Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants
Abstract
:1. Introduction
- the increase of the surface area by sandblasting;
- the surface coating with hydroxyapatite (HA) deposit; and
- the increase of macroporosity such as “metal spongiosa”.
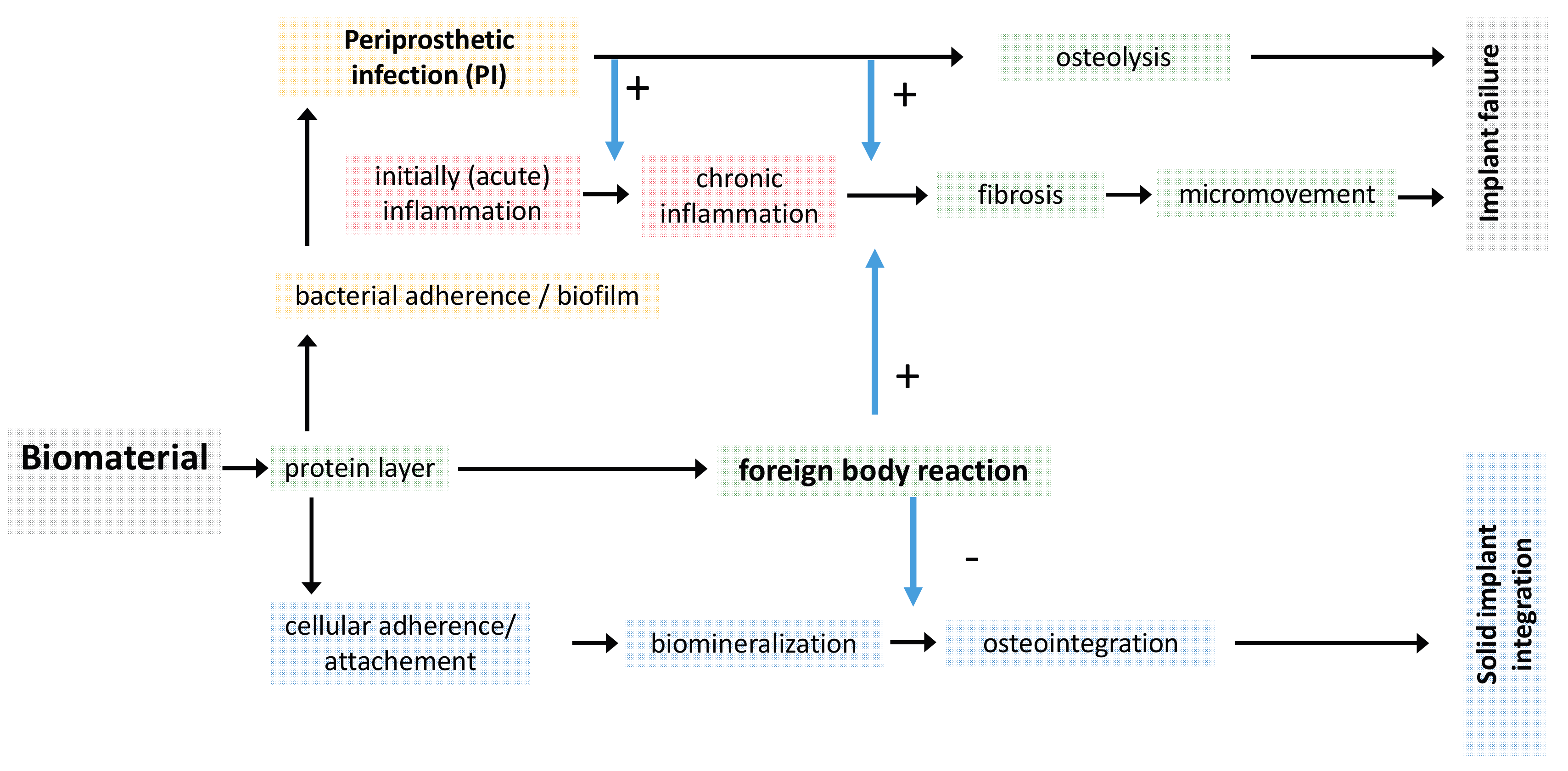
2. Bone Cells and Their Interactions
3. Titanium Surface Properties and Osteointegration
3.1. Local Biomolecular Reactions to Titanium Surfaces
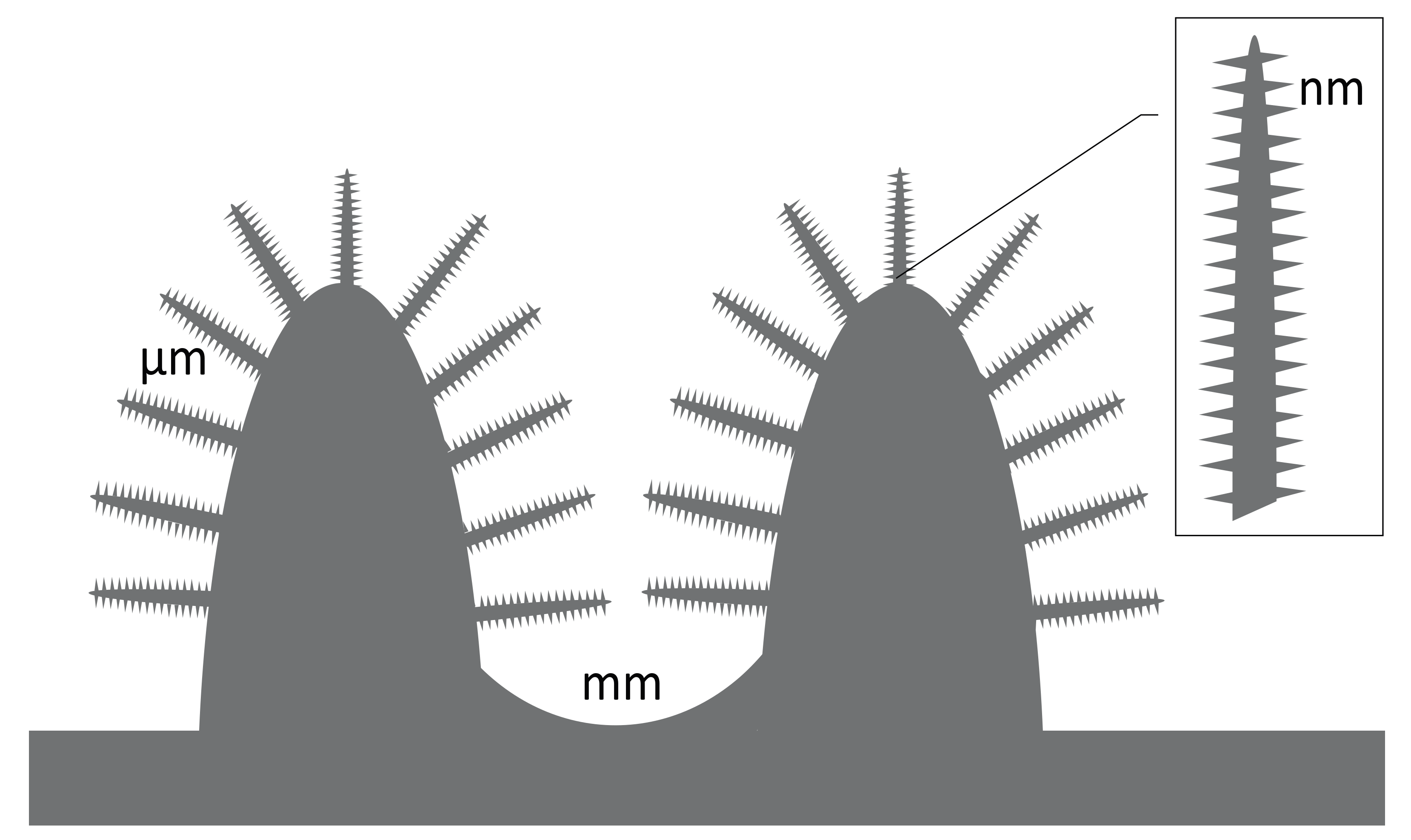
3.2. Titanium Nanostructures and Osteointegration
4. Periprosthetic Infections and Biofilm
5. Outlook and Recent Trends in Nanostructured Ti
- energy dependent efflux of toxic metals e.g., enzymatic transformations (oxidation, reduction, methylation, and demethylation);
- the expression of metal-binding proteins (metallothionein, SmtA, chaperone CopZ, SilE);
- inhibition of ions to enter the cell (downregulated expression of membrane transport proteins); and
- persisting phenotypes with slower growth or ceasing cell division [191].
Conflicts of Interest
Abbreviations
| AM | additive manufacturing |
| ASH | atomic-scale heating |
| Ag | argentum (silver) |
| Al | aluminium |
| ALP | alkaline phosphatase |
| B. | Bacillus |
| Ba | barium |
| BMP | bone morphogenic protein |
| BSA | bovine serum albumin anoparticles |
| C | carbon |
| Ca | calcium |
| CA | contact angle |
| CFRPEEK | carbon-fiber-reinforced polyetheretherketone |
| Cd | cadmium |
| copZ | copper transport protein, metallochaperone, Crk-associated substrate |
| cp | commercial pure |
| Cr | chromium |
| E. | Escherichia |
| EFG | epidermal growth factor |
| EPT | entangled porous titanium |
| F | flour |
| FAK | fokal adhesion kinase |
| FN | fibronectin |
| ECM | extracellular matrix |
| G− | gram negative |
| G+ | gram positive |
| Ga | gallium |
| Ga–CT | Ga-containing calcium titanate |
| GT | gallium titanate |
| ERK | extracellular signal-regulated kinase |
| Fe | ferrum (iron) |
| GLAD | glancing angle sputter deposition |
| H | hydrogen |
| Hg | hydrargyrum (mercury) |
| HA | hydroxyapatite |
| Hf | hafnium |
| HP | hydrophilic |
| HV | high voltage |
| HY | hydrophobic |
| K. | Klebsiella |
| LV | low voltage |
| Mo | molybdenum |
| MEK | MAPK/Erk kinase |
| MIC | minimal inhibitory concentration |
| MLC | myosin light chain |
| N | nitrogen |
| Nb | niobium |
| NMATF | nuclear matrix architectural transcription factors |
| NPs | nanoparticles |
| O | oxygen |
| OSA | oxidized alginate |
| P | phosphorus/phosphate |
| P. | Pseudomonas |
| PCL | polycaprolactone |
| PI | periprosthetic infection |
| PLLA | polylactide |
| PMB | polymer molecular brushes |
| RANKL | receptor activator of nuclear factor kappa-B ligand |
| Rho | Ras (Rat sarcom) homologue |
| RGD | Arginylglycylaspartic acid (tripeptide composed of l-arginine, glycine, and l-aspartic) |
| ROCK | Rho-associated protein kinase |
| S. | Staphylococcus |
| SAMs | self-assembled monolayers |
| SLA | sand-blasted, large grit, and acid-etched |
| Si | silicium |
| SilE | periplasmic Ag(I)-binding protein expressed by a sensor/responder two-component transcriptional regulatory system |
| SIRS | systemic inflammatory response syndrome |
| Sr | strontium |
| Ta | tantalum |
| Ti | titanium |
| V | vanadium, vascular endothelial growth factor (VEGF) |
| Zn | zinc |
| Zr | zirkonium |
References
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Qian, M.; Brandt, M.; Xie, Y.M. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lakes, R.S. Biomaterials. An Introduction; Springer: New York, NY, USA, 2007. [Google Scholar]
- Black, J.; Hastings, G. Handbook of Biomaterial Properties; Springer: New York, NY, USA, 1998. [Google Scholar]
- Tassani, S.; Ohman, C.; Baruffaldi, F.; Baleani, M.; Viceconti, M. Volume to density relation in adult human bone tissue. J. Biomech. 2011, 44, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Cuppone, M.; Seedhom, B.B.; Berry, E.; Ostell, A.E. The longitudinal young’s modulus of cortical bone in the midshaft of human femur and its correlation with CT scanning data. Calcif. Tissue Int. 2004, 74, 302–309. [Google Scholar] [PubMed]
- Rho, J.Y.; Ashman, R.B.; Turner, C.H. Young’s modulus of trabecular and cortical bone material: Ultrasonic and microtensile measurements. J. Biomech. 1993, 26, 111–119. [Google Scholar] [CrossRef]
- Brunette, D.M.; Tengvall, P.; Textor, M.; Thomsen, P. Titanium in Medicine: Material Science, Surface Science, Engineering, Biological Responses and Medical Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Bagno, A.; Di Bello, C. Surface treatments and roughness properties of Ti-based biomaterials. J. Mater. Sci. Mater. Med. 2004, 15, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Ramaswamy, N. Electrochemical surface modification of titanium in dentistry. Dent. Mater. J. 2009, 28, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Kamada, K.; Sato, K.; Hatada, R.; Baba, K.; Atsuta, M. Thin sol-gel-derived silica coatings on dental pure titanium casting. J. Biomed. Mater. Res. 1999, 48, 778–785. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Nanci, A.; Wuest, J.D.; Peru, L.; Brunet, P.; Sharma, V.; Zalzal, S.; McKee, M.D. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J. Biomed. Mater. Res. 1998, 40, 324–335. [Google Scholar] [CrossRef]
- Schliephake, H.; Scharnweber, D.; Dard, M.; Sewing, A.; Aref, A.; Roessler, S. Functionalization of dental implant surfaces using adhesion molecules. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Morra, M. Biochemical modification of titanium surfaces: Peptides and ECM proteins. Eur. Cells Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef]
- Rautray, T.R.; Narayanan, R.; Kwon, T.Y.; Kim, K.H. Surface modification of titanium and titanium alloys by ion implantation. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 93, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Shibata, Y.; Miyazaki, T. Anode glow discharge plasma treatment enhances calcium phosphate adsorption onto titanium plates. J. Dent. Res. 2002, 81, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Yu, G.P.; Huang, J.H. Role of process parameters in the texture evolution of tin films deposited by hollow cathode discharge ion plating. Surf. Coat. Technol. 2001, 141, 156–163. [Google Scholar] [CrossRef]
- Liu, X.; Chu, P.; Ding, C. Surface modification of titanium, titanium alloys, and related materials for biomedical applications. Mater. Sci. Eng. 2004, 47, 49–121. [Google Scholar] [CrossRef]
- Jung, M.J.; Nam, K.H.; Shaginyan, L.R.; Han, J.G. Deposition of Ti thin film using the magnetron sputtering method. Thin Solid Films 2003, 435, 145–149. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N. Apatite formation induced by silica gel in a simulated body fluid. J. Am. Ceram. Soc. 1992, 75, 2094–2097. [Google Scholar] [CrossRef]
- Li, P.; Kangasniemi, I.; de Groot, K.; Kokubo, T. Bonelike hydroxyapatite induction by a Gel-derived titania on a titanium substrate. J. Am. Ceram. Soc. 1994, 77, 1307–1312. [Google Scholar] [CrossRef]
- LeClair, P.; Berera, G.P.; Moodera, J.S. Titanium nitride thin films obtained by a modified physical vapor deposition process. Thin Solid Films 2000, 376, 9–15. [Google Scholar] [CrossRef]
- Kim, K.H.; Narayanan, R.; Rautray, R.T. Surface Modification of Titanium for Biomaterial Applications; Nova Publishers Science Inc.: Hauppauge, NY, USA, 2010. [Google Scholar]
- Gardon, M.; Guilemany, J.M. Milestones in functional titanium dioxide thermal spray coatings: A review. J. Therm. Srpay Technol. 2014, 23, 577–595. [Google Scholar] [CrossRef]
- Florencio-Silva, R.; Sasso, G.R.; Sasso-Cerri, E.; Simoes, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Kaspar, D.; Sarkar, M.R.; Claes, L.E.; Ignatius, A.A. A scanning electron microscopy study of human osteoblast morphology on five orthopedic metals. J. Biomed. Mater. Res. 2002, 63, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Penninger, J.M. Rank(L) as a key target for controlling bone loss. Adv. Exp. Med. Biol. 2009, 647, 130–145. [Google Scholar] [PubMed]
- Athanasou, N.A.; Quinn, J.; Bulstrode, C.J. Resorption of bone by inflammatory cells derived from the joint capsule of hip arthroplasties. J. Bone Jt. Surg. Br. 1992, 74, 57–62. [Google Scholar]
- Beauvais, S.; Drevelle, O.; Jann, J.; Lauzon, M.A.; Foruzanmehr, M.; Grenier, G.; Roux, S.; Faucheux, N. Interactions between bone cells and biomaterials: An update. Front. Biosci. (Sch. Ed.) 2016, 8, 227–263. [Google Scholar]
- Williams, D.F. On the mechanisms of biocompatibility. Biomaterials 2008, 29, 2941–2953. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Cleveland, OH, USA, 2000. [Google Scholar]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Brown, S.A. Medical Applications of Titanium and Its Alloys: The Material and Biological Issues; ASTM Special Technical Publication: Ann Arbor, MI, USA, 1996. [Google Scholar]
- Kulkarni, M.M.A.; Schmuki, P.; Iglič, A. Biomaterial surface modification of titanium and titanium alloys for medical applications. In Nanomedicine; Seifalian, A.D., Kalaskar, D.M., Eds.; One Central Press: Cheshire, UK, 2014; pp. 112–130. [Google Scholar]
- Hiromoto, S.; Inoue, M.; Taguchi, T.; Yamane, M.; Ohtsu, N. In vitro and in vivo biocompatibility and corrosion behaviour of a bioabsorbable magnesium alloy coated with octacalcium phosphate and hydroxyapatite. Acta Biomater. 2015, 11, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Hiromoto, S.; Hanawa, T.; Asami, K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials 2004, 25, 979–986. [Google Scholar] [CrossRef]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases 2015, 3, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, B.E.; Li, Y.; Min, Y.; Hao, J.Z.; Liang, C.Y.; Li, H.P.; Wang, G.C.; Liu, S.M.; Wang, H.S. Synergistic effects of hierarchical hybrid micro/nanostructures on the biological properties of titanium orthopaedic implants. RSC Adv. 2015, 5, 49552–49558. [Google Scholar] [CrossRef]
- Nazarov, D.V.; Zemtsova, E.G.; Valiev, R.Z.; Smirnov, V.M. Formation of micro- and nanostructures on the nanotitanium surface by chemical etching and deposition of titania films by atomic layer deposition (ALD). Materials 2015, 8, 8366–8377. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.D.; Hlady, V. Plasma protein adsorption: The big twelve. Ann. N. Y. Acad. Sci. 1987, 516, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Vroman, L. The importance of surfaces in contact phase reactions. Semin. Thromb. Hemost. 1987, 13, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Bakir, M. Haemocompatibility of titanium and its alloys. J. Biomater. Appl. 2012, 27, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Meyer, T.; Jones, D.B. Attachment kinetics, proliferation rates and vinculin assembly of bovine osteoblasts cultured on different pre-coated artificial substrates. J. Mater. Sci. Mater. Med. 1998, 9, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Rezania, A.; Healy, K.E. The effect of peptide surface density on mineralization of a matrix deposited by osteogenic cells. J. Biomed. Mater. Res. 2000, 52, 595–600. [Google Scholar] [CrossRef]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [PubMed]
- Jager, M.; Zilkens, C.; Zanger, K.; Krauspe, R. Significance of nano- and microtopography for cell-surface interactions in orthopaedic implants. J. Biomed. Biotechnol. 2007, 2007, 69036. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Laurent, S.; Huet, G.L.; Dupont-Gillain, C.C. Protein adsorption can be reversibly switched on and off on mixed PEO/PAA brushes. Acta Biomater. 2015, 11, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Demoustier-Champagne, S.; Dupont-Gillain, C.C. Quartz crystal microbalance study of ionic strength and pH-dependent polymer conformation and protein adsorption/desorption on PAA, PEO, and mixed PEO/PAA brushes. Langmuir 2014, 30, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Delcroix, M.F.; Huet, G.L.; Conard, T.; Demoustier-Champagne, S.; Du Prez, F.E.; Landoulsi, J.; Dupont-Gillain, C.C. Design of mixed PEO/PAA brushes with switchable properties toward protein adsorption. Biomacromolecules 2013, 14, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.; Soria, C.; Mirshahi, M.; Boucheix, C.; Aurengo, A.; Perrot, J.-Y.; Bernadou, A.; Samama, M.; Rosenfeld, C. Conformational change in fibrinogen induced by adsorption to a surface. J. Colloid Interface Sci. 1985, 107, 204–208. [Google Scholar] [CrossRef]
- Kusakawa, Y.; Yoshida, E.; Hayakawa, T. Protein adsorption to titanium and zirconia using a quartz crystal microbalance method. Biomed. Res. Int. 2017, 2017, 1521593. [Google Scholar] [CrossRef] [PubMed]
- Cei, S.; Karapetsa, D.; Aleo, E.; Graziani, F. Protein adsorption on a laser-modified titanium implant surface. Implant Dent. 2015, 24, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Jimbo, R.; Ivarsson, M.; Koskela, A.; Sul, Y.T.; Johansson, C.B. Protein adsorption to surface chemistry and crystal structure modification of titanium surfaces. J. Oral Maxillofac. Res. 2010, 1, e3. [Google Scholar] [CrossRef] [PubMed]
- Romero-Gavilan, F.; Gomes, N.C.; Rodenas, J.; Sanchez, A.; Azkargorta, M.; Iloro, I.; Elortza, F.; Garcia Arnaez, I.; Gurruchaga, M.; Goni, I.; et al. Proteome analysis of human serum proteins adsorbed onto different titanium surfaces used in dental implants. Biofouling 2017, 33, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, S.K.; Nishimoto, M.; Park, S.W.; Lee, K.M.; Kim, H.S.; Koh, J.T.; Ong, J.L.; Liu, Y.; Yang, Y. The effect of titanium surface roughening on protein absorption, cell attachment, and cell spreading. Int. J. Oral Maxillofac. Implants 2008, 23, 675–680. [Google Scholar] [PubMed]
- Paredes, J.A.; Polini, A.; Chrzanowski, W. Protein-based biointerfaces to control stem cell differentiation. In Biointerfaces: Where Material Meets Biology; Hutmacher, D.C.W., Ed.; Royal Society of Chemistry: London, UK, 2005. [Google Scholar]
- Biao, M.N.; Chen, Y.M.; Xiong, S.B.; Wu, B.Y.; Yang, B.C. Synergistic effects of fibronectin and bone morphogenetic protein on the bioactivity of titanium metal. J. Biomed. Mater. Res. A 2017, 105, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Park, J.; Gongadze, E.; Killian, M.S.; Kralj, S.; von der Mark, K.; Iglic, A.; Schmuki, P. Protein interactions with layers of TiO2 nanotube and nanopore arrays: Morphology and surface charge influence. Acta Biomater. 2016, 45, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Huber-Lang, M.; Ignatius, A.; Brenner, R.E. Role of complement on broken surfaces after trauma. Adv. Exp. Med. Biol. 2015, 865, 43–55. [Google Scholar] [PubMed]
- Hoon, J.L.; Tan, M.H.; Koh, C.G. The regulation of cellular responses to mechanical cues by Rho GTPases. Cells 2016, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.; Juodzbalys, G.; Vilkinis, V. Titanium surfaces with nanostructures influence on osteoblasts proliferation: A systematic review. J. Oral Maxillofac. Res. 2014, 5, e1. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Mazare, A.; Schneider, H.; von der Mark, K.; Fischer, M.J.; Schmuki, P. Electric field-induced osteogenic differentiation on TiO2 nanotubular layer. Tissue Eng. Part C Methods 2016, 22, 809–821. [Google Scholar] [CrossRef]
- Uskokovic, V. When 1 + 1 > 2: Nanostructured composites for hard tissue engineering applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 57, 434–451. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.A.; von Walter, M.; Wirtz, T.; Sellei, R.; Schmidt-Rohlfing, B.; Paar, O.; Erli, H.J. Structural, mechanical and in vitro characterization of individually structured Ti-6AL-4V produced by direct laser forming. Biomaterials 2006, 27, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Warnke, P.H.; Douglas, T.; Wollny, P.; Sherry, E.; Steiner, M.; Galonska, S.; Becker, S.T.; Springer, I.N.; Wiltfang, J.; Sivananthan, S. Rapid prototyping: Porous titanium alloy scaffolds produced by selective laser melting for bone tissue engineering. Tissue Eng. Part C Methods 2009, 15, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Van Bael, S.; Chai, Y.C.; Truscello, S.; Moesen, M.; Kerckhofs, G.; Van Oosterwyck, H.; Kruth, J.P.; Schrooten, J. The effect of pore geometry on the in vitro biological behavior of human periosteum-derived cells seeded on selective laser-melted Ti6AL4V bone scaffolds. Acta Biomater. 2012, 8, 2824–2834. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, B.; Idaszek, J.; Szlazak, K.; Strzelczyk, K.; Brynk, T.; Kurzydlowski, K.J.; Swieszkowski, W. Post processing and biological evaluation of the titanium scaffolds for bone tissue engineering. Materials 2016, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Choren, J.A.; Heinrich, S.M.; Silver-Thorn, M.B. Young’s modulus and volume porosity relationships for additive manufacturing applications. J. Mater. Sci. 2013, 48, 5103–5112. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Campoli, G.; Amin Yavari, S.; Sajadi, B.; Wauthle, R.; Schrooten, J.; Weinans, H.; Zadpoor, A.A. Mechanical behavior of regular open-cell porous biomaterials made of diamond lattice unit cells. J. Mech. Behav. Biomed. Mater. 2014, 34, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Heinl, P.; Muller, L.; Korner, C.; Singer, R.F.; Muller, F.A. Cellular Ti-6AL-4V structures with interconnected macro porosity for bone implants fabricated by selective electron beam melting. Acta Biomater. 2008, 4, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.G.; Myers, D.E.; Wallace, G.G.; Brandt, M.; Choong, P.F. Bioactive coatings for orthopaedic implants-recent trends in development of implant coatings. Int. J. Mol. Sci. 2014, 15, 11878–11921. [Google Scholar] [CrossRef] [PubMed]
- Dale, G.R.; Hamilton, J.W.; Dunlop, P.S.; Lemoine, P.; Byrne, J.A. Electrochemical growth of titanium oxide nanotubes: The effect of surface roughness and applied potential. J. Nanosci. Nanotechnol. 2009, 9, 4215–4219. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglic, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Park, J.; Schmuki, P.; Iglic, A. Adhesion of osteoblasts to a vertically aligned TiO2 nanotube surface. Mini Rev. Med. Chem. 2013, 13, 194–200. [Google Scholar] [PubMed]
- Ueno, T.; Yamada, M.; Suzuki, T.; Minamikawa, H.; Sato, N.; Hori, N.; Takeuchi, K.; Hattori, M.; Ogawa, T. Enhancement of bone-titanium integration profile with UV-photofunctionalized titanium in a gap healing model. Biomaterials 2010, 31, 1546–1557. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Herten, M.; Sager, M.; Wieland, M.; Dard, M.; Becker, J. Bone regeneration in dehiscence-type defects at chemically modified (slactive) and conventional SLA titanium implants: A pilot study in dogs. J. Clin. Periodontol. 2007, 34, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Schwartz, Z.; Wieland, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Boyan, B.D. High surface energy enhances cell response to titanium substrate microstructure. J. Biomed. Mater. Res. A 2005, 74, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.; Kammerer, P.W.; Al-Nawas, B.; Luszpinski, M.A.; Forch, R.; Brieger, J. The effect of extracellular matrix proteins on the cellular response of HUVECS and HOBS after covalent immobilization onto titanium. J. Biomed. Mater. Res. A 2015, 103, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Jager, M.; Boge, C.; Janissen, R.; Rohrbeck, D.; Hulsen, T.; Lensing-Hohn, S.; Krauspe, R.; Herten, M. Osteoblastic potency of bone marrow cells cultivated on functionalized biometals with cyclic RGD-peptide. J. Biomed. Mater. Res. A 2013, 101, 2905–2914. [Google Scholar] [CrossRef] [PubMed]
- Haversath, M.; Hulsen, T.; Boge, C.; Tassemeier, T.; Landgraeber, S.; Herten, M.; Warwas, S.; Krauspe, R.; Jager, M. Osteogenic differentiation and proliferation of bone marrow-derived mesenchymal stromal cells on PDLLA + BMP-2-coated titanium alloy surfaces. J. Biomed. Mater. Res. A 2016, 104, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Mas-Moruna, C.E.M.; Montufar, E.; Mestres, G.; Aparicio, D.; Javier, G.F.; Ginebra, M. Biomaterials Surface Science; Wiley-VCH Verlag: Weinheim, Germany, 2013. [Google Scholar]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 nanotubes: Synthesis and applications. Angew. Chem. Int. Ed. Engl. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Ji, S.; Liu, G.; Xu, G.; Ye, C. Understanding the growth behavior of titania nanotubes. Electrochem. Commun. 2011, 13, 454–457. [Google Scholar] [CrossRef]
- Schultze, J.W.; Lohrengel, M.M. Stability, reactivity and breakdown of passive films. Problems of recent and future research. Electrochim. Acta 2000, 45, 2499–2513. [Google Scholar] [CrossRef]
- Webster, T.J.; Yao, C. Anodizaton: A Promising Nano-Modification Technique of Titanium-Based Implants for Orthopedic Application; Springer: Heidelberg, Germany, 2007; pp. 21–47. [Google Scholar]
- Li, Y.; Ding, D.; Ning, C.; Bai, S.; Huang, L.; Li, M.; Mao, D. Thermal stability and in vitro bioactivity of Ti-Al-V-O nanostructures fabricated on Ti6AL4V alloy. Nanotechnology 2009, 20, 065708. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, T.T.; Zhang, Z.; Li, G. Fabrication of highly ordered TiO2 nanotube arrays via anodization of Ti-6AL-4V alloy sheet. J. Nanosci. Nanotechnol. 2010, 10, 8312–8321. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.H.; Lee, S.J.; Park, I.S.; Lee, M.H.; Soh, Y.J.; Bae, T.S.; Kim, H.S. Bioactivity of Ti-6AL-4V alloy implants treated with ibandronate after the formation of the nanotube TiO2 layer. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 2053–2059. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Lim, H.; Yun, K.D.; Park, S.; Jeong, C.; Lee, K. Effect of viscosities on the surface morphology and crystallographic properties of hydroxyapatite coated titanium dioxide nanotubes. J. Nanosci. Nanotechnol. 2015, 15, 5310–5313. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, Z.; Piszczek, P.; Radtke, A.; Jedrzejewski, T.; Kozak, W.; Sadowska, B. The evaluation of the impact of titania nanotube covers morphology and crystal phase on their biological properties. J. Mater. Sci. Mater. Med. 2015, 26, 163. [Google Scholar] [CrossRef] [PubMed]
- Lotz, E.M.; Olivares-Navarrete, R.; Berner, S.; Boyan, B.D.; Schwartz, Z. Osteogenic response of human mscs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J. Biomed. Mater. Res. A 2016, 104, 3137–3148. [Google Scholar] [CrossRef] [PubMed]
- Lattner, D.; Jennissen, H.P. Preparation and properties of ultra-hydrophilic surfaces on titanium and steel. Mater. Sci. Eng. Technol. 2009, 40, 109–116. [Google Scholar] [CrossRef]
- Jennissen, H.P.; Lüers, S. Lotus-effect and inverse lotus-effect in connection with extremely rough titanium surfaces. Mater. Sci. Eng. Technol. 2010, 41. [Google Scholar] [CrossRef]
- Park, J.; Bauer, S.; von der Mark, K.; Schmuki, P. Nanosize and vitality: TiO2 nanotube diameter directs cell fate. Nano Lett. 2007, 7, 1686–1691. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Bauer, S.; Schmuki, P.; Schlegel, K.; Neukam, F.; von der Mark, K. Nanotube diameter directs stem cell fate. J. Stem Cells Regen. Med. 2007, 2, 168. [Google Scholar] [PubMed]
- Park, J.; Bauer, S.; Pittrof, A.; Killian, M.S.; Schmuki, P.; von der Mark, K. Synergistic control of mesenchymal stem cell differentiation by nanoscale surface geometry and immobilized growth factors on TiO2 nanotubes. Small 2012, 8, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Von Wilmowsky, C.; Bauer, S.; Roedl, S.; Neukam, F.W.; Schmuki, P.; Schlegel, K.A. The diameter of anodic TiO2 nanotubes affects bone formation and correlates with the bone morphogenetic protein-2 expression in vivo. Clin. Oral Implants Res. 2012, 23, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Fagan, J.A.; Bauer, B.J.; Hobbie, E.K.; Becker, M.L.; Hight Walker, A.R.; Simpson, J.R.; Chun, J.; Obrzut, J.; Bajpai, V.; Phelan, F.R.; et al. Carbon nanotubes: Measuring dispersion and length. Adv. Mater. 2011, 23, 338–348. [Google Scholar] [CrossRef] [PubMed]
- Iwata, N.; Nozaki, K.; Horiuchi, N.; Yamashita, K.; Tsutsumi, Y.; Miura, H.; Nagai, A. Effects of controlled micro-/nanosurfaces on osteoblast proliferation. J. Biomed. Mater. Res. A 2017, 105, 2589–2596. [Google Scholar] [CrossRef] [PubMed]
- Palin, E.; Liu, H.; Webster, T.J. Mimicking the nanofeatures of bone increases bone-forming cell adhesion and proliferation. Nanotechnology 2005, 16, 1828–1835. [Google Scholar] [CrossRef]
- Gao, L.; Feng, B.; Wang, J.; Lu, X.; Liu, D.; Qu, S.; Weng, J. Micro/nanostructural porous surface on titanium and bioactivity. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Zinger, O.; Anselme, K.; Denzer, A.; Habersetzer, P.; Wieland, M.; Jeanfils, J.; Hardouin, P.; Landolt, D. Time-dependent morphology and adhesion of osteoblastic cells on titanium model surfaces featuring scale-resolved topography. Biomaterials 2004, 25, 2695–2711. [Google Scholar] [CrossRef] [PubMed]
- Kane, R.; Ma, P.X. Mimicking the nanostructure of bone matrix to regenerate bone. Mater. Today 2013, 16, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Weiner, S.; Wagner, H.D. The material bone: Structure-mechanical function relations. Annu. Rev. Mater. Sci. 1998, 28, 271–298. [Google Scholar] [CrossRef]
- Kheradmandfard, M.; Kashani-Bozorg, S.F.; Kim, C.L.; Hanzaki, A.Z.; Pyoun, Y.S.; Kim, J.H.; Amanov, A.; Kim, D.E. Nanostructured beta-type titanium alloy fabricated by ultrasonic nanocrystal surface modification. Ultrason. Sonochem. 2017, 39, 698–706. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dai, X.; Bai, Y.; Liu, Y.; Wang, Y.; Liu, O.; Yan, F.; Tang, Z.; Zhang, X.; Deng, X. Electroactive BaTiO3 nanoparticle-functionalized fibrous scaffolds enhance osteogenic differentiation of mesenchymal stem cells. Int. J. Nanomed. 2017, 12, 4007–4018. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xi, X.; Shen, X.; Liu, P.; Hu, Y.; Cai, K. Titania nanotubes dimensions-dependent protein adsorption and its effect on the growth of osteoblasts. J. Biomed. Mater. Res. A 2014, 102, 3598–3608. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Faltenbacher, J.; Berger, S.; von der Mark, K.; Schmuki, P. Size selective behavior of mesenchymal stem cells on ZrO2 and TiO2 nanotube arrays. Integr. Biol. 2009, 1, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Salou, L.; Hoornaert, A.; Louarn, G.; Layrolle, P. Enhanced osseointegration of titanium implants with nanostructured surfaces: An experimental study in rabbits. Acta Biomater. 2015, 11, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Bjursten, L.M.; Rasmusson, L.; Oh, S.; Smith, G.C.; Brammer, K.S.; Jin, S. Titanium dioxide nanotubes enhance bone bonding in vivo. J. Biomed. Mater. Res. A 2010, 92, 1218–1224. [Google Scholar] [PubMed]
- Zhao, X.; Wang, T.; Qian, S.; Liu, X.; Sun, J.; Li, B. Silicon-doped titanium dioxide nanotubes promoted bone formation on titanium implants. Int. J. Mol. Sci. 2016, 17, 292. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, T.; Hu, J.; Li, S.; Zou, Q.; Li, Y.; Jiang, N.; Li, H.; Li, J. Modified surface morphology of a novel Ti-24Nb-4Zr-7.9Sn titanium alloy via anodic oxidation for enhanced interfacial biocompatibility and osseointegration. Colloids Surf. B Biointerfaces 2016, 144, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Hegedus, C.; Ho, C.C.; Csik, A.; Biri, S.; Ding, S.J. Enhanced physicochemical and biological properties of ion-implanted titanium using electron cyclotron resonance ion sources. Materials 2016, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qiao, Y.; Cheng, M.; Jiang, G.; He, G.; Chen, Y.; Zhang, X.; Liu, X. Tantalum implanted entangled porous titanium promotes surface osseointegration and bone ingrowth. Sci. Rep. 2016, 6, 26248. [Google Scholar] [CrossRef] [PubMed]
- Heo, D.N.; Ko, W.K.; Lee, H.R.; Lee, S.J.; Lee, D.; Um, S.H.; Lee, J.H.; Woo, Y.H.; Zhang, L.G.; Lee, D.W.; et al. Titanium dental implants surface-immobilized with gold nanoparticles as osteoinductive agents for rapid osseointegration. J. Colloid Interface Sci. 2016, 469, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Csarnovics, I.; Hajdu, P.; Biri, S.; Hegedus, C.; Kökényesi, S.; Rácz, R.; Csik, A. Preliminary studies of creation of gold nanoparticles on titanium surface towards biomedical applications. Vaccum 2017, 126, 55–58. [Google Scholar] [CrossRef]
- Zainali, K.; Danscher, G.; Jakobsen, T.; Baas, J.; Moller, P.; Bechtold, J.E.; Soballe, K. Assessment of modified gold surfaced titanium implants on skeletal fixation. J. Biomed. Mater. Res. A 2013, 101, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Park, J.; Pittrof, A.; Song, Y.; von der Mark, K.; Schmuki, P. Covalent functionalization of TiO2 nanotube arrays with EGF and BMP-2 for modified behavior towards mesenchymal stem cell. Integr. Biol. 2011, 3, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Cai, K.; Zhao, L.; Chen, X.; Hou, Y.; Yang, Z. Surface functionalization of TiO2 nanotubes with bone morphogenetic protein 2 and its synergistic effect on the differentiation of mesenchymal stem cells. Biomacromolecules 2011, 12, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Neoh, K.G.; Zhang, J.; Kang, E.T.; Wang, W. Immobilization strategy for optimizing vegf’s concurrent bioactivity towards endothelial cells and osteoblasts on implant surfaces. Biomaterials 2012, 33, 8082–8093. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglic, V.; Milosev, I.; Schmuki, P.; Iglic, A.; Mozetic, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Chen, Y.M.; Biao, M.N.; Zhang, X.D.; Yang, B.C. Bio-functionalization of biomedical metals. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Chrastil, J.; Patel, A.A. Complications associated with posterior and transforaminal lumbar interbody fusion. J. Am. Acad. Orthop. Surg. 2012, 20, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Jennissen, H.P. A macrophage model of osseointegration. Curr. Dir. Biomed. Eng. 2016, 2, 53–56. [Google Scholar] [CrossRef]
- Chatzinikolaidou, M.; Lichtinger, T.K.; Muller, R.T.; Jennissen, H.P. Peri-implant reactivity and osteoinductive potential of immobilized rhbmp-2 on titanium carriers. Acta Biomater. 2010, 6, 4405–4421. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.; Kirsch, A.; Schwarz, F.; Chatzinikolaidou, M.; Rothamel, D.; Lekovic, V.; Laub, M.; Jennissen, H.P. Bone apposition to titanium implants biocoated with recombinant human bone morphogenetic protein-2 (rhbmp-2). A pilot study in dogs. Clin. Oral Investig. 2006, 10, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Koo, A.N.; Lee, S.W.; Lee, M.H.; Lee, S.C. Catechol-functionalized adhesive polymer nanoparticles for controlled local release of bone morphogenetic protein-2 from titanium surface. J. Control. Release 2013, 170, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Reddi, A.H. Growth and morphogenetic factors in bone induction: Role of osteogenin and related bone morphogenetic proteins in craniofacial and periodontal bone repair. Crit. Rev. Oral Biol. Med. 1992, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.A.; Vehof, J.W.; Ruhe, P.Q.; Kroeze-Deutman, H.; Kuboki, Y.; Takita, H.; Hedberg, E.L.; Mikos, A.G. Growth factor-loaded scaffolds for bone engineering. J. Control. Release 2005, 101, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, S.; Mochizuki, M.; Fukushima, S.; Ito, T.; Nozaki, K.; Iwai, T.; Takahashi, K.; Yokota, S.; Miyata, K.; Sasaki, N. Long-term stability of bone tissues induced by an osteoinductive biomaterial, recombinant human bone morphogenetic protein-2 and a biodegradable carrier. Biomaterials 2004, 25, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- La, W.G.; Park, S.; Yoon, H.H.; Jeong, G.J.; Lee, T.J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Biao, M.; Chen, Y.; Xie, M.; Yang, B. Regulating the osteogenic function of rhBMP 2 by different titanium surface properties. J. Biomed. Mater. Res. A 2016, 104, 1882–1893. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, N.; Ivanovski, S.; Prasadam, I.; Crawford, R.; Oloyede, A.; Xiao, Y. The microrna expression signature on modified titanium implant surfaces influences genetic mechanisms leading to osteogenic differentiation. Acta Biomater. 2012, 8, 3516–3523. [Google Scholar] [CrossRef] [PubMed]
- Babuska, V.; Moztarzadeh, O.; Kubikova, T.; Moztarzadeh, A.; Hrusak, D.; Tonar, Z. Evaluating the osseointegration of nanostructured titanium implants in animal models: Current experimental methods and perspectives. Biointerphases 2016, 11, 030801. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, M.; Sun, H.; Li, P.; Wang, K.; Ren, F.; Lu, X. Porous titanium scaffolds with self-assembled micro/nano hierarchical structure for dual functions of bone regeneration and anti-infection. J. Biomed. Mater. Res. A 2017. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Shirai, T.; Nishida, H.; Murakami, H.; Kabata, T.; Yamamoto, N.; Watanabe, K.; Nakase, J. Innovative antimicrobial coating of titanium implants with iodine. J. Orthop. Sci. 2012, 17, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef] [PubMed]
- Dziedzic, D.M.; Savva, I.H.; Wilkinson, D.S.; Davies, J.E. Osteoconduction on, and bonding to, calcium phosphate ceramic implants. Mater. Res. Soc. Symp. Proc. 1996, 414, 147–156. [Google Scholar] [CrossRef]
- Wengler, A.; Nimptsch, U.; Mansky, T. Hip and knee replacement in germany and the USA: Analysis of individual inpatient data from german and us hospitals for the years 2005 to 2011. Dtsch. Ärzteblatt Int. 2014, 111, 407–416. [Google Scholar]
- Kurtz, S.M.; Ong, K.L.; Lau, E.; Bozic, K.J.; Berry, D.; Parvizi, J. Prosthetic joint infection risk after TKA in the medicare population. Clin. Orthop. Relat. Res. 2010, 468, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.L.; Kurtz, S.M.; Lau, E.; Bozic, K.J.; Berry, D.J.; Parvizi, J. Prosthetic joint infection risk after total hip arthroplasty in the medicare population. J. Arthroplast. 2009, 24, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Bohl, D.D.; Samuel, A.M.; Basques, B.A.; Della Valle, C.J.; Levine, B.R.; Grauer, J.N. How much do adverse event rates differ between primary and revision total joint arthroplasty? J. Arthroplast. 2016, 31, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Kienapfel, H.; Ascherl, R.; Matziolis, G.; Hassenpflug, J.; Thomsen, M.; Thomas, P.; Huber, M.; Schuh, C.; et al. Revised consensus classification. Histopathological classification of diseases associated with joint endoprostheses. Z. Rheumatol. 2013, 72, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.L.; Scarponi, S.; Gallazzi, E.; Romano, D.; Drago, L. Antibacterial coating of implants in orthopaedics and trauma: A classification proposal in an evolving panorama. J. Orthop. Surg. Res. 2015, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Kindrachuk, J.; Duan, K.; Jenssen, H.; Hancock, R.E.; Wang, R. Antimicrobial peptides on calcium phosphate-coated titanium for the prevention of implant-associated infections. Biomaterials 2010, 31, 9519–9526. [Google Scholar] [CrossRef] [PubMed]
- Taylor, E.; Webster, T.J. Reducing infections through nanotechnology and nanoparticles. Int. J. Nanomed. 2011, 6, 1463–1473. [Google Scholar]
- Gallo, J.; Panacek, A.; Prucek, R.; Kriegova, E.; Hradilova, S.; Hobza, M.; Holinka, M. Silver nanocoating technology in the prevention of prosthetic joint infection. Materials 2016, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Kummer, K.M.; Tarquinio, K.M.; Webster, T.J. Decreased staphylococcus aureus biofilm growth on anodized nanotubular titanium and the effect of electrical stimulation. Acta Biomater. 2011, 7, 3003–3012. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.N.; Liang, E.I.; Rodriguez, L.A.; Vollereaux, N.; Yee, A.F. Nanopatterned polymer surfaces with bactericidal properties. Biointerphases 2015, 10, 021010. [Google Scholar] [CrossRef] [PubMed]
- Gorth, D.J.; Puckett, S.; Ercan, B.; Webster, T.J.; Rahaman, M.; Bal, B.S. Decreased bacteria activity on Si(3)N(4) surfaces compared with peek or titanium. Int. J. Nanomed. 2012, 7, 4829–4840. [Google Scholar]
- Sjöström, T.; Nobbs, A.H.; Su, B. Bactericidal nanospike surfaces via thermal oxidation of ti alloy substrates. Mater. Lett. 2016, 167, 22–26. [Google Scholar] [CrossRef]
- Mathew, D.; Bhardwaj, G.; Wang, Q.; Sun, L.; Ercan, B.; Geetha, M.; Webster, T.J. Decreased staphylococcus aureus and increased osteoblast density on nanostructured electrophoretic-deposited hydroxyapatite on titanium without the use of pharmaceuticals. Int. J. Nanomed. 2014, 9, 1775–1781. [Google Scholar]
- Hasan, J.; Webb, H.K.; Truong, V.K.; Pogodin, S.; Baulin, V.A.; Watson, G.S.; Watson, J.A.; Crawford, R.J.; Ivanova, E.P. Selective bactericidal activity of nanopatterned superhydrophobic cicada psaltoda claripennis wing surfaces. Appl. Microbiol. Biotechnol. 2013, 97, 9257–9262. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, K.M.; Watson, J.A.; Qu, X.; Liu, F.; Watson, G.S.; Chen, C.H. Self-cleaning of superhydrophobic surfaces by self-propelled jumping condensate. Proc. Natl. Acad. Sci. USA 2013, 110, 7992–7997. [Google Scholar] [CrossRef] [PubMed]
- Sengstock, C.; Lopian, M.; Motemani, Y.; Borgmann, A.; Khare, C.; Buenconsejo, P.J.; Schildhauer, T.A.; Ludwig, A.; Koller, M. Structure-related antibacterial activity of a titanium nanostructured surface fabricated by glancing angle sputter deposition. Nanotechnology 2014, 25, 195101. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Zhu, Z.; Mo, A.; Li, L.; Zhang, J. Deposition of silver nanoparticles on titanium surface for antibacterial effect. Int. J. Nanomed. 2010, 5, 261–267. [Google Scholar]
- Wan, W.; Yeow, J.T. Antibacterial properties of poly(quaternary ammonium) modified gold and titanium dioxide nanoparticles. J. Nanosci. Nanotechnol. 2012, 12, 4601–4606. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Ward, B.C.; Webster, T.J. Increased osteoblast and decreased staphylococcus epidermidis functions on nanophase zno and TiO2. J. Biomed. Mater. Res. Part A 2006, 78, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cai, X.M.; Tang, H.Q.; Liu, T.; Gu, H.Q.; Cui, R.Z. Bactericidal and biocompatible properties of TiN/Ag multilayered films by ion beam assisted deposition. J. Mater. Sci. Mater. Med. 2009, 20 (Suppl. 1), S101–S105. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.S.; Amna, T.; Mishra, A.; Yun, S.I.; Kim, H.C.; Kim, H.Y.; Khil, M.S. Fabrication, characterization and antibacterial effect of novel electrospun TiO2 nanorods on a panel of pathogenic bacteria. J. Biomed. Nanotechnol. 2012, 8, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Amna, T.; Hassan, M.S.; Barakat, N.A.; Pandeya, D.R.; Hong, S.T.; Khil, M.S.; Kim, H.Y. Antibacterial activity and interaction mechanism of electrospun zinc-doped titania nanofibers. Appl. Microbiol. Biotechnol. 2012, 93, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zhang, W.; Qiao, Y.; Jiang, X.; Liu, X.; Ding, C. Antibacterial activity and increased bone marrow stem cell functions of Zn-incorporated TiO2 coatings on titanium. Acta Biomater. 2012, 8, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, G. Potent antibacterial activities of ag/TiO2 nanocomposite powders synthesized by a One-Pot Sol-Gel method. Environ. Sci. Technol. 2009, 43, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Uota, M.; Torikai, T.; Watari, T.; Noda, I.; Hotokebuchi, T.; Yada, M. Antibacterial properties of nanostructured silver titanate thin films formed on a titanium plate. J. Biomed. Mater. Res. Part A 2010, 92, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Nasajpour, A.; Mandla, S.; Shree, S.; Mostafavi, E.; Sharifi, R.; Khalilpour, A.; Saghazadeh, S.; Hassan, S.; Mitchell, M.J.; Leijten, J.; et al. Nanostructured fibrous membranes with rose spike-like architecture. Nano Lett. 2017, 17, 6235–6240. [Google Scholar] [CrossRef] [PubMed]
- Uklejewski, R.; Rogala, P.; Winiecki, M.; Toklowicz, R.; Ruszkowski, P.; Wolun-Cholewa, M. Biomimetic multispiked connecting Ti-alloy scaffold prototype for entirely-cementless resurfacing arthroplasty endoprostheses-exemplary results of implantation of the Ca-P surface-modified scaffold prototypes in animal model and osteoblast culture evaluation. Materials 2016, 9, 532. [Google Scholar]
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef] [PubMed]
- De Giglio, E.; Cafagna, D.; Cometa, S.; Allegretta, A.; Pedico, A.; Giannossa, L.C.; Sabbatini, L.; Mattioli-Belmonte, M.; Iatta, R. An innovative, easily fabricated, silver nanoparticle-based titanium implant coating: Development and analytical characterization. Anal. Bioanal. Chem. 2013, 405, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, A.; Yeung, K.W.; Kao, R.Y.; Wu, G.; Hu, T.; Xu, Z.; Chu, P.K. Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl. Mater. Interfaces 2011, 3, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Nath, S.; Sugawara, Y.; Divakarla, K.; Das, T.; Manos, J.; Chrzanowski, W.; Matsushita, T.; Kokubo, T. Two-in-one biointerfaces-antimicrobial and bioactive nanoporous gallium titanate layers for titanium implants. Nanomaterials 2017, 7, 229. [Google Scholar] [CrossRef] [PubMed]
- Valappil, S.P.; Ready, D.; Abou Neel, E.A.; Pickup, D.M.; O’Dell, L.A.; Chrzanowski, W.; Pratten, J.; Newport, R.J.; Smith, M.E.; Wilson, M.; et al. Controlled delivery of antimicrobial gallium ions from phosphate-based glasses. Acta Biomater. 2009, 5, 1198–1210. [Google Scholar] [CrossRef] [PubMed]
- Cochis, A.; Azzimonti, B.; Della Valle, C.; De Giglio, E.; Bloise, N.; Visai, L.; Cometa, S.; Rimondini, L.; Chiesa, R. The effect of silver or gallium doped titanium against the multidrug resistant acinetobacter baumannii. Biomaterials 2016, 80, 80–95. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Thoendel, M.; Olakanmi, O.; Britigan, B.E.; Singh, P.K. The transition metal gallium disrupts pseudomonas aeruginosa iron metabolism and has antimicrobial and antibiofilm activity. J. Clin. Investig. 2007, 117, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Liu, X.; Qian, S.; Cao, H.; Qiao, Y.; Mei, Y.; Chu, P.K.; Ding, C. Multilevel surface engineering of nanostructured TiO2 on carbon-fiber-reinforced polyetheretherketone. Biomaterials 2014, 35, 5731–5740. [Google Scholar] [CrossRef] [PubMed]
- Toniatto, T.V.; Rodrigues, B.V.M.; Marsi, T.C.O.; Ricci, R.; Marciano, F.R.; Webster, T.J.; Lobo, A.O. Nanostructured poly (lactic acid) electrospun fiber with high loadings of TiO2 nanoparticles: Insights into bactericidal activity and cell viability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 71, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, F.; Karumidze, N.; Kusradze, I.; Goderdzishvili, M.; Teixeira, P.; Gouveia, I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomedicine 2017, 13, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zheng, W.; Kuang, L.; Ma, H.; Liang, H. Hydrophilic phage-mimicking membrane active antimicrobials reveal nanostructure-dependent activity and selectivity. ACS Infect. Dis. 2017, 3, 676–687. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Diu, T.; Faruqui, N.; Sjostrom, T.; Lamarre, B.; Jenkinson, H.F.; Su, B.; Ryadnov, M.G. Cicada-inspired cell-instructive nanopatterned arrays. Sci. Rep. 2014, 4, 7122. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y.; Juodkazis, S.; Crawford, R.J.; Ivanova, E.P. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef] [PubMed]
- Hizal, F.; Zhuk, I.; Sukhishvili, S.; Busscher, H.J.; van der Mei, H.C.; Choi, C.H. Impact of 3D hierarchical nanostructures on the antibacterial efficacy of a bacteria-triggered self-defensive antibiotic coating. ACS Appl. Mater. Interfaces 2015, 7, 20304–20313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, T.; Wen, J.; Xu, L.; Zeng, D.; Wu, Q.; Cao, L.; Lin, S.; Liu, X.; Jiang, X. Selective responses of human gingival fibroblasts and bacteria on carbon fiber reinforced polyetheretherketone with multilevel nanostructured TiO2. Biomaterials 2016, 83, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, A.; Klein, A.; Ritz, U.; Ackermann, A.; Anthonissen, J.; Kaufmann, K.B.; Brendel, C.; Gotz, H.; Rommens, P.M.; Hofmann, A. Surface functionalization of orthopedic titanium implants with bone sialoprotein. PLoS ONE 2016, 11, e0153978. [Google Scholar] [CrossRef] [PubMed]
- Graves, J.L., Jr.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in escherichia coli. Front. Genet. 2015, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Soto-Quintero, A.; Romo-Uribe, A.; Bermudez-Morales, V.H.; Quijada-Garrido, I.; Guarrotxena, N. 3D-hydrogel based polymeric nanoreactors for silver nano-antimicrobial composites generation. Nanomaterials 2017, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Li, J.P.; de Wijn, J.R.; van Blitterswijk, C.A.; de Groot, K. Porous Ti6AL4V scaffolds directly fabricated by 3D fibre deposition technique: Effect of nozzle diameter. J. Mater. Sci. Mater. Med. 2005, 16, 1159–1163. [Google Scholar] [CrossRef] [PubMed]
- Murr, L.E.; Esquivel, E.V.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Martinez, E.Y.; Medina, F.; Hernandez, D.H.; Martinez, E.; Stafford, S.W.; et al. Microstructures and mechanical properties of electron beam-rapid manufactured Ti-6AL-4V biomedical prototypes compared to wrought Ti-6AL-4V. Mater. Charact. 2009, 60, 96–105. [Google Scholar] [CrossRef]
- Mullen, L.; Stamp, R.C.; Brooks, W.K.; Jones, E.; Sutcliffe, C.J. Selective laser melting: A regular unit cell approach for the manufacture of porous, titanium, bone in-growth constructs, suitable for orthopedic applications. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Vandenbroucke, B.K.; Kruth, J.-P. Selective laser melting of biocompatible metals for rapid manufacturing of medical parts. Rapid Prototyp. J. 2007, 13, 196–203. [Google Scholar] [CrossRef]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Hotchkiss, K.M.; Reddy, G.B.; Hyzy, S.L.; Schwartz, Z.; Boyan, B.D.; Olivares-Navarrete, R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater. 2016, 31, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Stenlund, P.; Martinelli, A.; Thomsen, P.; Palmquist, A. Direct communication between osteocytes and acid-etched titanium implants with a sub-micron topography. J. Mater. Sci. Mater. Med. 2016, 27, 167. [Google Scholar] [CrossRef] [PubMed]


| Material | Density (Mg/m3) | Young’s Modulus, E (GPa) |
|---|---|---|
| Cp-Ti grade II | 4.2 | 100–110 |
| Ti-6Al-4V | 4.5 | 100–130 |
| Ti-6Al-7Nb | 4.52 | 110–130 |
| Surgical CrNiMo-Steel 316L | 7.8 | 195–210 |
| CoCrMo alloys | 8.5 | 210–230 |
| Cortical bone | 1.19–1.85 | 18.6–20.7 |
| Technique | Modified Layer | Objective | |
|---|---|---|---|
| Mechanical |
| Rough or smooth surface formed by the subtraction process | Produce specific surface topographies; Clean and roughen surface; Improve adhesion in bonding |
| To fabricate nanophase surface layers on Ti of commercial purity which improve the tensile properties and surface hardness of Ti | Produce materials with nanometre size grains (1–100 nm); To produce rough morphology and higher hydrophilicity | |
| Chemical |
| <10 nm of surface oxide layer ~1 μm of sodium titanate gel ~5 nm of dense inner oxide and porous outer layer | Remove oxide scales and contamination. Improve biocompatibility, bioactivity or bone conductivity. Improve biocompatibility, bioactivity or bone conductivity |
| ~10 μm of thin film, such as calcium phosphate, TiO2 and silica | Improve biocompatibility, bioactivity or bone conductivity | |
| ~1 μm of TiN, TiC, TiCN, diamond and diamond-like carbon thin film | Improve wear resistance, corrosion resistance and blood compatibility | |
| ~10 nm–40 μm of TiO2 layer, adsorption and incorporation of electrolyte anions | Produce specific surface topographies; improve corrosion resistance; improve biocompatibility, bioactivity or bone conductivity | |
| Coating deposition; modification through silanized Ti, photochemistry, self-assembled monolayers, protein-resistance, etc. | Induce specific cell and tissue response by means of surface immobilized peptides, proteins, or growth factors | |
| Physical |
| ~30 to ~200 μm of coatings, such as titanium, HA, calcium silicate, Al2O3, ZrO2, TiO2 | Improve wear resistance, corrosion resistance and biological properties (osteoblast adhesion) |
| ~1 μm of TiN, TiC, TiCN, diamond and diamond-like carbon thin film Hydroxyapatite coating by sputtering | Improve wear resistance, corrosion resistance and blood compatibility. | |
| ~10 nm of surface modified layer and/or um of thin film | Modify surface composition; improve wear, corrosion resistance, and biocompatibility | |
| ~1 nm to ~100 nm of surface modified layer | Cleaning, sterilizing or oxidizing the surface; surface nitridation; removal of the native oxide layer |
| Method | Effect | ||
|---|---|---|---|
| Coating |
| Decreased viabilitiy and adhesion of Escherichia coli and Staphylococcus aureus in vitro | [161] |
| Decreased viability of Escherichia coli (5 logs in 10 min) in vitro | [162] | |
| Surface structure |
| Decreased adhesion of Staphylococcus epidermidis in vitro | [163] |
| Bactericidal in Escherichia coli in vitro | [164] | |
| Disruption of cell membrane in Escherichia coli, Salmonella Typhimurium, Klebsiella pneumoniae, Staphylococcus aureus in vitro | [165] | |
| Disruption of cell membrane in Escherichia coli, Staphylococcus aureus in vitro | [166] | |
| Disruption of cell membrane in Escherichia coli in vitro | [159] | |
| Decreased growth of Escherichia coli and Staphylococcus aureus in vitro | [167] | |
| Complete growth inhibition of Escherichia coli in vitro | [168] | |
| Antibacterial against MRSA in vitro | [169] |
| Surface | Surface Feature | Method | Wettability (CA) | Bactericidal and Fungicidal Efficacy |
|---|---|---|---|---|
| Cicada wing | Nanoneedles, height 200 nm, diameter 60 nm size at the top, 100 nm at the base of the pillar, and spacing 170 nm | natural | HY [159°] | Lethal to P. aeruginosa (G−) |
| Gecko skin | Hair (spinules) like structures with sub-micron spacing and a tip radius of curvature <20 nm | natural | HY [151°–155°] | Lethal to Porphyromonas gingivalis (G−) |
| Dragon fly wing | Nanograss, diameter 50–70 nm, height 240 nm | natural | HY [153°] | Lethal to P. aeruginosa (G−), S. aureus (G+), B. subtilis (G+) |
| Periodical cicada | Hemispherical nano features with height 83.5 nm, diameter 167 nm, pitch 252 nm | natural | HP [80.1°] | Caused cell wall rupturing of Saccharomyces cerevisiae |
| Annual DD cicada | Spherical nanocones with height 183 nm, base diameter 104 nm, cap diameter 104 nm, pitch 175 nm | natural | HY [132°] | Caused cell wall rupturing of Saccharomyces cerevisiae |
| Sanddragon dragonfly | High-aspect ratio spherical capped nanocylinders with height 241 nm, diameter 53 nm, pitch 123 nm | natural | HY [119°] | Caused cell wall rupturing of Saccharomyces cerevisiae |
| Megapomponia intermedia | Nanopillars with height 241 nm, diameter 156 nm, pitch 165 nm | natural | HY [135.5°] | Bactericidal against Pseudomonas fluorescens (G−) |
| Cryptotympana aguila | Nanopillars with height 182 nm, diameter 159 nm, pitch 187 nm | natural | HY [113.2°] | Bactericidal against G− P. fluorescens |
| Ayuthia spectabile | Nanopillars with height 182 nm, diameter 207 nm, pitch 251 nm | natural | HY [95.65°] | Bactericidal against P. fluorescens (but more than Megapomponia intermedia and Cryptotympana aguila) |
| Titania nanowire arrays | Nanowires, brush type: Diameter 100 nm | Hydrothermal | - | Effective in killing motile bacteria (P. aeruginosa, Escherichia coli (G−), B. subtilis), less lethal against non-motile bacteria (S. aureus, Enterococcus faecalis (G+), K. pneumoniae (G−)) |
| Titania nanowire arrays | Nanowires, niche type: Diameter 10–15 μm | Hydrothermal | - | Effective in killing motile bacteria (P. aeruginosa, Escherichia coli and B. subtilis), less lethal against non-motile bacteria (S. aureus, Enterococcus faecalis, and Klebsiella pneumoniae) |
| Ti nanopatterned arrays | Nanopatterned arrays, average diameter 40.3 nm | Hydrothermal etching | HP [73°] | Effective in killing P. aeruginosa, less lethal against S. aureus |
| Ti alloy nanospike surface | Nanospikes, average diameter 10 nm, spacing 2 μm, height 2 μm | Anodization | - | Lethal to S. aureus |
| Ti alloy anospike surface | Nanospikes, average diameter 20 nm | Thermal oxidation | - | Lethal to E. coli |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. https://doi.org/10.3390/ma10111302
Jäger M, Jennissen HP, Dittrich F, Fischer A, Köhling HL. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials. 2017; 10(11):1302. https://doi.org/10.3390/ma10111302
Chicago/Turabian StyleJäger, Marcus, Herbert P. Jennissen, Florian Dittrich, Alfons Fischer, and Hedda Luise Köhling. 2017. "Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants" Materials 10, no. 11: 1302. https://doi.org/10.3390/ma10111302





