1. Introduction
As interests over the impact of the emissions of greenhouse gases on global warming continue to increase, especially the rising concentration of carbon dioxide (CO
2) primarily from the use of fossil fuels, it is imperative to alleviate CO
2 emissions by development of pollution prevention technologies. The CO
2 capture and sequestration (CCS) is a set of technologies that can greatly reduce CO
2 emissions and is considered an effective approach in mitigating global warming [
1,
2,
3,
4]. A typical untreated flue gas composition from a power plant burning low sulfur eastern bituminous coal consists of approximately 15–16% CO
2, 70–75% N
2, 5–7% H
2O, 3–4% O
2, and several trace amounts of constituents [
5]. Various CO
2 capture technologies, such as absorption, adsorption, cryogenics, and membranes have been widely investigated [
4,
6]. Although the absorption–regeneration technologies are recognized as the most effective methods and are widely implemented, the high energy density of the absorption processes has restricted their applications. On the other hand, adsorption has been considered as one of the most cost-effective options for CO
2 separation because of the low energy requirement, cost advantage, and ease of applicability over a relatively wide range of temperatures and pressures [
7].
The key point to success of adsorption technology depends on the development of the adsorbents with a high CO
2 selectivity and adsorption capacity, easy regeneration, and high stability. Several possible adsorbents, including activated carbon fibers (ACFs) [
4], activated carbon [
8], zeolite [
9], silica adsorbents [
10], metal organic frameworks (MOFs) [
11], or carbon nanotubes (CNTs) [
12], have been considered as potential candidates for CO
2 capture. In addition, surface modification techniques are usually requisite and have been used to improve the CO
2 adsorption capacity of carbonaceous materials. For instance, the chemical activation of ACFs by potassium hydroxide (KOH) [
4] or the functionalization of mesoporous capsules using oligomeric amine [
13]. Lee and Park [
4] observed that the KOH-modified ACFs had the narrowest microporosity ranging from 0.5 nm to 0.7 nm and exhibited higher CO
2 adsorption capacity. The KOH-activated nitrogen-containing granular porous carbons with developed microporosity between 0.43 and 1 nm and high N content showed high CO
2 adsorption capacity at one bar and 25 °C [
14]. However, the CO
2 adsorption capacity was dominated by its surface area and pore volume when the adsorption pressure was higher than five bar. The tetraethylenepentamine (TEPA)-loaded TiO
2 nanotubes exhibited a better CO
2 adsorption capacity due to their higher amino-group content and the CO
2 adsorption capacity was enhanced under varying moisture conditions [
15].
In order to estimate carbon bed breakthrough time (service time) for a given gas or vapor which is removed from flowing air by physical adsorption, both the adsorption capacity and adsorption rate need to be known [
16]. Several theoretical or empirical equations have been proposed to fit the breakthrough curves in fixed-bed adsorption, such as the Wheeler equation [
17,
18] or the Wheeler–Jonas equation [
19], the modified Wheeler equation [
20], the Mecklenburg equation [
21], and the Yoon and Nelson equation [
22]. The Wheeler equation was originally used to describe the behavior of adsorbable poison gas on catalytic reactors [
23] and modified by Jonas and Svirbely [
17] to study tetrachloride and chloroform adsorption on activated carbon. Wood [
24], however, introduced the skew parameter to quantify the effect of unsymmetrical breakthrough curves. The Wheeler equation can be used successfully to describe any type of adsorption of a single vapor by a suitable adsorbent [
19]. The reason that this equation was widely used could be attributed to its apparent simplicity: The combination of a single capacity term and an overall kinetic effect, which strongly enhanced its applicability to different adsorption circumstances. Zhou et al. [
25] used the Wheeler equation to discuss xenon breakthrough on activated carbon, and found that the adsorption rate coefficient was proportional to the values of flow rate, but was independent of that of temperature and inlet concentration.
It has been reported that water vapor would be competitively adsorbed on zeolite and blocked the access for CO
2 [
26]. In addition, the MOFs had high capacity of CO
2 at high pressure, however, their capacities were lower at atmospheric pressures [
27]. Although various aminopropylsilanes have been grafted onto mesoporous silicas to produce excellent adsorption capacities of CO
2 [
28], the costs of these aminopropylsilanes and mesoporous silicas (adsorbents) were too high for large-scale applications in CO
2 capture [
29]. In contrast to the poor adsorption capacity of CO
2 on zeolites in the presence of moisture, the carbon materials were relatively insensitive to moisture [
30] and were suitable candidates for CO
2 capture in flue gas.
Generally, ACFs exhibit a high surface area, large surface density, high mass transfer rates (or smaller mass transfer resistance) for both adsorption and desorption and are easier to handle compared to granular and powdered activated carbons [
31]. Cheng et al. [
32] proposed a new mathematical model to predict the breakthrough curves of volatile organic compounds on ACFs fixed beds, where both pore diffusion and surface diffusion were considered. They estimated the pore diffusion coefficient was similar to the surface diffusion coefficient. Brasquet and Cloirec [
31] observed that the main resistance to the mass transfer for adsorption on ACFs might be due to the diffusion through micropores within the ACFs and the effect of the external mass transfer was insignificant.
The objective of this study was to investigate the breakthrough characteristics of CO2 on ACFs under varying moisture conditions. Once the adsorption capacity and adsorption rate were obtained, the service time in ACF application for CO2 capture could be estimated. To the best of our knowledge, the studies investigating the effects of relative humidity for CO2 adsorption on carbon materials were sparse and their equations of breakthrough curves have not been studied. One commercial ACF cloth was modified by KOH activation or TEPA amination and the materials’ properties were characterized. Their adsorption breakthrough curves for carbon dioxide (CO2) were measured and the effect of relative humidity in the gas stream was discussed.
3. Results and Discussion
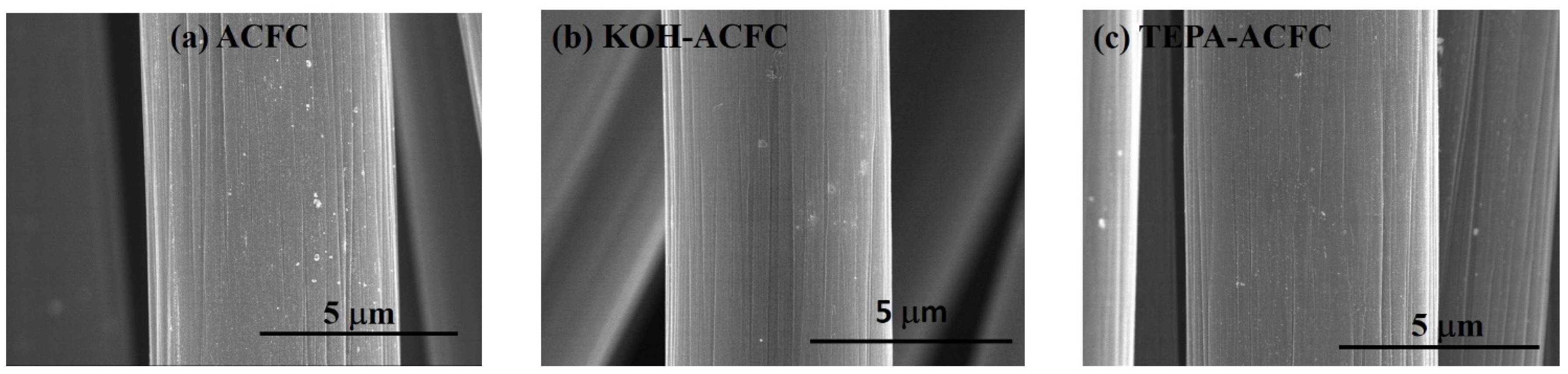
The FESEM images of the adsorbents are shown in
Figure 1. The diameter of a single fiber of ACFC as-received was approximately 6.0 μm and the surface was smooth with typically well-defined striations almost parallel to the fiber axis (
Figure 1a). After KOH activation process, there was no significant change in morphology and the diameters of the fibers were narrowed slightly (
Figure 1b). This could be due to the etching of the carbon framework by the redox reactions between various K-containing compounds with carbonaceous materials, which was responsible for further generating pore network [
36]. The TEPA amination also did not make a visible change in the apparent morphology of the fibers (
Figure 1c). TEPA was featured with higher amino-groups content and less viscous nature compared to several amines [
13,
15].
Table 1 shows the surface characteristics of the adsorbents, which were determined from the N
2 adsorption/desorption isotherms of the samples at –196 °C. The adsorption isotherms for all samples were essentially type I according to the classification proposed by Brunauer, Emmett, and Teller [
37]. Since the c value in Brunauer–Emmett–Teller (BET) surface area report was negative, it implied the BET surface area was invalid. The c value is the BET constant, which is defined as 1 + A/I, where A and I are the slope and the
y-intercept in a BET plot. Therefore, the Langmuir model was used to find the specific surface areas in this study. The Langmuir specific surface areas of ACFC, KOH–ACFC and TEPA–ACFC were 1385, 2304, and 1051 m
2/g, respectively. The total pore volume and micropore volume for all samples have the similar trend. Furthermore, the ratios of micropore volume to total pore volume were 0.80 (ACFC), 0.79 (KOH–ACFC), and 0.73 (TEPA–ACFC). As seen from the data, KOH activation of ACFs generated a lot of mesopore and micropore volumes. Moreover, KOH–ACFC exhibited the highest specific surface area, and its microporosity still retained.
Table 1 also recorded the volume of micropores with diameter less than 1 nm (fine pores), which followed the order KOH–ACFC > ACFC > TEPA–ACFC. The fact that KOH–ACFC had the largest fine pore volumes could be due to a series of processes [
36,
38]: The chemical activation resulted in the generation of pore network, the physical activation contributed to the further development of porosity through carbon gasification, and the intercalation of metallic K into the carbon matrix. The metallic K was very active and mobile at the activation temperature so that the lattices of carbon matrix expanded and leading to microporosity. It was expected that the long chain structure of TEPA molecules could block the original micropores and further decrease the available surface area and pore volume. The ratios of fine pore volume to micropore volume were 0.51 (ACFC), 0.55 (KOH–ACFC), and 0.47 (TEPA–ACFC), respectively. It is believed that KOH activation generated a high fraction of fine pores (<1 nm) [
39]. The mean equivalent pore widths from Dubinin–Astakhov (DA) method were approximately 1.6 nm, corresponding to the optimum pore width for CO
2 adsorption in literature [
40], though Thiruvenkatachari et al. [
41] reported them as 1.8 nm.
The XPS survey scan spectra of the samples revealed that the major peaks in the scan spectra were due to the C
1s, O
1s, and N
1s photoelectrons.
Table 2 summarized the surface atomic contents and atomic ratios. The as-received ACFC contained about 2.43 at.% of N
1s because of the PAN precursor and 8.20 at.% of O
1s. The nitrogen content on KOH–ACFC significantly decreased compared to ACFC, implying the loss of N-moieties during KOH activation at high temperature, which was consistent with the results in several literature [
39,
42,
43,
44]. On the other hand, the TEPA amination of ACFC resulted in a large increase in N
1s contents, although it suffered a heat treatment at 500 °C. It verified that TEPA has been grafted onto the surface of ACFC.
In order to understand the chemical bonding states, the deconvolution of high-resolution XPS spectra over the C
1s, O
1s, and N
1s regions for all samples was conducted.
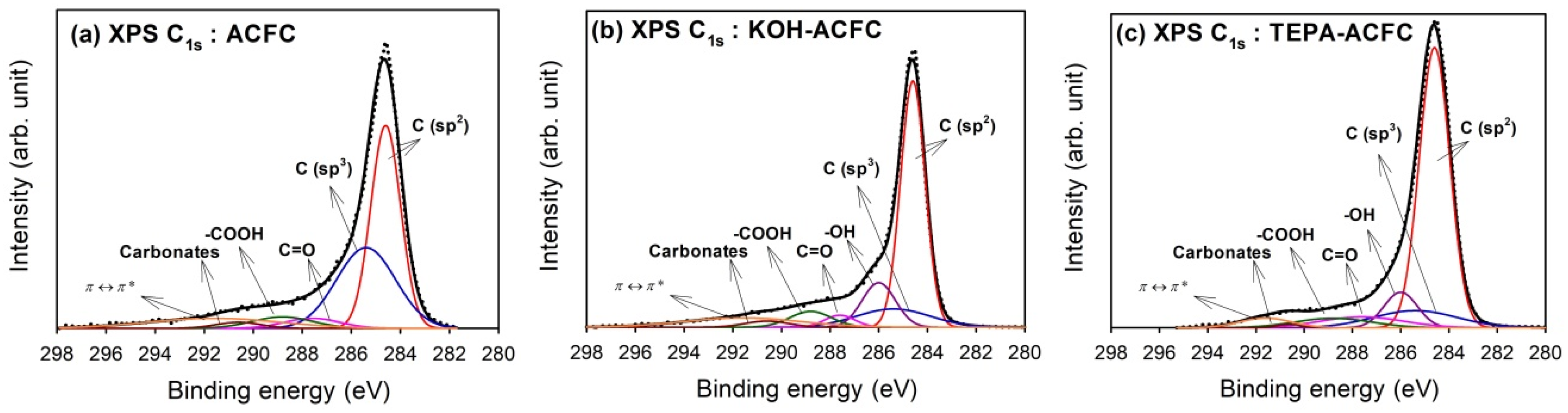
Figure 2 illustrates the optimum curve fitting of the high-resolution XPS C
1s spectra for all samples and
Table 3 shows the calculated percentages of non-functional and functional carbon atoms. We found that the C
1s spectra were decomposed into at most seven identified components that represented carbon atoms in polyaromatic structures (C (sp
2), B.E. = 284.6 eV) and in aliphatic structures (C (sp
3), B.E. = 285.4 eV), and carbon presented in phenolic, alcohol, ether or C=N groups (B.E. = 286.0 eV), carbonyl or quinone groups (B.E. = 287.6 eV), carboxyl, lactone, or ester groups (B.E. = 288.8 eV), carbonate groups (B.E. = 290.6 eV), and satellite peaks due to π–π* transitions in aromatic rings (B.E. = 291.6 eV) [
45]. The C (sp
2) and C (sp
3) were predominant in C
1s region. An increase in C (sp
2) and a decrease in C (sp
3) displayed the improvement of graphite-like structure after modification. The –COOH groups were the major functional groups in ACFC. After KOH activation, the –OH groups increased significantly. On the other hand, the TEPA amination resulted in the increase of –OH groups and C=O, which indicated the grafting of TEPA molecules [
46].
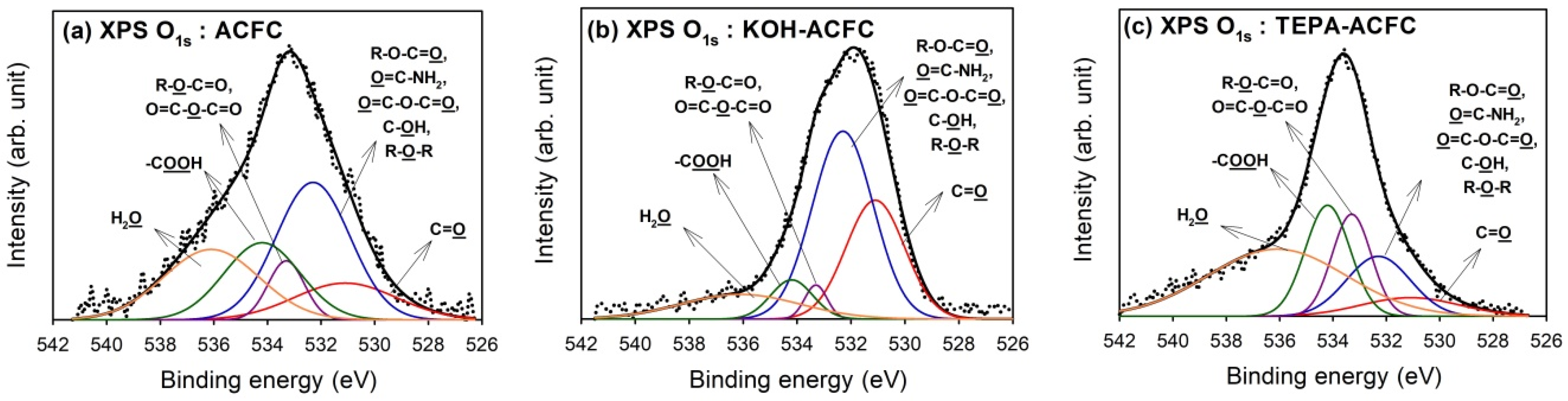
Deconvolution of XPS O
1s peaks gave additional information on the nature of the surface oxygen-containing groups. The optimum curve fitting of the O
1s peak for all samples are shown in
Figure 3 and the calculated percentages of oxygen-containing functional groups are shown in
Table 4. Four different O functionalities and the contribution of chemisorbed water can be identified [
45]. The peak at 531.1 eV corresponded to the carbonyl oxygen atoms; the peak at 532.3 eV to the carbonyl oxygen atoms in esters, amides and anhydrides as well as oxygen atoms in hydroxyls or ethers; the peak at 533.3 eV to the ether oxygen atoms in esters and anhydrides; and the peak at 534.2 eV to the oxygen atoms in the carboxyl groups. The contribution of chemisorbed water located at 536.1 eV (H
2O) was found in all samples. As seen from the data, the oxygen atoms in KOH–ACFC were mainly the –OH and C=O groups. However, the chemisorbed water was the primary surface oxide on TEPA–ACFC.
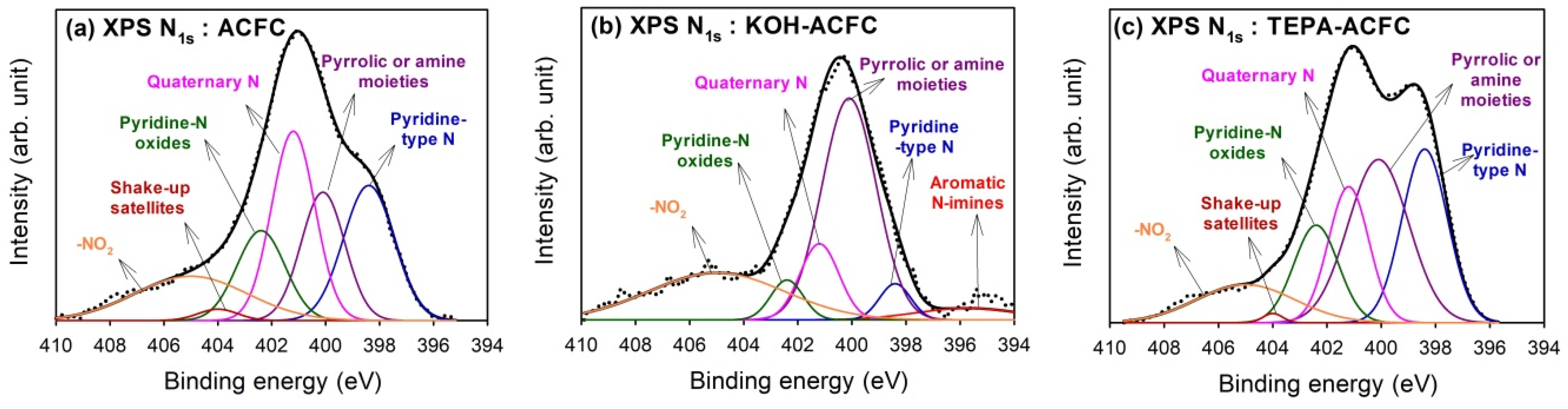
Figure 4 shows the results of curve-fitting for the high-resolution XPS N
1s spectra for all samples, which found that the N
1s spectra were decomposed into at most six identified components [
47]. The calculated percentages of nitrogen-containing functional groups are shown in
Table 5. The peak at 395.7 eV represented the aromatic N-imines; the peak at 398.4 eV was the pyridine-type N; the peak at 400.1 eV showed the pyrrolic or amine moieties; the peak at 401.2 eV was the quarternary N; and the peak at 402.4 eV exhibited the pyridine-N oxides. The contribution of chemisorbed NO
2 was located at 405 eV. Except for the chemisorbed NO
2, the quarternary N and the pyridine-type N were the predominant N-functionalities on ACFC, while the grafting of TEPA would lead to the increase in pyrrolic or amine moieties which implied the linear TEPA resulted in the increase of pyridine-like structures of six-member or five-member rings. Although KOH activation resulted in the decrease in N content, the major N groups on KOH–ACFC were the pyrrolic or amine moieties and the chemisorbed NO
2. It was believed that the pyrrolic or amine moieties were stable compared to the pyridine-type N and quaternary N, and that the KOH activation destroyed the pyridine N structure.
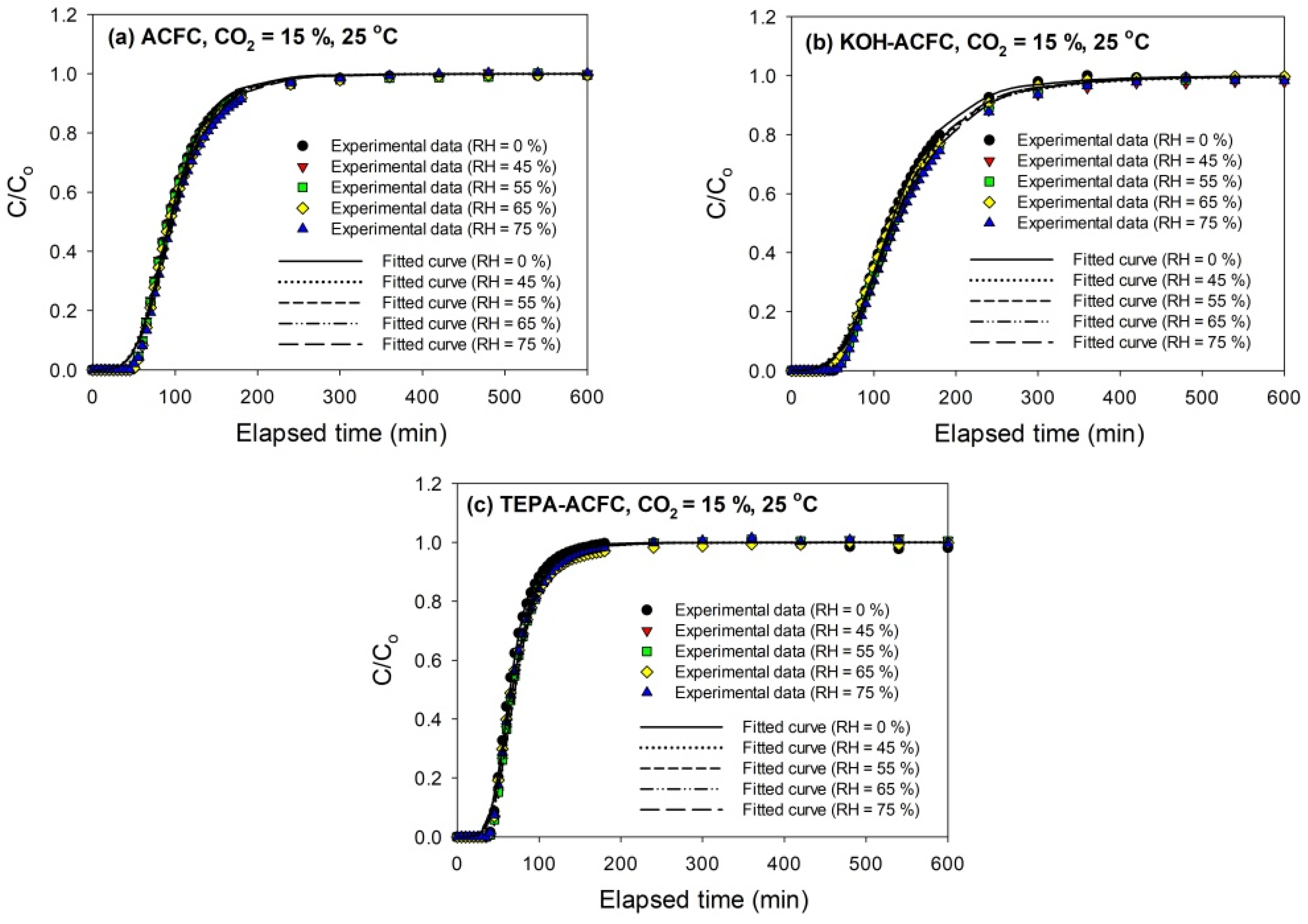
The breakthrough curves of CO
2 (15%) on all adsorbents at 25 °C are sketched in
Figure 5. The breakthrough curves obtained with KOH–ACFC were less steep, suggesting a higher pore diffusion resistance than with the other two adsorbents [
31], which could be attributed to its large micropore volume. As seen in
Figure 5, it implies that the relative humidity within the studied range (45–75%) had almost no effects on the breakthrough curves, which is consistent with the study of Li et al. [
48]. It was expected that the interactions between CO
2 molecules and the ACFs were stronger than those between the water vapor molecules and the ACFs such that the competitive adsorption of water vapor on ACFs was insignificant [
49,
50].
Since the original Wheeler equation (Equation (1)) could not fit the data well around the breakthrough point (i.e.,
C/
Co = 0.1) and the
We was highly overestimated, another parameter,
n (the power of time), was added to the model. To determine an appropriate value of
n, the adsorption isotherms of CO
2 were required.
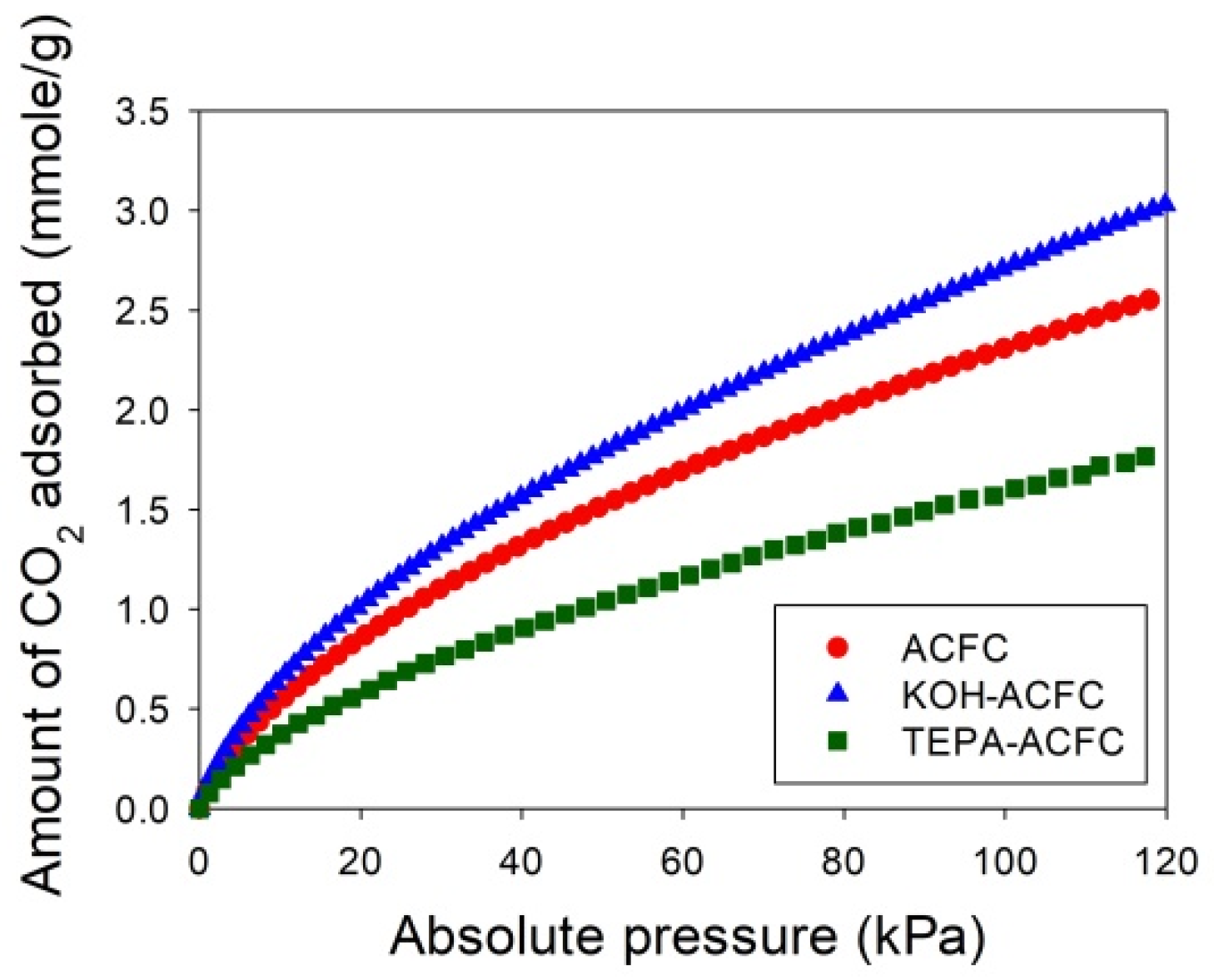
Figure 6 depicts the adsorption isotherms of CO
2 collected on the adsorbents at 25 °C for low CO
2 pressures less than 120 kPa, measured using an ASAP 2020 (Micromeritics, Norcross, GA, USA). The temperature during the CO
2 adsorption process was maintained by a circular water bath thermostat. The uptakes of CO
2 had typical monotonic curves, which were increasing with increasing pressures and indicative of the favorable adsorption. The adsorption amounts of CO
2 at 25 °C and 15 kPa followed the order KOH–ACFC (0.855 mmole/g) > ACFC (0.713 mmole/g) > TEPA–ACFC (0.486 mmole/g). These equilibrium data were utilized to calibrate the values of
n. After the curve fitting process, it produced
n = 0.1 for ACFC,
n = 0.08 for KOH–ACFC, and
n = 0.001 for TEPA–ACFC, respectively. The reason that impregnating amine did not result in increase of CO
2 uptake could be attributed to the low TEPA loading [
13]. Therefore, the increase in CO
2 uptake due to carbamate formation was limited.
The above fitted values of
n were kept unchanged for each adsorbent. Then the modified Wheeler equation was used further to fit the breakthrough data measured at different relative humidity for each adsorbent. The fitting reports are shown in
Table 6. The high values of
kv for TEPA–ACFC were responsible for the steep breakthrough curves. The equilibrium adsorption amounts,
We, exhibited a little increase with increasing relative humidity on ACFC and KOH–ACFC. However, the values of
kv, the overall adsorption rate coefficient, was decreased in the presence of water vapor. This appeared that the adsorption rate was surface adsorption limited because the active sites were occupied by water vapor molecules or it was pore diffusion limited such that the adsorption rate slowed down and the values of
kv decreased [
18].
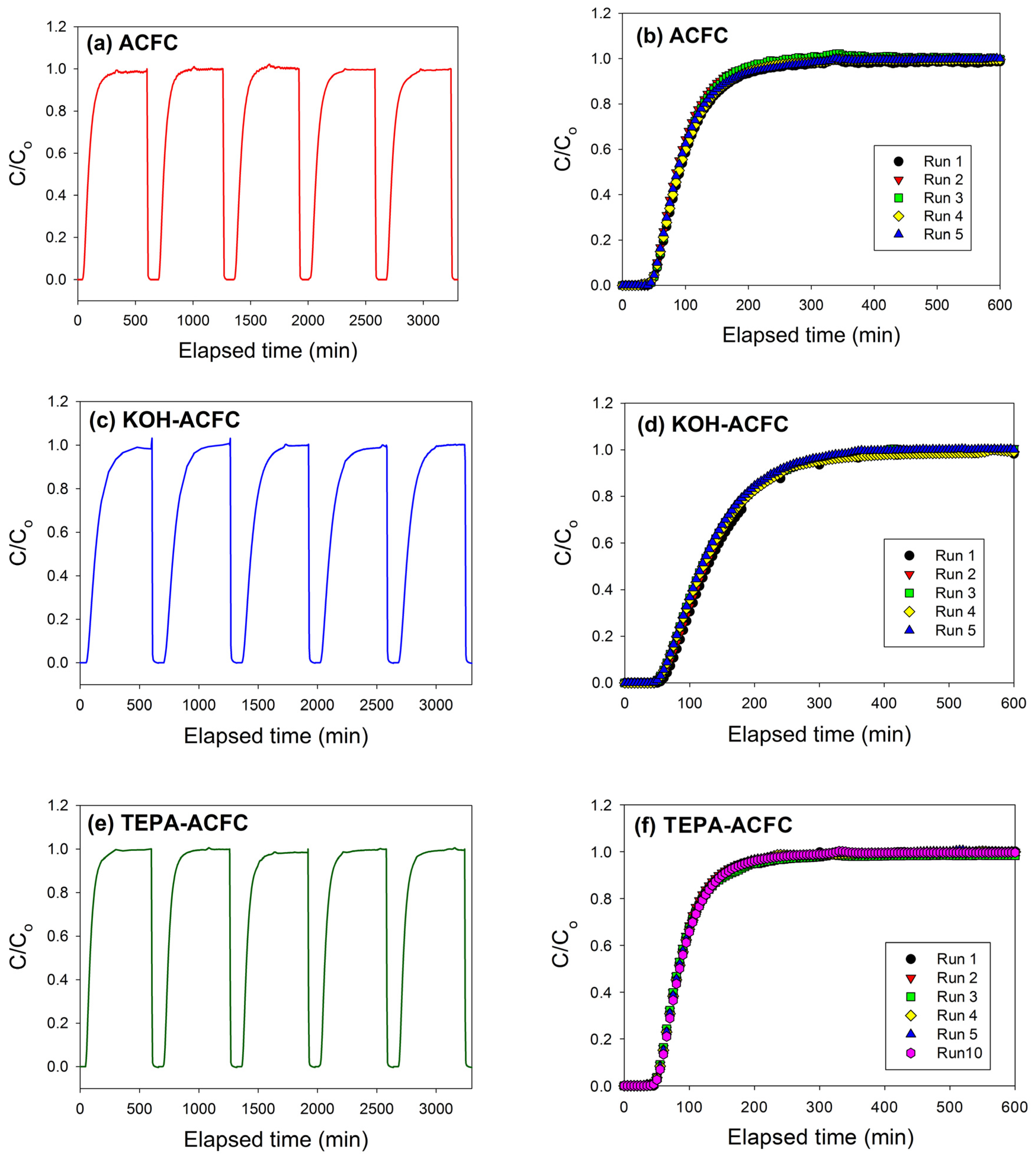
In order to check the recyclability of the adsorbents, cycle tests were performed for these three adsorbents. As shown in
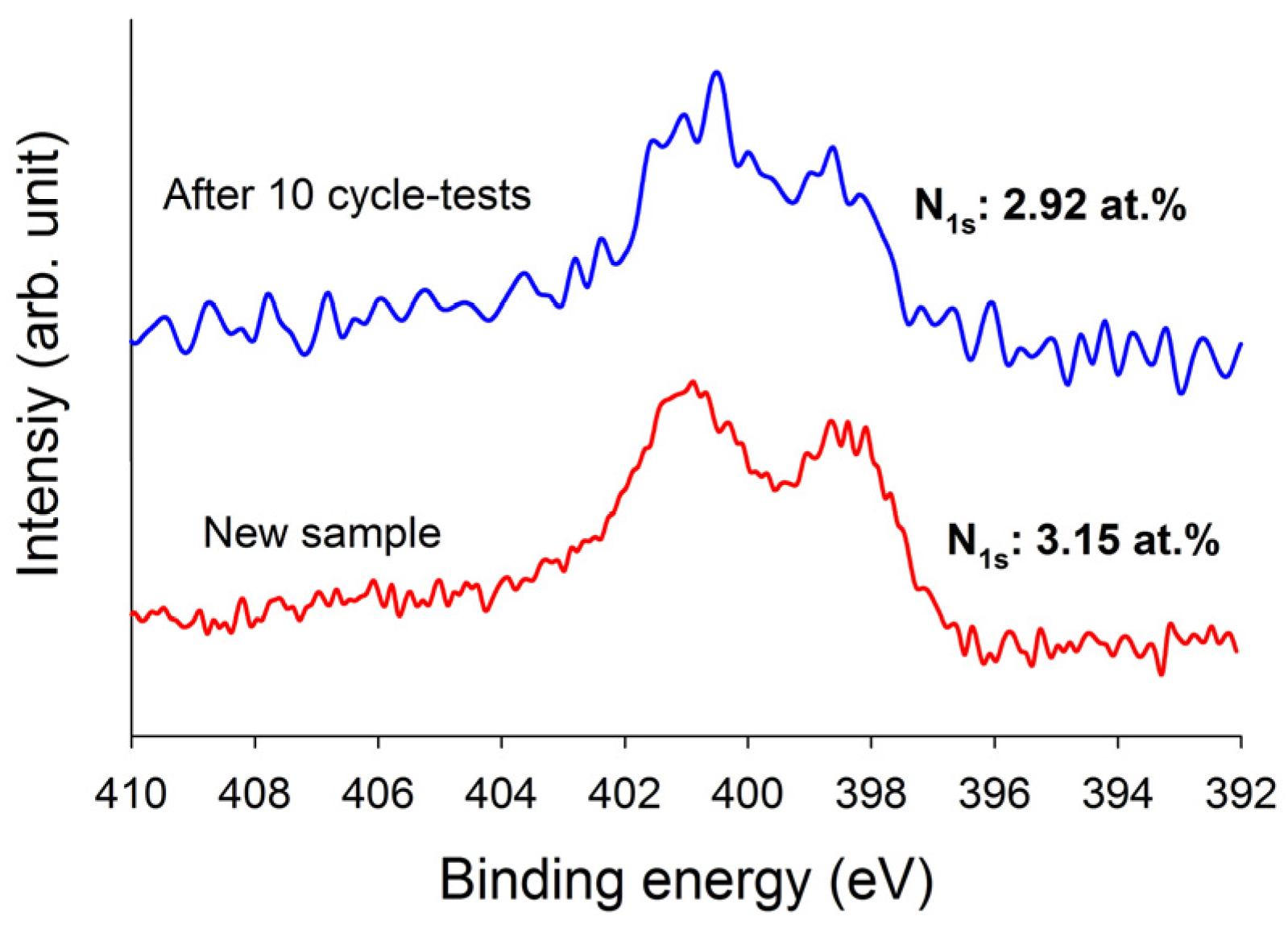
Figure 7, it is evident that these three adsorbents can be used up to five adsorption/desorption cycles almost without any changes in breakthrough time and dynamic adsorption capacities. These revealed that all adsorbents were fairly stable in five adsorption/desorption cycles and the heel (the residual adsorbate present in the bed following regeneration) was insignificant. In addition, the stability of the N-functionalities on TEPA–ACFC during thermal regeneration was analyzed. The nitrogen contents for the new TEPA–ACFC sample and the samples after 10 cycle-tests (by thermal regeneration) were probed using XPS. Their breakthrough curves and XPS N1s spectra are compared in
Figure 7f and
Figure 8, respectively. It can be observed that two patterns are similar. And the loss of nitrogen atoms on TEPA–ACFC after ten adsorption/thermal regenerations processes was estimated about 7%. It is believed that the N-functionalities were grafted and fixed strongly on ACFC.
Table 7 compares the CO
2 dynamic adsorption capacities on carbonaceous adsorbents. The KOH–ACFC showed a higher CO
2 uptake compared to those published data in literature under similar operation conditions.