An Investigation of Polyoxometalate Hybrid Materials as Patternable Dielectrics and Lithographic Resists
Abstract
:1. Introduction
2. Materials and Methods
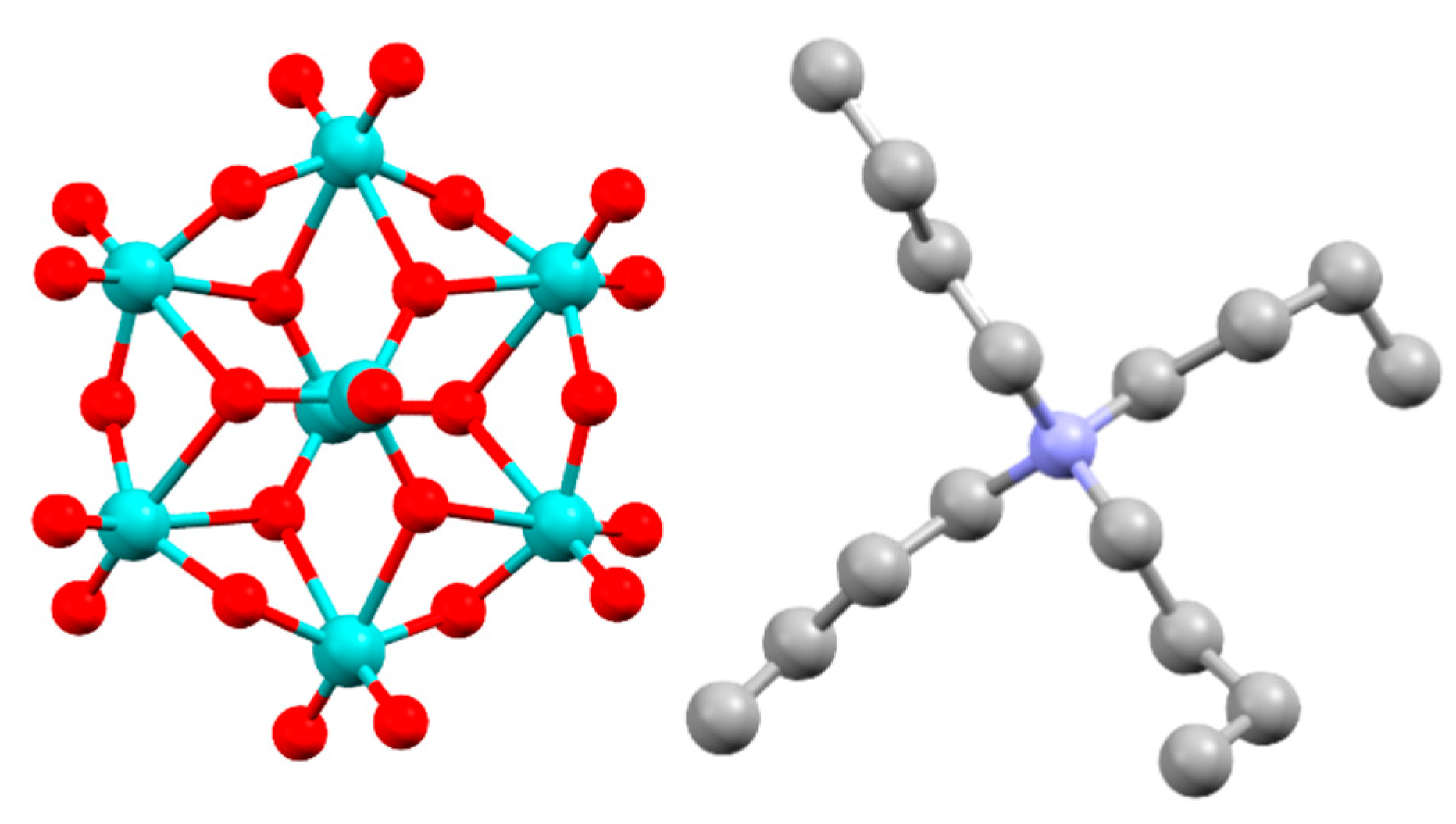
2.1. Octamolybdate Synthesis and Blends
2.2. Spin-Coating Optimization
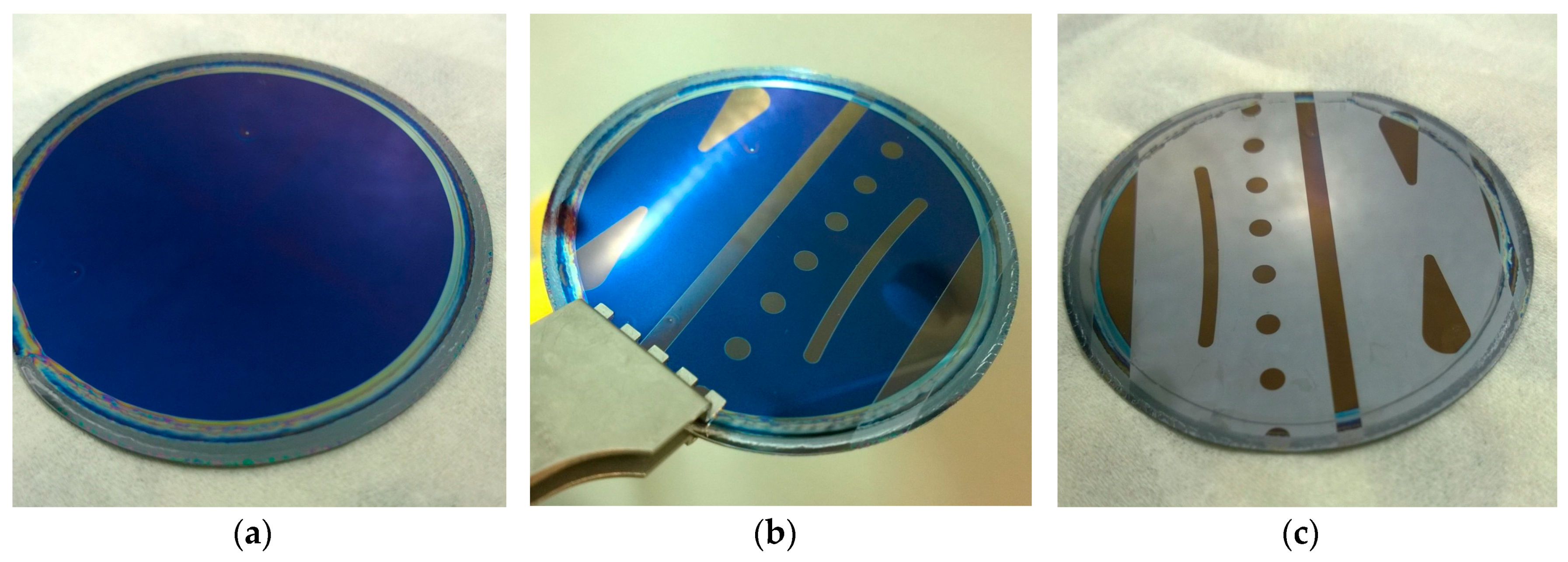
2.3. Patterning and Development Methods
2.4. Film Characterization
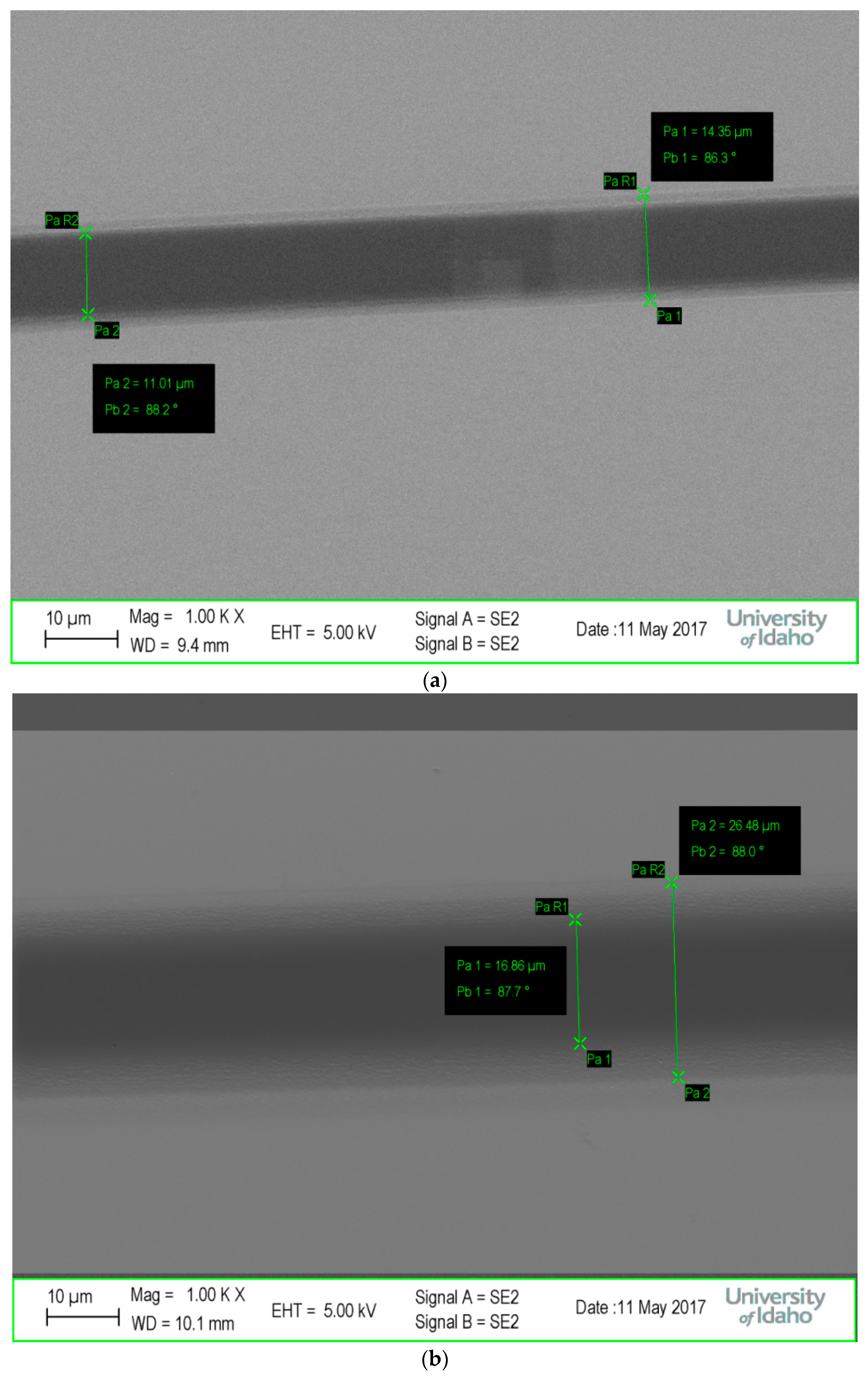
2.5. SEM Imaging
3. Results and Discussion
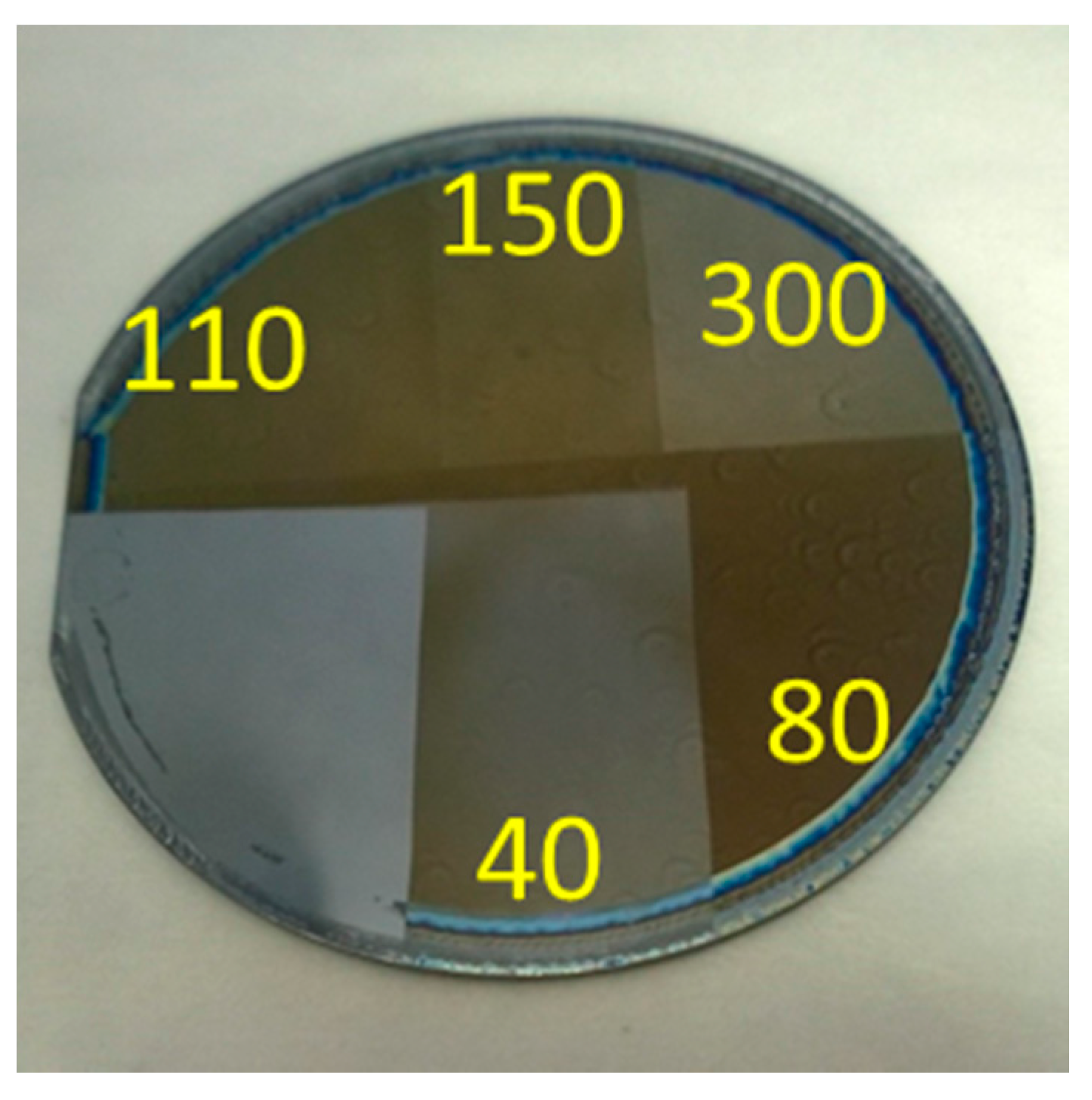
3.1. Spin-Coating Optimization
3.2. Patterning and Lithography: Mo8 Alone
3.2.1. UV Curing of Mo8-Only Resists
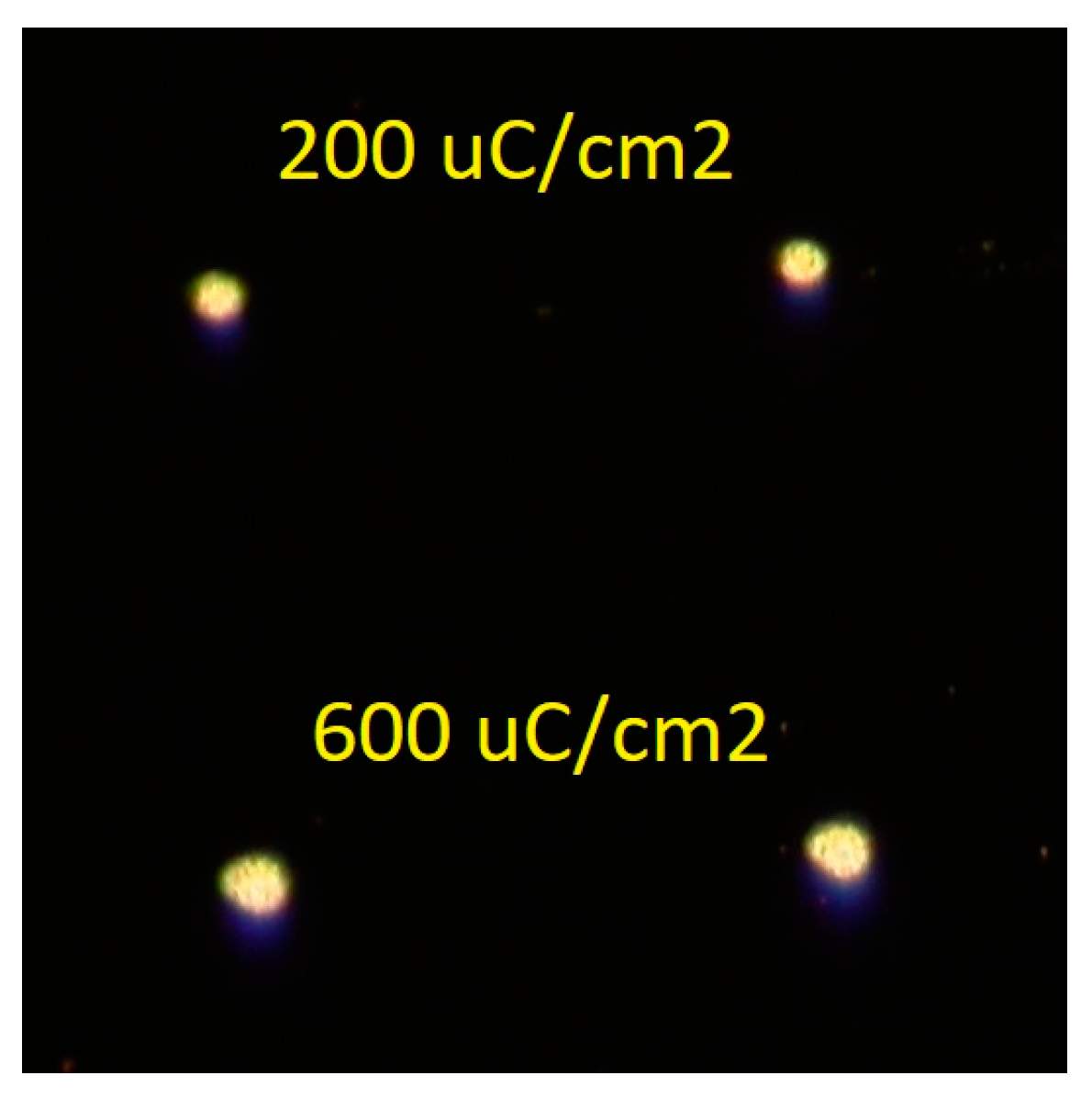
3.2.2. Electron-Beam Lithography (EBL) of Mo8-Only Resists
3.3. Patterning and Lithography: Blended Resists
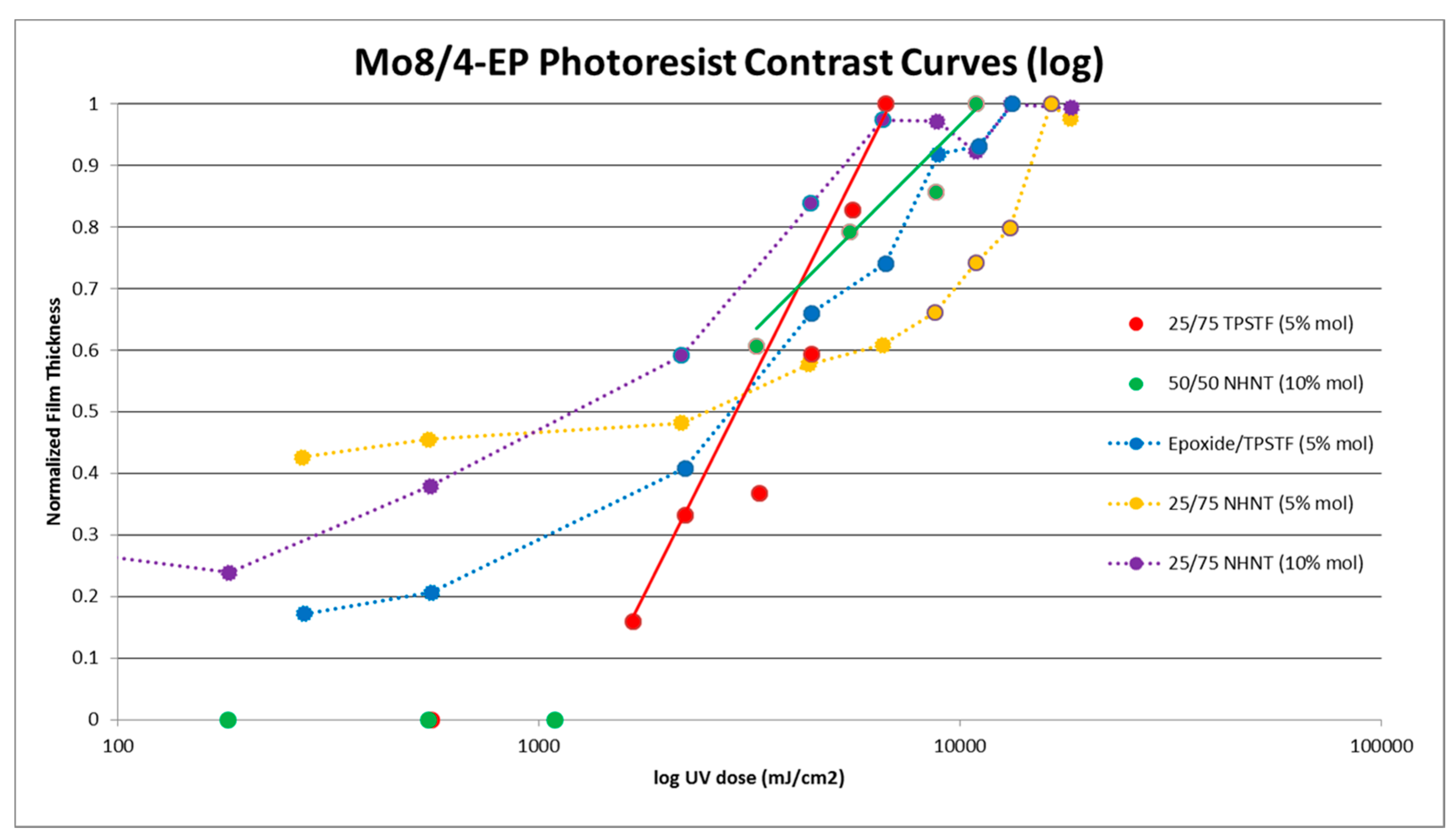
3.3.1. UV Mask Aligner of Blended Resists
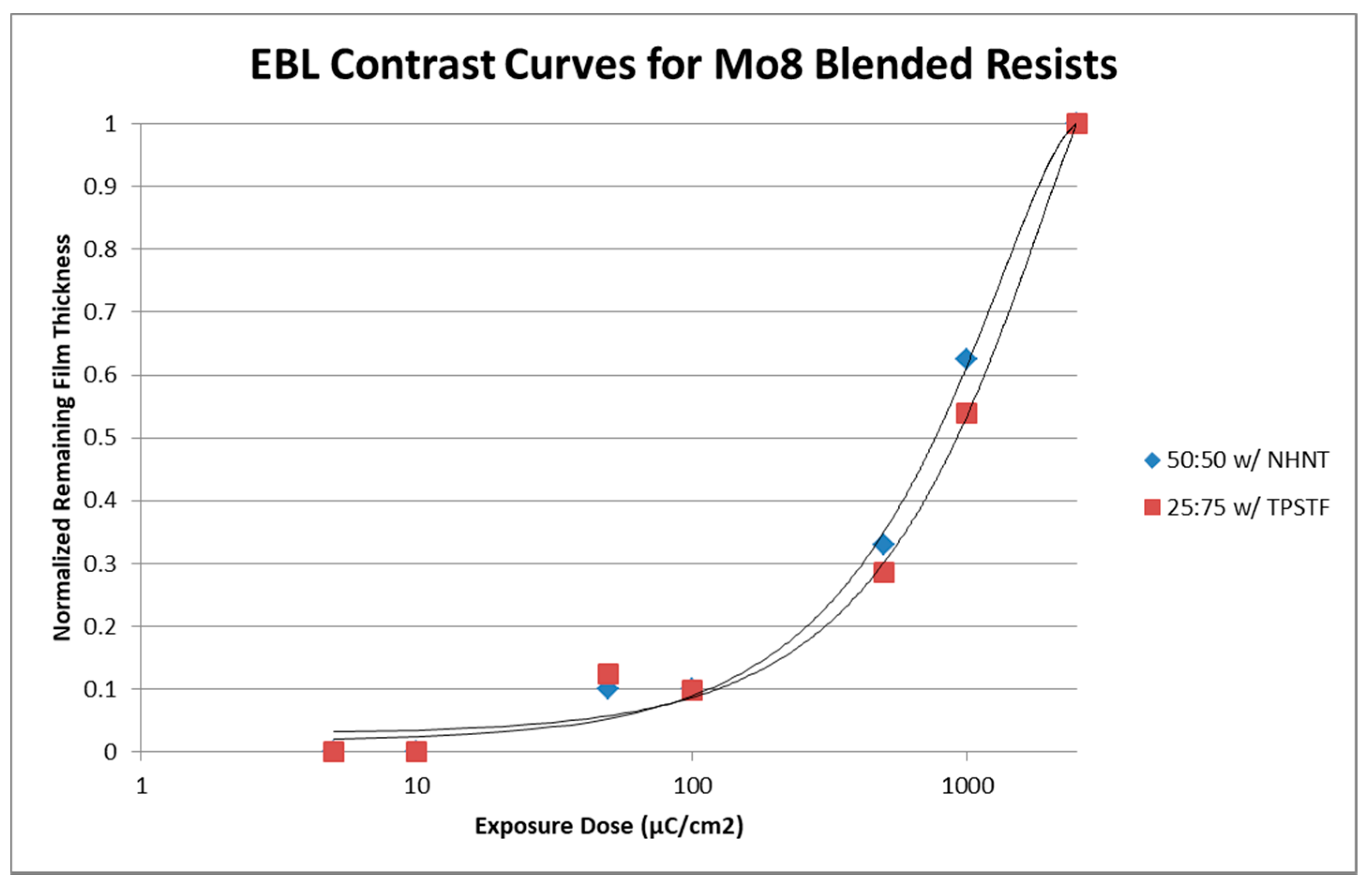
3.3.2. Electron-Beam Lithography (EBL) of Blended Resists
3.4. Mechanistic Analysis
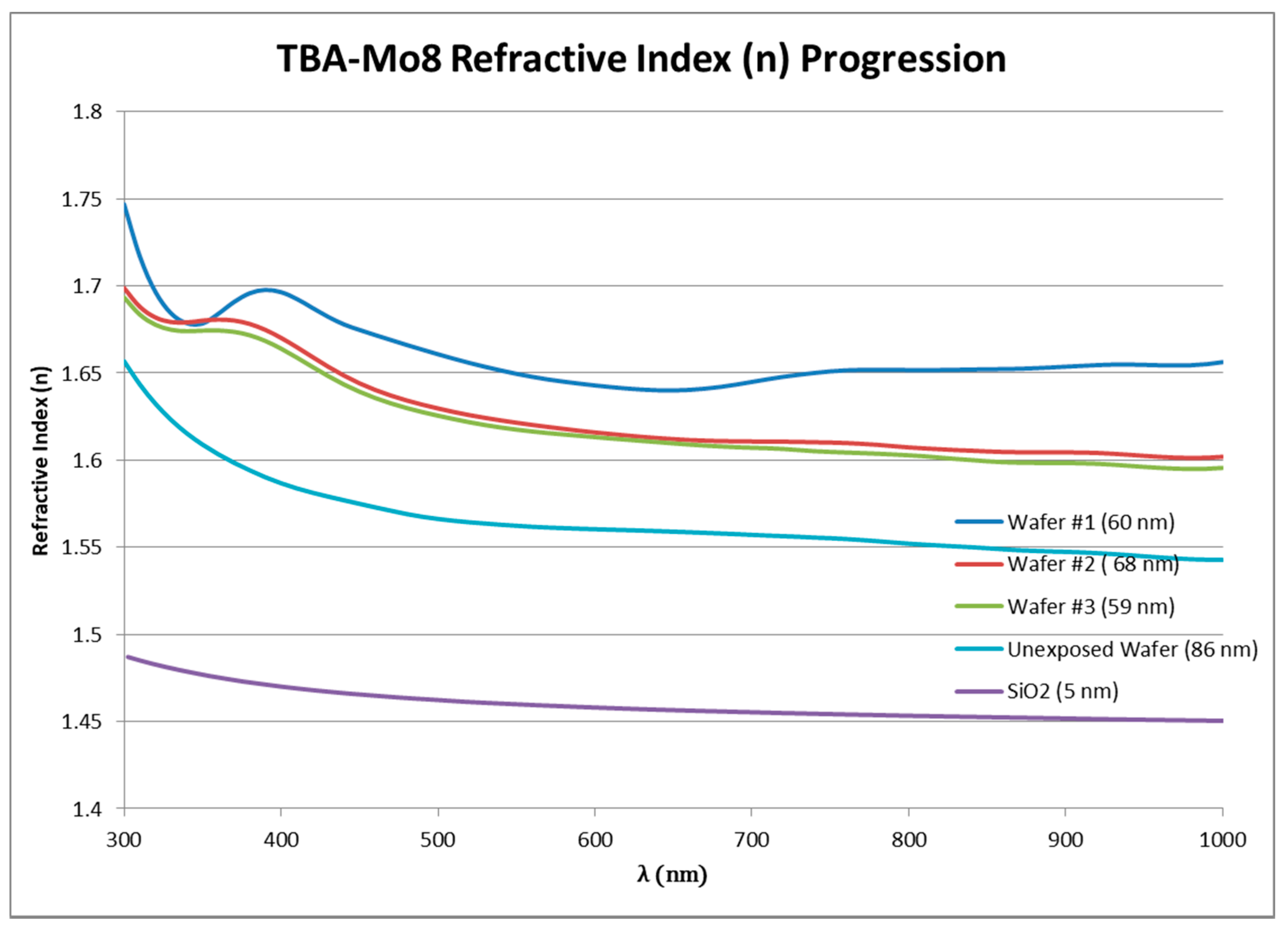
3.5. Ellipsometry
3.6. Future Work
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
Appendix A.1. Elemental Analysis Results
References
- Song, Y.-F.; Tsunashima, R. Recent advances on polyoxometalate-based molecular and composite materials. Chem. Soc. Rev. 2012, 41, 7384–7402. [Google Scholar] [CrossRef] [PubMed]
- Dolbecq, A.; Dumas, E.; Mayer, C.R.; Mialane, P. Hybrid organic-inorganic polyoxometalate compounds: From structural diversity to applications. Chem. Rev. 2010, 110, 6009–6048. [Google Scholar] [CrossRef] [PubMed]
- Long, D.-L.; Tsunashima, R.; Cronin, L. Polyoxometalates: Building blocks for functional nanoscale systems. Angew. Chem. Int. Ed. 2010, 49, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, A.K.; Devi, M.; Gonsalves, K.E.; Pradeep, C.P. Engineering multifunctionality in hybrid polyoxometalates: Aromatic sulfonium octamolybdates as excellent photochromic materials and self-separating catalysts for epoxidation. Inorg. Chem. 2017, 56, 10325–10336. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Ishikawa, A.; Okamoto, H.; Miyauchi, K.; Murai, F.; Mochiji, K.; Umezaki, H. Spin-coatable inorganic resists based on novel peroxopolyniobotungstic acids for bilayer lithography. J. Electrochem. Soc. 1987, 134, 2607–2613. [Google Scholar] [CrossRef]
- Heller, A.; Carls, J.C.; Argitis, P.; Meaux, J.J. Pattern Forming and Transferring Processes. U.S. Patent 5,178,989, 12 January 1993. [Google Scholar]
- Kalyani, V.; Satyanarayana, V.S.V.; Singh, V.; Pradeep, C.P.; Ghosh, S.; Sharma, S.K.; Gonsalves, K.E. New polyoxometalates containing hybrid polymers and their potential for nano-patterning. Chem. Eur. J. 2014, 21, 2250–2258. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.; Stampka, T.; Roll, M.F. A direct synthesis of the styrylimido derivative of the hexamolybdate diaion. J. Ceram. Soc. Jpn. 2015, 23, 813–815. [Google Scholar] [CrossRef]
- Hardie, B.; Fischer, J.; Fitzgerald, M.; Roll, M.F. Cation effects on imidization of the hexamolybdate dianion via direct dehydration using the green combination of dimethoxypropane in dimethylsulfoxide. Inorg. Chem. Commun. 2017, 84, 84–88. [Google Scholar] [CrossRef]
- Xu, L.; Lu, M.; Xu, B.; Wei, Y.; Peng, Z.; Powell, D.R. Towards main-chain-polyoxometalate-contaning hybrid polymers: A highly efficient approach to bifunctionalized organoimido derivatives of hexamolybdates. Angew. Chem. Int. Ed. 2002, 41, 4129–4132. [Google Scholar] [CrossRef]
- Fuchs, J.; Hartl, H. Anion Structure of Tetrabutylammonium Octamolybdate [N(C4H9)4]4Mo8O26. Angew. Chem. Int. Ed. Engl. 1976, 15, 375–376. [Google Scholar] [CrossRef]
- Yeh, W.; Noga, D.E.; Lawson, R.A. Comparison of positive tone versus negative tone resist pattern collapse behavior. J. Vac. Sci. Technol. 2010, 28, C6S6–C6S11. [Google Scholar] [CrossRef]
- Cruywagen, J.J. Protonation, oligomerization, and condensation reactions of vanadate (v), molybdate (vi), and tungstate (vi). Adv. Inorg. Chem. 1999, 49, 127–182. [Google Scholar]
- Dui, X.; Yang, W.; Wu, X.; Kuang, X.; Liao, J.; Yu, R.; Lu, C. Two novel POM-based inorganic-organic hybrid compounds: Synthesis, structures, magnetic properties, photodegradation and selective absorption of organic dyes. Dalton Trans. 2015, 44, 9496–9505. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Zhang, L.Z.; Wang, R.; Chou, S.R.; Dong, Z.L. A new integrated approach for dye removal from wastewater by polyoxometalates functionalized membranes. J. Hazard. Mater. 2016, 301, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Yu, J.; Pang, H.; Ma, H.; Zhao, C. “Pinwheel” subunit-directed polyoxometalate-based hybrid compound [Cu(bimb)2]2[β-Mo8O26] with both helixes and chiral layers. New J. Chem. 2016, 40, 2944–2948. [Google Scholar] [CrossRef]
- Kang, S.; Prabhu, V.M.; Wu, W.; Lin, E.K.; Choi, K.; Chandhok, M.; Younkin, T.R.; Yueh, W. Characterization of the photoacid diffusion length. Proc. SPIE 2009, 7273. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Lykakis, I.N.; Orfanopoulos, M. Decatungstate as an efficient photocatalyst in organic chemistry. Chem. Soc. Rev. 2009, 38, 2609–2621. [Google Scholar] [CrossRef] [PubMed]


| Resist Components | Sensitivity (mJ/cm2) | Contrast |
|---|---|---|
| 25:75 TPSTF | 4000 | 1.66 |
| 50:50 NHNT | 2500 | 1.00 |
| Resists, Mo8:4-EP | Sensitivity (μC/cm2) | Contrast |
|---|---|---|
| 25:75 TPSTF (5 mol %) | 900 | 1.4 |
| 50:50 (10 mol % NHNT) | 750 | 1.4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hardie, B.; Roll, M. An Investigation of Polyoxometalate Hybrid Materials as Patternable Dielectrics and Lithographic Resists. Materials 2017, 10, 1309. https://doi.org/10.3390/ma10111309
Hardie B, Roll M. An Investigation of Polyoxometalate Hybrid Materials as Patternable Dielectrics and Lithographic Resists. Materials. 2017; 10(11):1309. https://doi.org/10.3390/ma10111309
Chicago/Turabian StyleHardie, Brandon, and Mark Roll. 2017. "An Investigation of Polyoxometalate Hybrid Materials as Patternable Dielectrics and Lithographic Resists" Materials 10, no. 11: 1309. https://doi.org/10.3390/ma10111309




