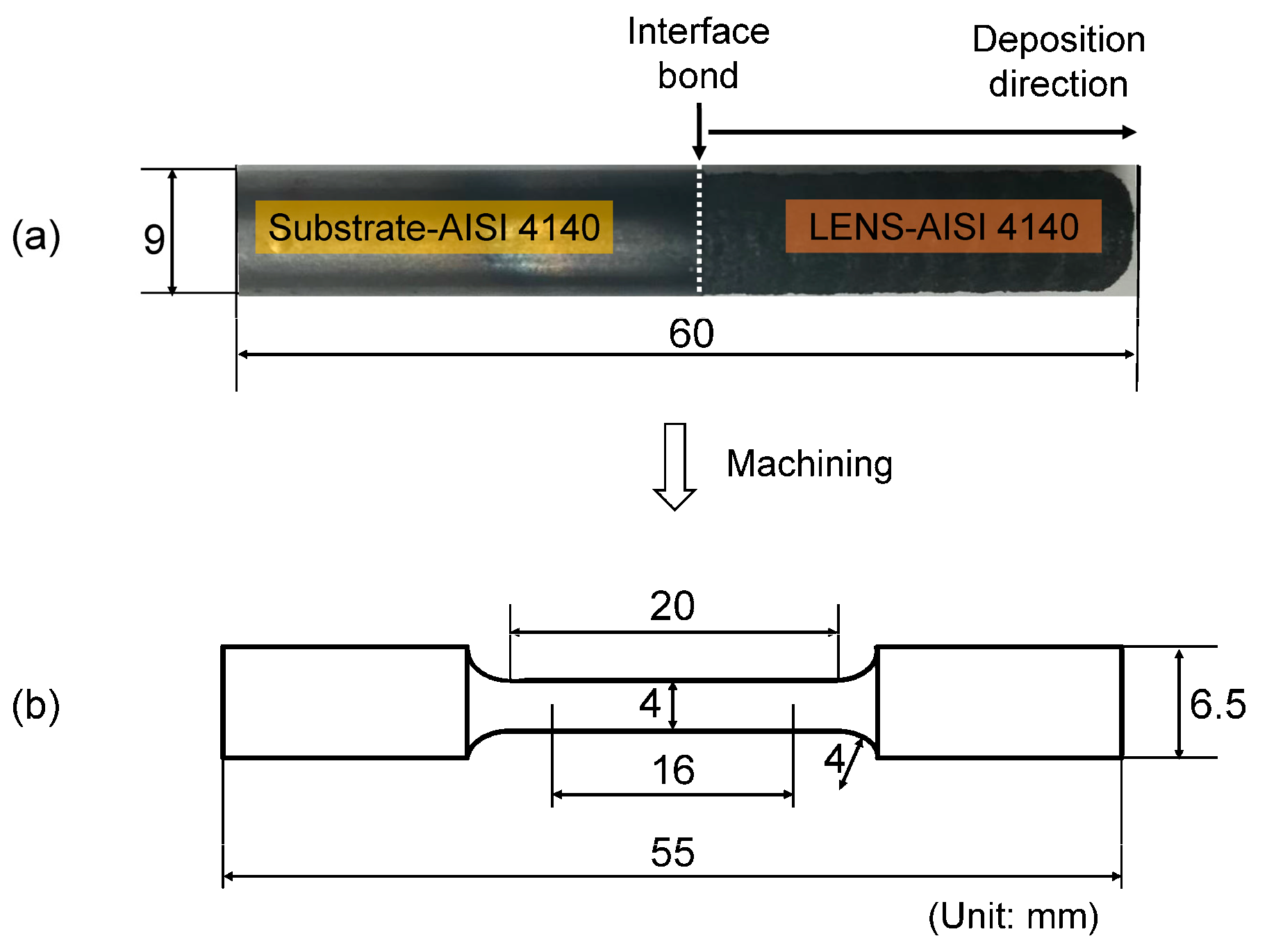
2. Materials and Methods
3. Results and Discussion
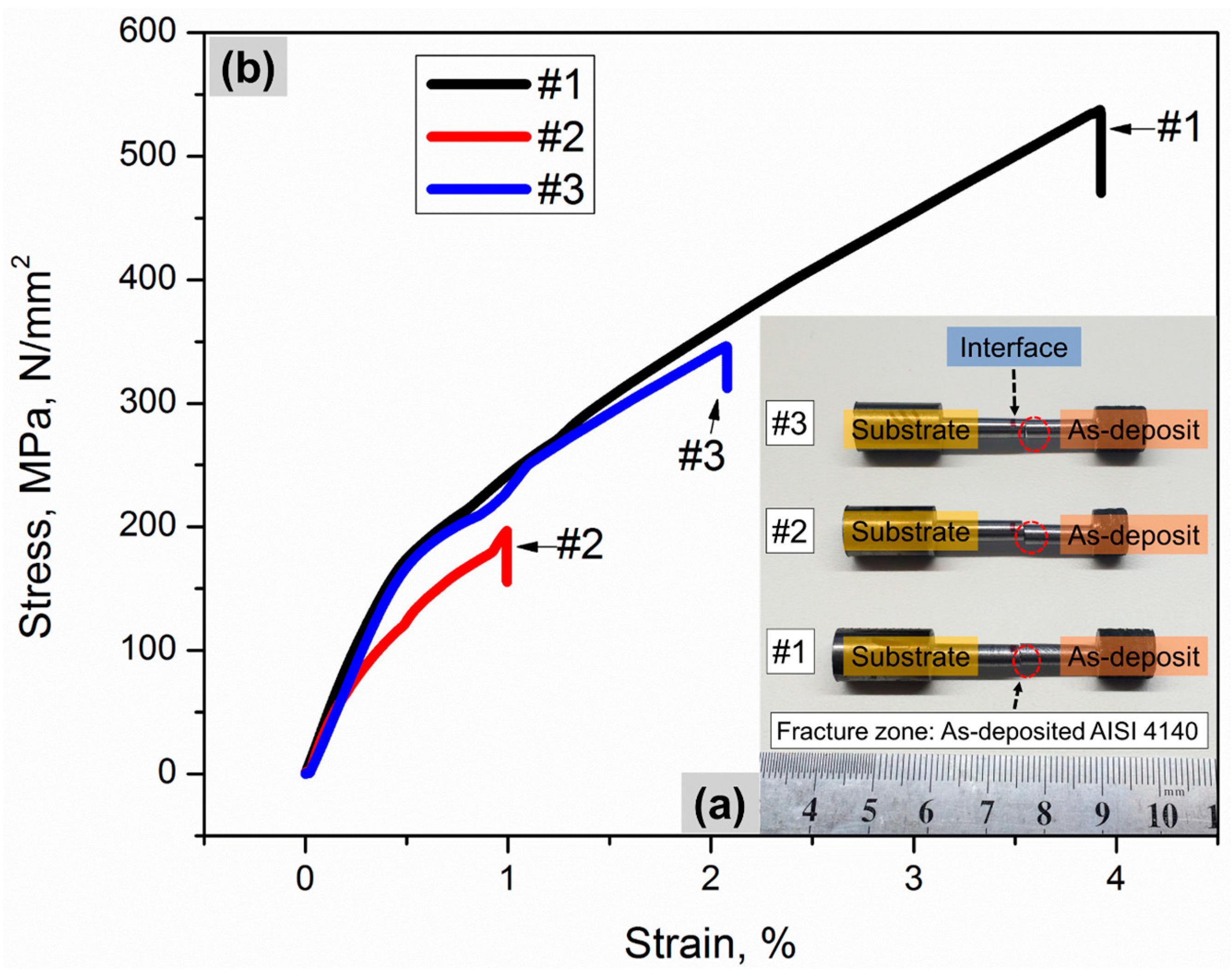
3.1. Interface Bond and Tensile Fracture Behavior
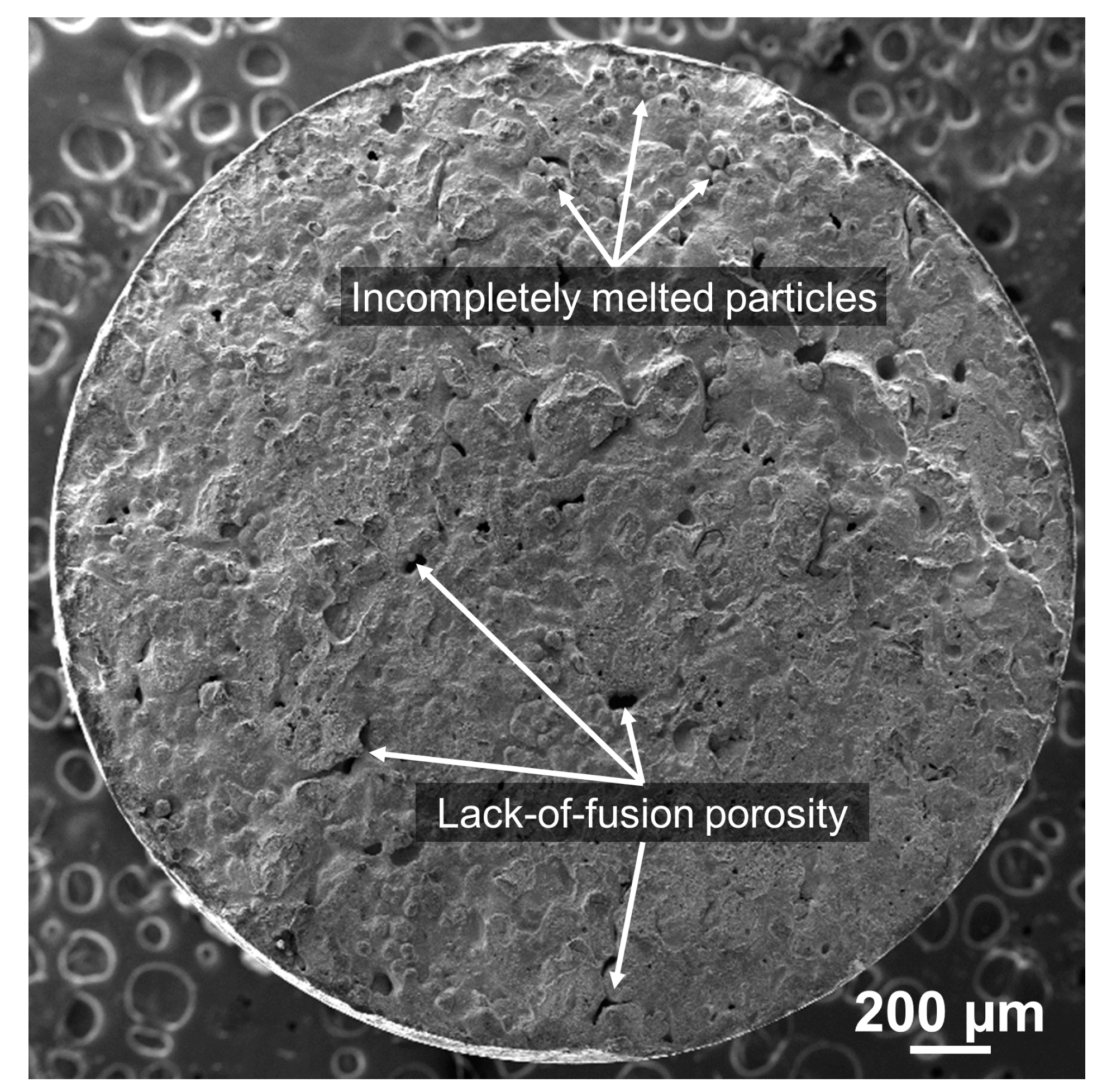
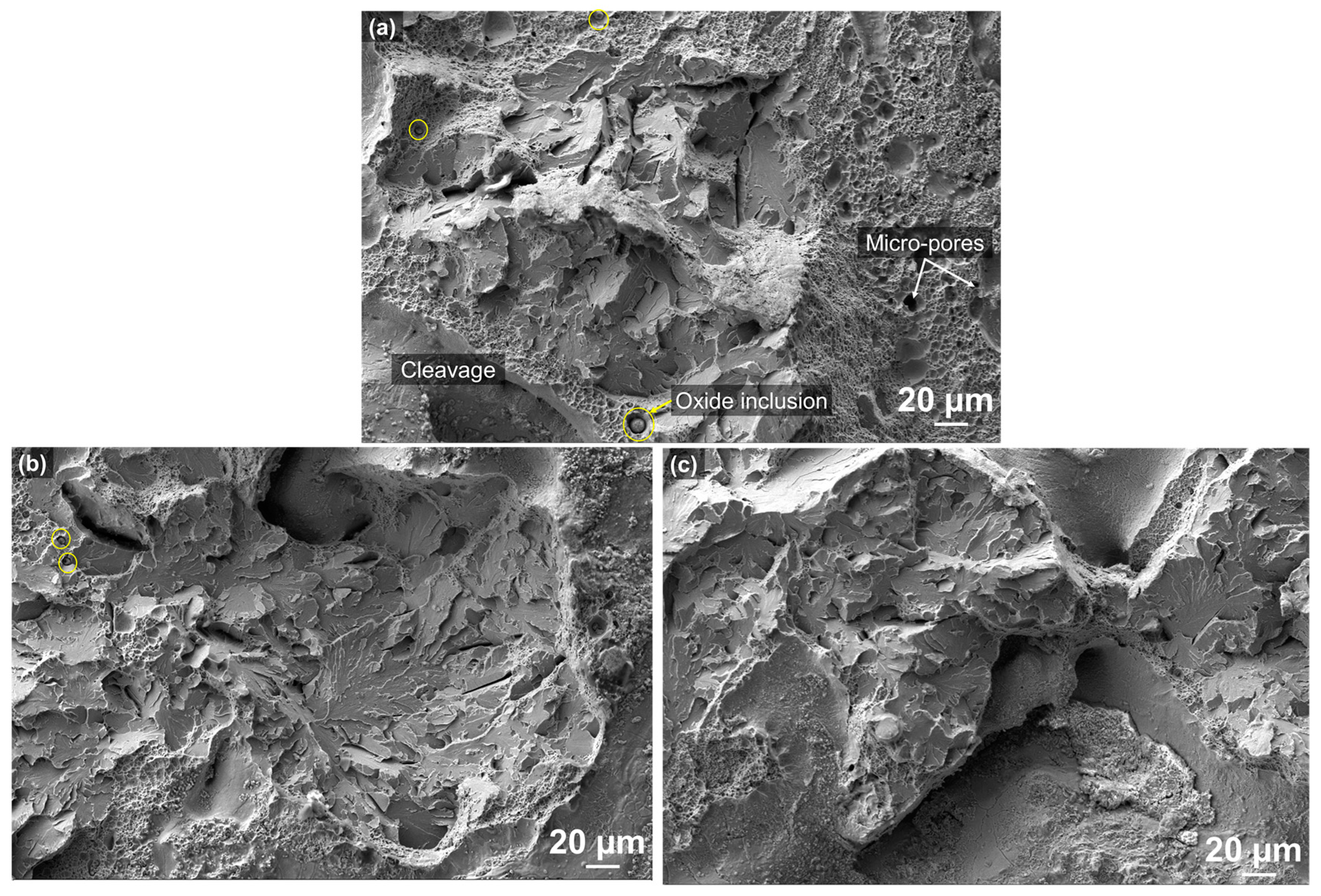
3.2. Fracture Surface and Failure Mechanism
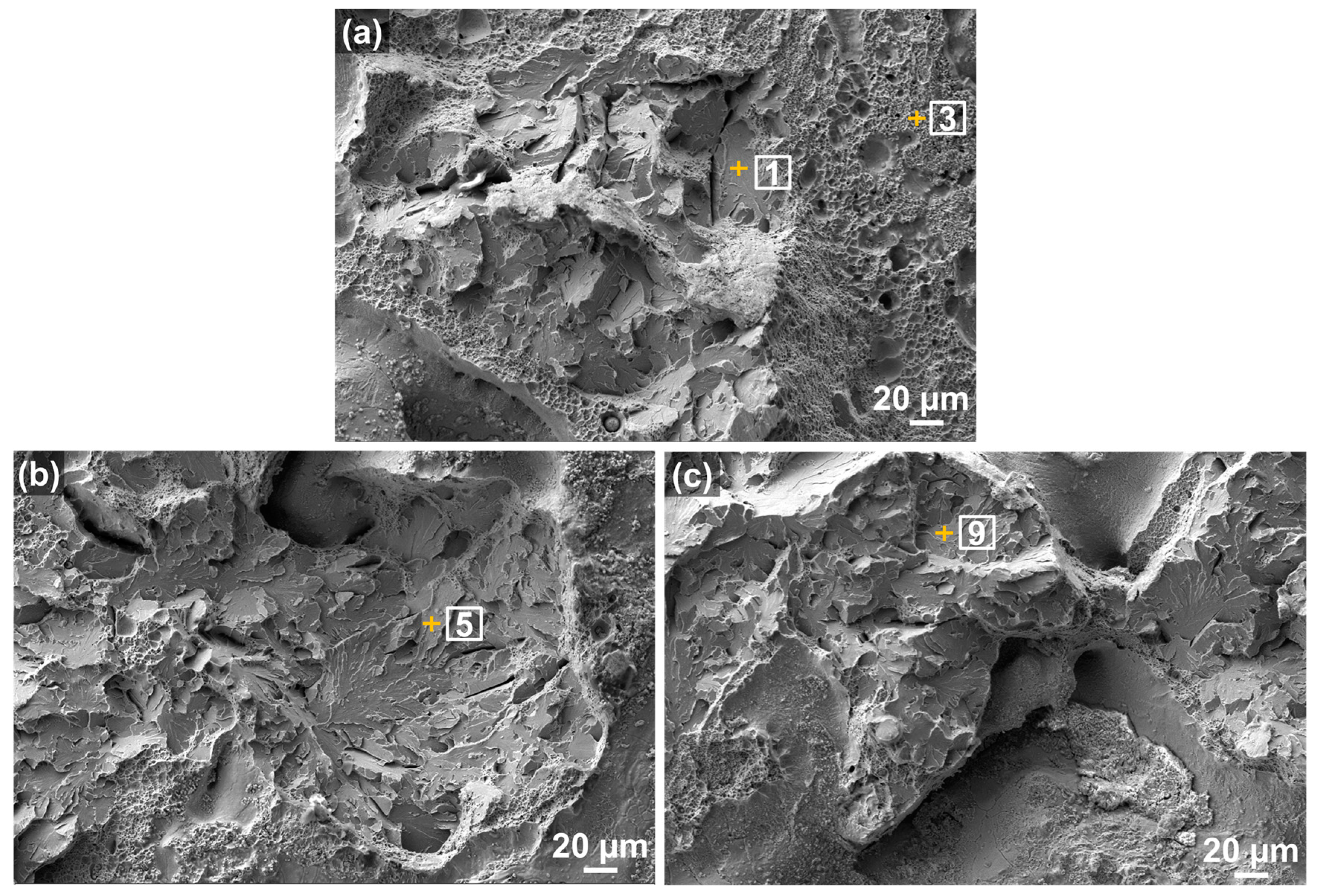
3.2.1. Fracture Morphology and Defects
3.2.2. Phase Formation
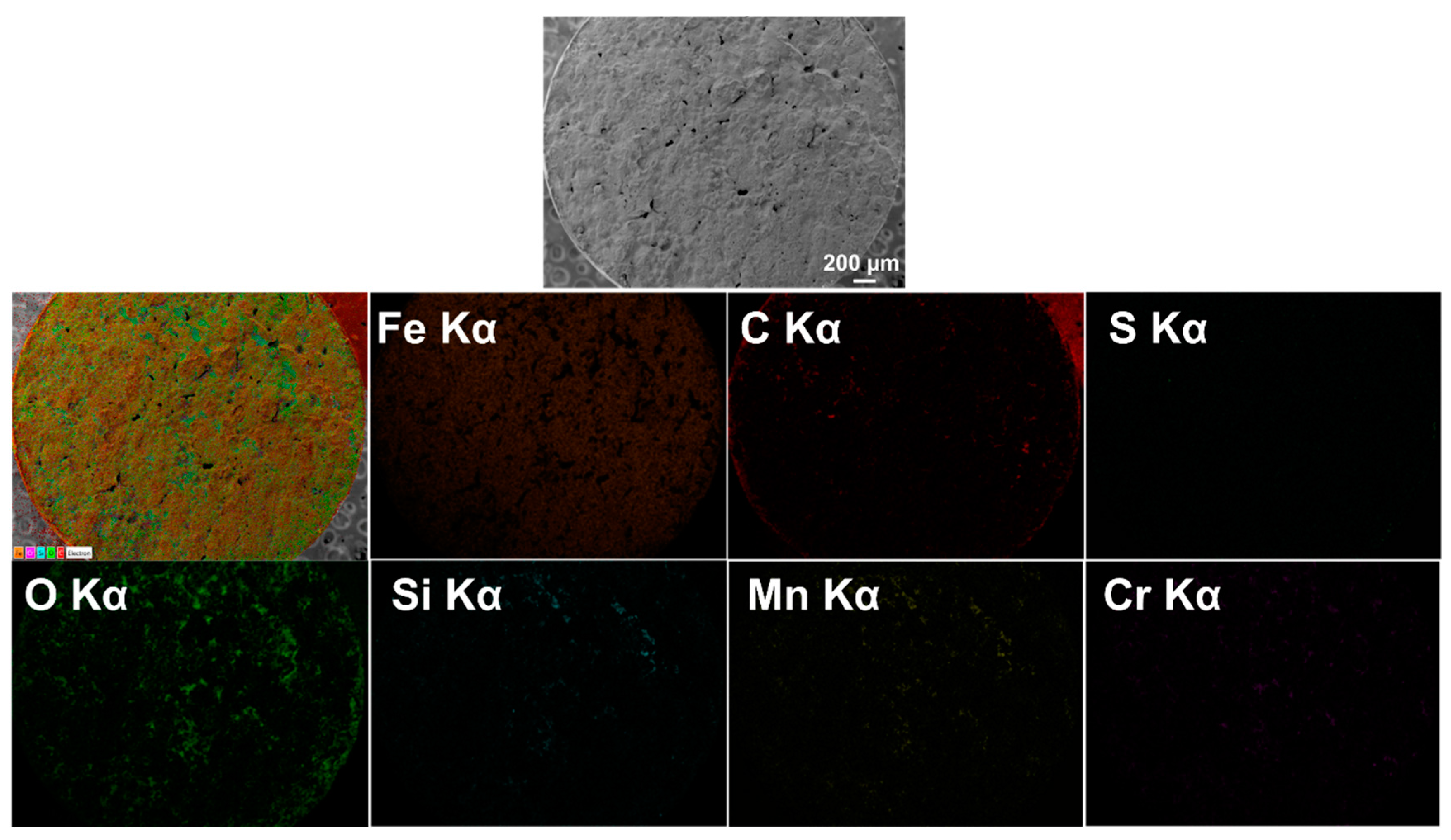
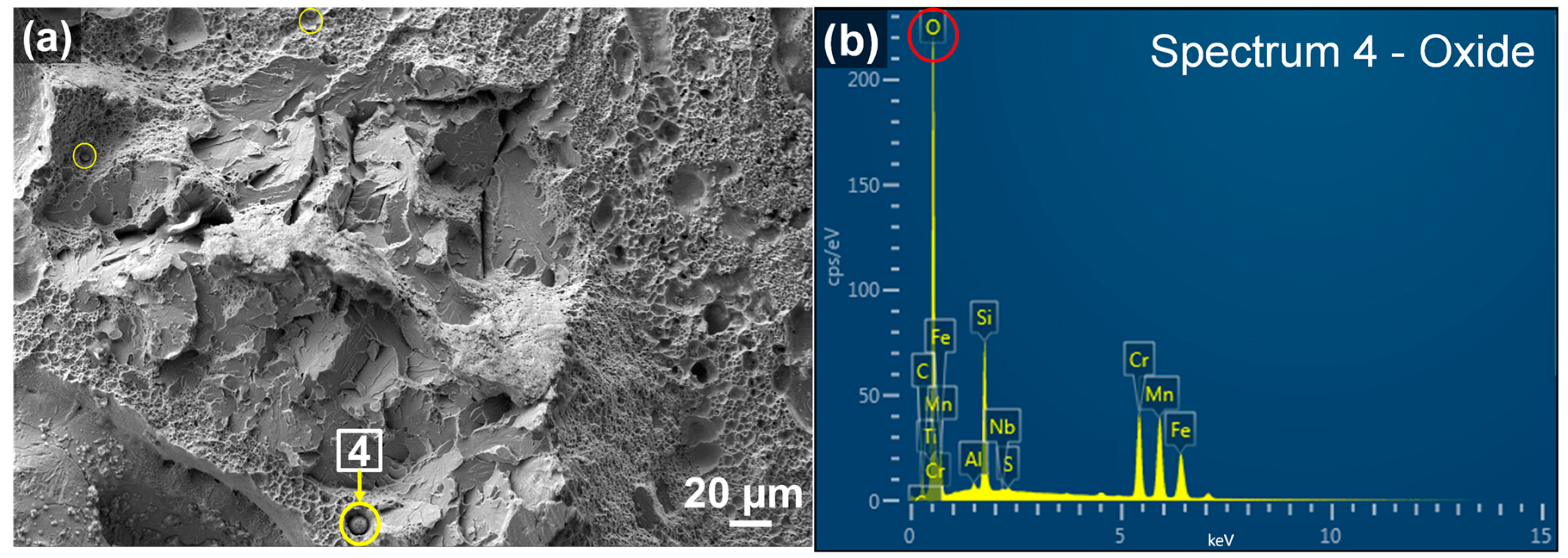
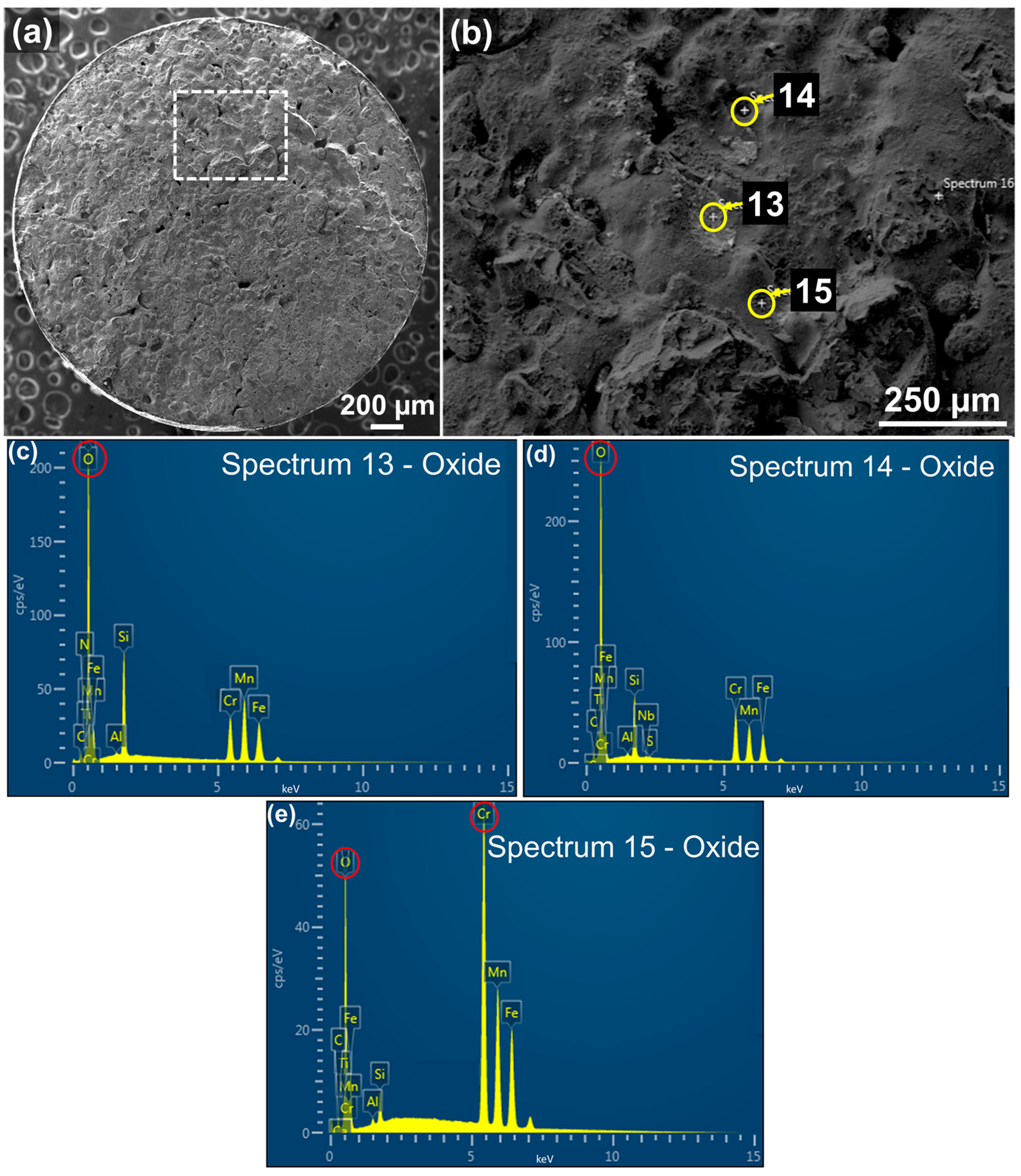
3.2.3. Oxide Formation
4. Conclusions
- (1)
- The interface between the as-deposited part and the corresponding substrate counterpart exhibits good metallurgical bonding.
- (2)
- Through the tensile tests, all the specimens fractured within the laser-deposited region instead of the interface or the substrate, indicating that the interlayer bonding is weaker than the interface bonding.
- (3)
- From the fractography analysis, the main causes of the fracture failure in the as-deposited part are lack-of-fusion defects, carbide precipitation, and oxide particles inclusions.
- (4)
- The fracture failure mechanism is associated with all these factors, which deteriorates the mechanical properties and structural integrity, and causes premature failure of critical components during service.
Author Contributions
Conflicts of Interest
References
- Wang, L.; Qian, D.; Guo, J.; Pan, Y. Austenite grain growth behavior of aisi 4140 alloy steel. Adv. Mech. Eng. 2015, 5, 762890. [Google Scholar] [CrossRef]
- Chunping, H.; Xin, L.; Fencheng, L.; Jun, C.; Fenggang, L.; Weidong, H. Effects of cooling condition on microstructure and mechanical properties in laser rapid forming of 34crnimo6 thin-wall component. Int. J. Adv. Manuf. Technol. 2016, 82, 1269–1279. [Google Scholar] [CrossRef]
- Terres, M.A.; Laalai, N.; Sidhom, H. Effect of nitriding and shot-peening on the fatigue behavior of 42crmo4 steel: Experimental analysis and predictive approach. Mater. Des. 2012, 35, 741–748. [Google Scholar] [CrossRef]
- Koehler, H.; Partes, K.; Seefeld, T.; Vollertsen, F. Influence of laser reconditioning on fatigue properties of crankshafts. Phys. Procedia 2011, 12, 512–518. [Google Scholar] [CrossRef]
- Al-Tubi, I.S.; Long, H.; Zhang, J.; Shaw, B. Experimental and analytical study of gear micropitting initiation and propagation under varying loading conditions. Wear 2015, 328–329, 8–16. [Google Scholar] [CrossRef]
- Greco, A.; Mistry, K.; Sista, V.; Eryilmaz, O.; Erdemir, A. Friction and wear behaviour of boron based surface treatment and nano-particle lubricant additives for wind turbine gearbox applications. Wear 2011, 271, 1754–1760. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, K.; Huang, J.; Hosseini, S.R.E.; Li, Z. Characterization of heat affected zone liquation cracking in laser additive manufacturing of inconel 718. Mater. Des. 2016, 90, 586–594. [Google Scholar] [CrossRef]
- Kandukuri, S.T.; Klausen, A.; Karimi, H.R.; Robbersmyr, K.G. A review of diagnostics and prognostics of low-speed machinery towards wind turbine farm-level health management. Renew. Sustain. Energy Rev. 2016, 53, 697–708. [Google Scholar] [CrossRef]
- Sarker, B.R.; Faiz, T.I. Minimizing maintenance cost for offshore wind turbines following multi-level opportunistic preventive strategy. Renew. Energy 2016, 85, 104–113. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhuang, W.; Zhang, M. Effect of the thickness of cold sprayed aluminium alloy coating on the adhesive bond strength with an aluminium alloy substrate. Surf. Coat. Technol. 2015, 270, 259–265. [Google Scholar] [CrossRef]
- Arias-González, F.; del Val, J.; Comesaña, R.; Penide, J.; Lusquiños, F.; Quintero, F.; Riveiro, A.; Boutinguiza, M.; Pou, J. Fiber laser cladding of nickel-based alloy on cast iron. Appl. Surf. Sci. 2015, 374, 197–205. [Google Scholar] [CrossRef]
- Kaiming, W.; Yulong, L.; Hanguang, F.; Yongping, L.; Zhenqing, S.; Pengfei, M. A study of laser cladding nicrbsi/mo composite coatings. Surf. Eng. 2016, 1–8. [Google Scholar] [CrossRef]
- Kattire, P.; Paul, S.; Singh, R.; Yan, W. Experimental characterization of laser cladding of cpm 9v on h13 tool steel for die repair applications. J. Manuf. Process. 2015, 20, 492–499. [Google Scholar] [CrossRef]
- Sen, S.; Sen, U.; Bindal, C. The growth kinetics of borides formed on boronized aisi 4140 steel. Vacuum 2005, 77, 195–202. [Google Scholar] [CrossRef]
- Limodin, N.; Verreman, Y. Fatigue strength improvement of a 4140 steel by gas nitriding: Influence of notch severity. Mater. Sci. Eng. A 2006, 435–436, 460–467. [Google Scholar] [CrossRef]
- Serres, N.; Hlawka, F.; Costil, S.; Langlade, C.; Machi, F.; Cornet, A. Dry coatings and ecodesign. Surf. Coat. Technol. 2009, 204, 197–204. [Google Scholar] [CrossRef]
- Bolelli, G.; Lusvarghi, L.; Barletta, M. Heat treatment effects on the corrosion resistance of some vof-sprayed metal alloy coatings. Surf. Coat. Technol. 2008, 202, 4839–4847. [Google Scholar] [CrossRef]
- Abioye, T.E.; McCartney, D.G.; Clare, A.T. Laser cladding of inconel 625 wire for corrosion protection. J. Mater. Process. Technol. 2015, 217, 232–240. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Wu, X.; Dargusch, M.S. Comparative study of commercially pure titanium produced by laser engineered net shaping, selective laser melting and casting processes. Mater. Sci. Eng. A 2017, 705, 385–393. [Google Scholar] [CrossRef]
- Attar, H.; Ehtemam-Haghighi, S.; Kent, D.; Okulov, I.V.; Wendrock, H.; Bӧnisch, M.; Volegov, A.S.; Calin, M.; Eckert, J.; Dargusch, M.S. Nanoindentation and wear properties of ti and ti-tib composite materials produced by selective laser melting. Mater. Sci. Eng. A 2017, 688, 20–26. [Google Scholar] [CrossRef]
- Attar, H.; Bönisch, M.; Calin, M.; Zhang, L.C.; Scudino, S.; Eckert, J. Selective laser melting of in situ titanium-titanium boride composites: Processing, microstructure and mechanical properties. Acta Mater. 2014, 76, 13–22. [Google Scholar] [CrossRef]
- Attar, H.; Löber, L.; Funk, A.; Calin, M.; Zhang, L.C.; Prashanth, K.G.; Scudino, S.; Zhang, Y.S.; Eckert, J. Mechanical behavior of porous commercially pure Ti and Ti-TiB composite materials manufactured by selective laser melting. Mater. Sci. Eng. A 2015, 625, 350–356. [Google Scholar] [CrossRef]
- Scudino, S.; Unterdörfer, C.; Prashanth, K.G.; Attar, H.; Ellendt, N.; Uhlenwinkel, V.; Eckert, J. Additive manufacturing of Cu-10sn bronze. Mater. Lett. 2015, 156, 202–204. [Google Scholar] [CrossRef]
- Němeček, S.; Fidler, L.; Fišerová, P. Corrosion resistance of laser clads of inconel 625 and metco 41c. Phys. Procedia 2014, 56, 294–300. [Google Scholar] [CrossRef]
- Gu, D.D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Wang, Q.; Spencer, K.; Birbilis, N.; Zhang, M.-X. The influence of ceramic particles on bond strength of cold spray composite coatings on az91 alloy substrate. Surf. Coat. Technol. 2010, 205, 50–56. [Google Scholar] [CrossRef]
- El Kadiri, H.; Wang, L.; Horstemeyer, M.F.; Yassar, R.S.; Berry, J.T.; Felicelli, S.; Wang, P.T. Phase transformations in low-alloy steel laser deposits. Mater. Sci. Eng. A 2008, 494, 10–20. [Google Scholar] [CrossRef]
- Foroozmehr, E.; Kovacevic, R. Thermokinetic modeling of phase transformation in the laser powder deposition process. Metall. Mater. Trans. A 2009, 40, 1935–1943. [Google Scholar] [CrossRef]
- Blackwell, P.L. The mechanical and microstructural characteristics of laser-deposited IN718. J. Mater. Process. Technol. 2005, 170, 240–246. [Google Scholar] [CrossRef]
- Qi, H.; Azer, M.; Ritter, A. Studies of standard heat treatment effects on microstructure and mechanical properties of laser net shape manufactured inconel 718. Metall. Mater. Trans. A 2009, 40, 2410–2422. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Fang, Y.; Wang, H. Microstructure and mechanical properties of hybrid fabricated 1Cr12Ni2WMoVNb steel by laser melting deposition. Chin. J. Aeronaut. 2013, 26, 481–486. [Google Scholar] [CrossRef]
- 42crmo4/1.7225 Alloy Special Steel—Equivalent, Chemical Composition, Properties. Available online: http://www.steelnumber.com/en/steel_composition_eu.php?name_id=335 (accessed on 4 December 2016).
- Zhang, K.; Liu, W.; Shang, X. Research on the processing experiments of laser metal deposition shaping. Opt. Laser Technol. 2007, 39, 549–557. [Google Scholar] [CrossRef]
- Wang, L.; Pratt, P.; Felicelli, S.D.; El Kadiri, H.; Berry, J.T.; Wang, P.T.; Horstemeyer, M.F. Pore formation in laser-assisted powder deposition process. J. Manuf. Sci. Eng. 2009, 131, 051008. [Google Scholar] [CrossRef]
- Pineau, A.; Benzerga, A.A.; Pardoen, T. Failure of metals i: Brittle and ductile fracture. Acta Mater. 2016, 107, 424–483. [Google Scholar] [CrossRef]
- Vilar, R. Laser powder deposition. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherland, 2014; Volume 10, pp. 163–216. [Google Scholar]
- Zhong, C.; Chen, J.; Linnenbrink, S.; Gasser, A.; Sui, S.; Poprawe, R. A comparative study of inconel 718 formed by high deposition rate laser metal deposition with ga powder and prep powder. Mater. Des. 2016, 107, 386–392. [Google Scholar] [CrossRef]
- Pinkerton, A.J. Laser direct metal deposition: Theory and applications in manufacturing and maintenance. In Advances in Laser Materials Processing; Elsevier: Amsterdam, The Netherland, 2010; Volume 16, pp. 461–491. [Google Scholar]
- Amano, R.S.; Rohatgi, P.K. Laser engineered net shaping process for sae 4140 low alloy steel. Mater. Sci. Eng. A 2011, 528, 6680–6693. [Google Scholar] [CrossRef]
- Ma, M.; Wang, Z.; Zeng, X. Effect of energy input on microstructural evolution of direct laser fabricated in718 alloy. Mater. Charact. 2015, 106, 420–427. [Google Scholar] [CrossRef]
- Zeng, C.; Tian, W.; Liao, W.H.; Hua, L. Microstructure and porosity evaluation in laser-cladding deposited ni-based coatings. Surf. Coat. Technol. 2016, 294, 122–130. [Google Scholar] [CrossRef]
- Casati, R.; Lemke, J.; Vedani, M. Microstructure and fracture behavior of 316l austenitic stainless steel produced by selective laser melting. J. Mater. Sci. Technol. 2016, 32, 738–744. [Google Scholar] [CrossRef]
- Shim, D.-S.; Baek, G.-Y.; Lee, E.-M. Effect of substrate preheating by induction heater on direct energy deposition of aisi m4 powder. Mater. Sci. Eng. A 2017, 682, 550–562. [Google Scholar] [CrossRef]
- Carlton, H.D.; Haboub, A.; Gallegos, G.F.; Parkinson, D.Y.; MacDowell, A.A. Damage evolution and failure mechanisms in additively manufactured stainless steel. Mater. Sci. Eng. A 2016, 651, 406–414. [Google Scholar] [CrossRef]
- Sun, G.; Zhou, R.; Lu, J.; Mazumder, J. Evaluation of defect density, microstructure, residual stress, elastic modulus, hardness and strength of laser-deposited aisi 4340 steel. Acta Mater. 2015, 84, 172–189. [Google Scholar] [CrossRef]
- Suryawanshi, J.; Prashanth, K.G.; Ramamurty, U. Tensile, fracture, and fatigue crack growth properties of a 3d printed maraging steel through selective laser melting. J. Alloys Compd. 2017, 725, 355–364. [Google Scholar] [CrossRef]
- Sun, S.D.; Liu, Q.; Brandt, M.; Luzin, V.; Cottam, R.; Janardhana, M.; Clark, G. Effect of laser clad repair on the fatigue behaviour of ultra-high strength aisi 4340 steel. Mater. Sci. Eng. A 2014, 606, 46–57. [Google Scholar] [CrossRef]
- Bai, Y.; Yang, Y.; Wang, D.; Zhang, M. Influence mechanism of parameters process and mechanical properties evolution mechanism of maraging steel 300 by selective laser melting. Mater. Sci. Eng. A 2017, 703, 116–123. [Google Scholar] [CrossRef]
- Kudzal, A.; McWilliams, B.; Hofmeister, C.; Kellogg, F.; Yu, J.; Taggart-Scarff, J.; Liang, J. Effect of scan pattern on the microstructure and mechanical properties of powder bed fusion additive manufactured 17-4 stainless steel. Mater. Des. 2017, 133, 205–215. [Google Scholar] [CrossRef]
- Wang, K.; Dai, Y.; Spätig, P. Microstructure and fracture behavior of f82h steel under different irradiation and tensile test conditions. J. Nucl. Mater. 2016, 468, 246–254. [Google Scholar] [CrossRef]
- Shibanuma, K.; Aihara, S.; Suzuki, K. Prediction model on cleavage fracture initiation in steels having ferrite–cementite microstructures—Part I: Model presentation. Eng. Fract. Mech. 2016, 151, 161–180. [Google Scholar] [CrossRef]
- Chen, J.H.; Cao, R. Chapter 1—Introduction. In Micromechanism of Cleavage Fracture of Metals; Butterworth-Heinemann: Boston, MA, USA, 2015; pp. 1–54. [Google Scholar]
- Alam, M.M.; Kaplan, A.F.H.; Tuominen, J.; Vuoristo, P.; Miettinen, J.; Poutala, J.; Näkki, J.; Junkala, J.; Peltola, T.; Barsoum, Z. Analysis of the stress raising action of flaws in laser clad deposits. Mater. Des. 2013, 46, 328–337. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Lin, X.; Huang, W. Study on microstructure and mechanical properties of laser rapid forming inconel 718. Mater. Sci. Eng. A 2008, 478, 119–124. [Google Scholar] [CrossRef]
- Vargas-Arista, B.; Teran-Guillen, J.; Solis, J.; García-Cerecero, G.; Martínez-Madrid, M. Normalizing effect on fatigue crack propagation at the heat-affected zone of aisi 4140 steel shielded metal arc weldings. Mater. Res. 2013, 16, 722–778. [Google Scholar] [CrossRef]
- Vazdirvanidis, A.; Pantazopoulos, G.; Louvaris, A. Failure analysis of a hardened and tempered structural steel (42CrMo4) bar for automotive applications. Eng. Fail. Anal. 2009, 16, 1033–1038. [Google Scholar] [CrossRef]
- Salemi, A.; Abdollah-Zadeh, A.; Mirzaei, M.; Assadi, H. A study on fracture properties of multiphase microstructures of a crmo steel. Mater. Sci. Eng. A 2008, 492, 45–48. [Google Scholar] [CrossRef]
- Darwish, F.A.; Pereira, L.C.; Gatts, C.; Graça, M.L. On the tempered martensite embrittlement in aisi 4140 low alloy steel. Mater. Sci. Eng. A 1991, 132, L5–L9. [Google Scholar] [CrossRef]
- Sun, G.; Bhattacharya, S.; Dinda, G.P.; Dasgupta, A.; Mazumder, J. Microstructure evolution during laser-aided direct metal deposition of alloy tool steel. Scr. Metall. 2011, 64, 454–457. [Google Scholar] [CrossRef]
- Balan, K.P.; Reddy, A.V. Aero steels: Part 1—Low alloy steels. In Aerospace Materials and Material Technologies; Springer: Singapore, 2017; pp. 149–171. [Google Scholar]
- Maciejewski, J.; Regulski, C. Fracture assessment of martempered and quenched and tempered alloy steel. J. Fail. Anal. Prev. 2009, 9, 397–408. [Google Scholar] [CrossRef]
- Feng, J.; Frankenbach, T.; Wettlaufer, M. Strengthening 42crmo4 steel by isothermal transformation below martensite start temperature. Mater. Sci. Eng. A 2017, 683, 110–115. [Google Scholar] [CrossRef]
- Krauss, G. Tempering of lath martensite in low and medium carbon steels: Assessment and challenges. Steel Res. Int. 2017, 1700038, 1–18. [Google Scholar] [CrossRef]
- Qin, R.; Zhang, X.; Guo, S.; Sun, B.; Tang, S.; Li, W. Laser cladding of high Co–Ni secondary hardening steel on 18Cr2Ni4WA steel. Surf. Coat. Technol. 2016, 285, 242–248. [Google Scholar] [CrossRef]
- Oh, M.C.; Yeom, H.; Jeon, Y.; Ahn, B. Microstructural characterization of laser heat treated aisi 4140 steel with improved fatigue behavior. Arch. Metall. Mater. 2015, 60, 5–8. [Google Scholar] [CrossRef]
- Hussein, A.-H.A.; Abdu, M.T.; El-Banna, E.-S.M.; Soliman, S.E.; Tash, M.M. Interrelation of steel composition, hardening route, and tempering response of medium carbon low-alloy steels. J. Mater. Eng. Perform. 2016, 25, 1463–1473. [Google Scholar] [CrossRef]
- Clarke, D.R. Stress generation during high-temperature oxidation of metallic alloys. Curr. Opin. Solid State Mater. Sci. 2002, 6, 237–244. [Google Scholar] [CrossRef]
- Song, M.; Lin, X.; Liu, F.; Yang, H.; Huang, W. Effect of environmental oxygen content on the oxide inclusion in laser solid formed aisi 420 stainless steel. Mater. Des. 2016, 90, 459–467. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, G.; He, X.; Ning, W.; Zheng, C. Numerical and experimental investigation of multilayer ss410 thin wall built by laser direct metal deposition. J. Mater. Process. Technol. 2012, 212, 106–112. [Google Scholar] [CrossRef]
- Yadollahi, A.; Shamsaei, N.; Thompson, S.M.; Seely, D.W. Effects of process time interval and heat treatment on the mechanical and microstructural properties of direct laser deposited 316l stainless steel. Mater. Sci. Eng. A 2015, 644, 171–183. [Google Scholar] [CrossRef]
- Gaskell, D.R. Discussion of “representation of mixed reactive gases on free energy (ellingham-richardson) diagrams”. Metall. Mater. Trans. B 1996, 27, 693. [Google Scholar] [CrossRef]
- Stratton, P. Ellingham diagrams—Their use and misuse. Int. Heat Treat. Surf. Eng. 2013, 7, 70–73. [Google Scholar] [CrossRef]
- Jia, Q.; Gu, D. Selective laser melting additive manufactured inconel 718 superalloy parts: High-temperature oxidation property and its mechanisms. Opt. Laser Technol. 2014, 62, 161–171. [Google Scholar] [CrossRef]
- He, B.; Li, D.; Zhang, A.; Lu, Z.; Ge, J.; Tat Khoa, D. Influence of oxidation on the cracks of dz125l nickel-based superalloy thin-walled parts in laser metal direct forming. Rapid Prototyp. J. 2013, 19, 446–451. [Google Scholar] [CrossRef]
- Caplan, D.; Sproule, G.I. Effect of oxide grain structure on the high-temperature oxidation of cr. Oxid. Met. 1975, 9, 459–472. [Google Scholar] [CrossRef]
| Material | UTS (MPa) | YS (MPa) | Elongation (%) |
|---|---|---|---|
| Hybrid samples in this paper * | 360 ± 170 | 235 ± 75 | 2.3 ± 1.5 |
| AISI 4140 wrought [32] | 720 | 655 | 4 |
| Spot | C | Si | Mn | S | P | Cr | Mo | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 5.30 | 0.31 | 1.12 | 0.00 | 0.00 | 1.32 | 0.31 | 91.65 | 100.00 |
| 3 | 5.30 | 0.10 | 0.21 | 0.00 | 0.00 | 0.42 | 0.42 | 93.56 | 100.00 |
| 5 | 5.06 | 0.10 | 0.62 | 0.10 | 0.00 | 1.24 | 0.00 | 92.88 | 100.00 |
| 9 | 4.06 | 0.10 | 0.71 | 0.00 | 0.00 | 1.12 | 0.00 | 94.02 | 100.00 |
| Nominal | 0.44 | 0.35 | 1.00 | 0.04 | 0.03 | 1.10 | 0.25 | 96.78 | 100.00 |
| Spot | C | Si | Mn | S | P | Cr | Mo | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 20.55 | 0.40 | 1.01 | 0.00 | 0.00 | 1.21 | 0.20 | 76.62 | 100.00 |
| 3 | 20.78 | 0.11 | 0.21 | 0.00 | 0.00 | 0.32 | 0.21 | 78.37 | 100.00 |
| 5 | 19.89 | 0.21 | 0.53 | 0.11 | 0.00 | 1.06 | 0.00 | 78.20 | 100.00 |
| 9 | 16.28 | 0.20 | 0.61 | 0.00 | 0.00 | 1.01 | 0.00 | 81.90 | 100.00 |
| Spot | O | C | Si | Mn | S | P | Cr | Mo | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 28.50 | 5.07 | 7.51 | 22.92 | 0.20 | 0.00 | 19.68 | 0.00 | 16.13 | 100.00 |
| 13 | 28.10 | 2.56 | 7.79 | 26.46 | 0.00 | 0.00 | 14.97 | 0.00 | 20.10 | 100.00 |
| 14 | 31.96 | 4.13 | 5.75 | 18.15 | 0.10 | 0.00 | 21.07 | 0.00 | 18.85 | 100.00 |
| 15 | 8.90 | 1.82 | 1.11 | 20.02 | 0.00 | 0.00 | 45.50 | 0.00 | 22.65 | 100.00 |
| Nominal | - | 0.44 | 0.35 | 1.00 | 0.04 | 0.03 | 1.10 | 0.25 | 96.78 | 100.00 |
| Spot | O | C | Si | Mn | S | P | Cr | Mo | Fe | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| 4 | 50.10 | 11.82 | 7.58 | 11.72 | 0.10 | 0.00 | 10.61 | 0.00 | 8.08 | 100.00 |
| 13 | 52.10 | 6.29 | 8.18 | 14.26 | 0.00 | 0.00 | 8.49 | 0.00 | 10.69 | 100.00 |
| 14 | 55.23 | 9.46 | 5.63 | 9.15 | 0.00 | 0.00 | 11.17 | 0.00 | 9.36 | 100.00 |
| 15 | 23.26 | 6.47 | 1.62 | 15.17 | 0.00 | 0.00 | 36.60 | 0.00 | 16.89 | 100.00 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Liu, Z.; Cong, W.; Zhang, H.-C. Tensile Fracture Behavior and Failure Mechanism of Additively-Manufactured AISI 4140 Low Alloy Steel by Laser Engineered Net Shaping. Materials 2017, 10, 1283. https://doi.org/10.3390/ma10111283
Kim H, Liu Z, Cong W, Zhang H-C. Tensile Fracture Behavior and Failure Mechanism of Additively-Manufactured AISI 4140 Low Alloy Steel by Laser Engineered Net Shaping. Materials. 2017; 10(11):1283. https://doi.org/10.3390/ma10111283
Chicago/Turabian StyleKim, Hoyeol, Zhichao Liu, Weilong Cong, and Hong-Chao Zhang. 2017. "Tensile Fracture Behavior and Failure Mechanism of Additively-Manufactured AISI 4140 Low Alloy Steel by Laser Engineered Net Shaping" Materials 10, no. 11: 1283. https://doi.org/10.3390/ma10111283





