Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles
Abstract
:1. Introduction
2. Materials and Methods
3. Results
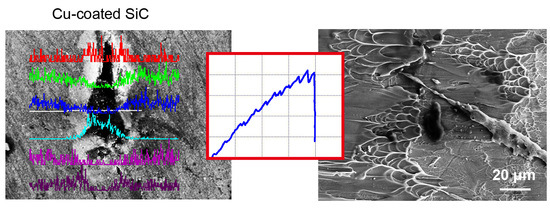
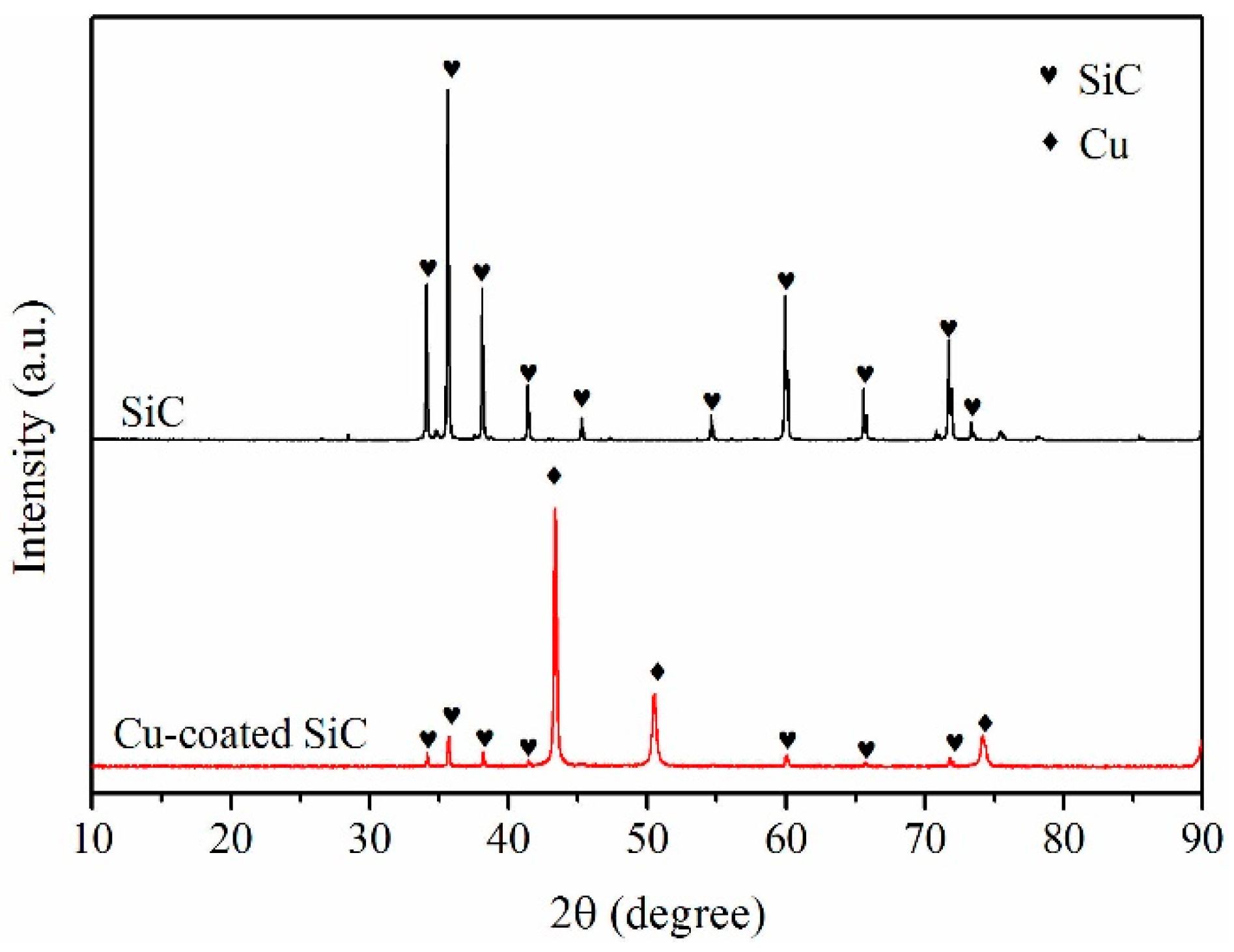
3.1. Electroless Cu Plating on SiC Particles
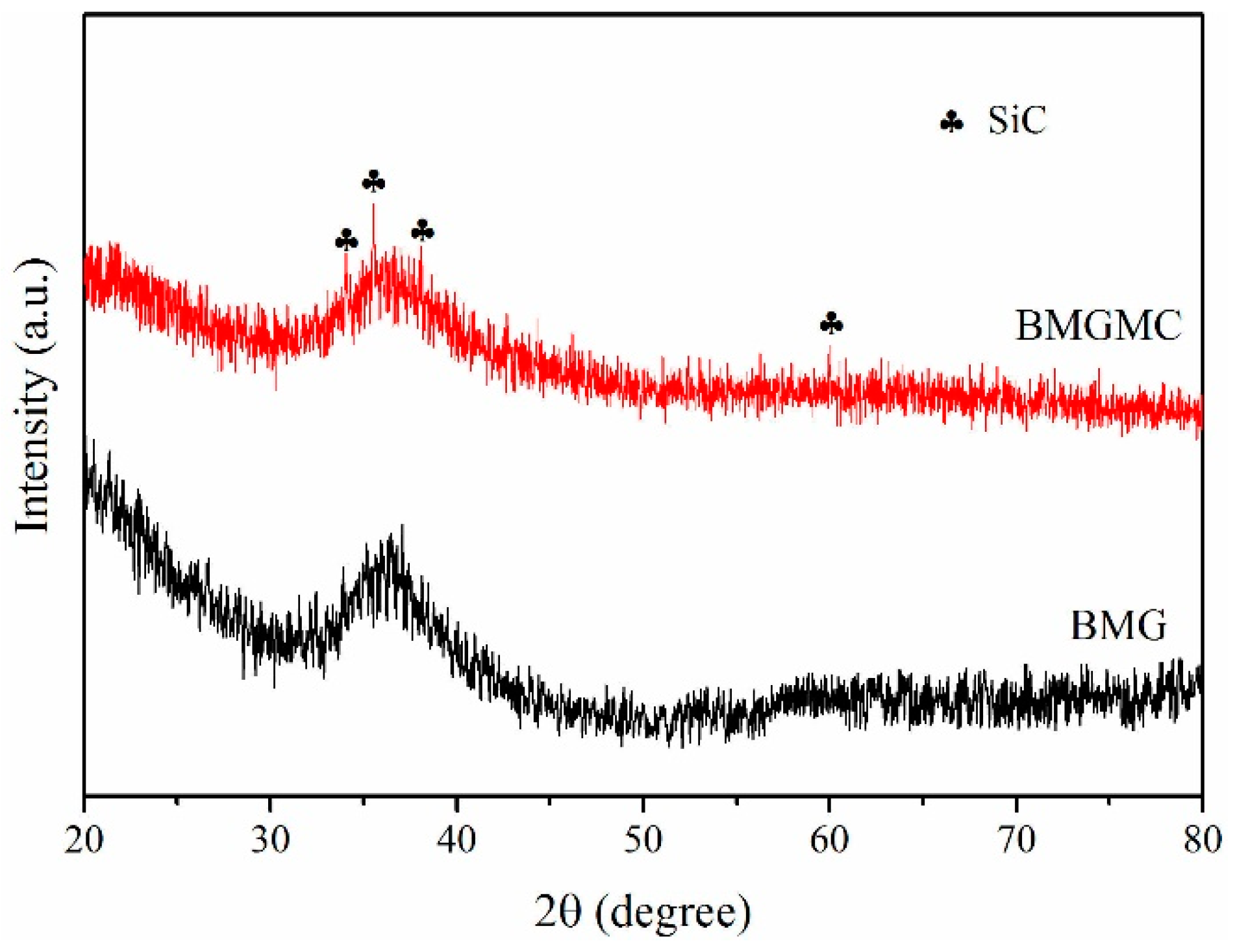
3.2. Microstructure of the Fabricated BMGMC
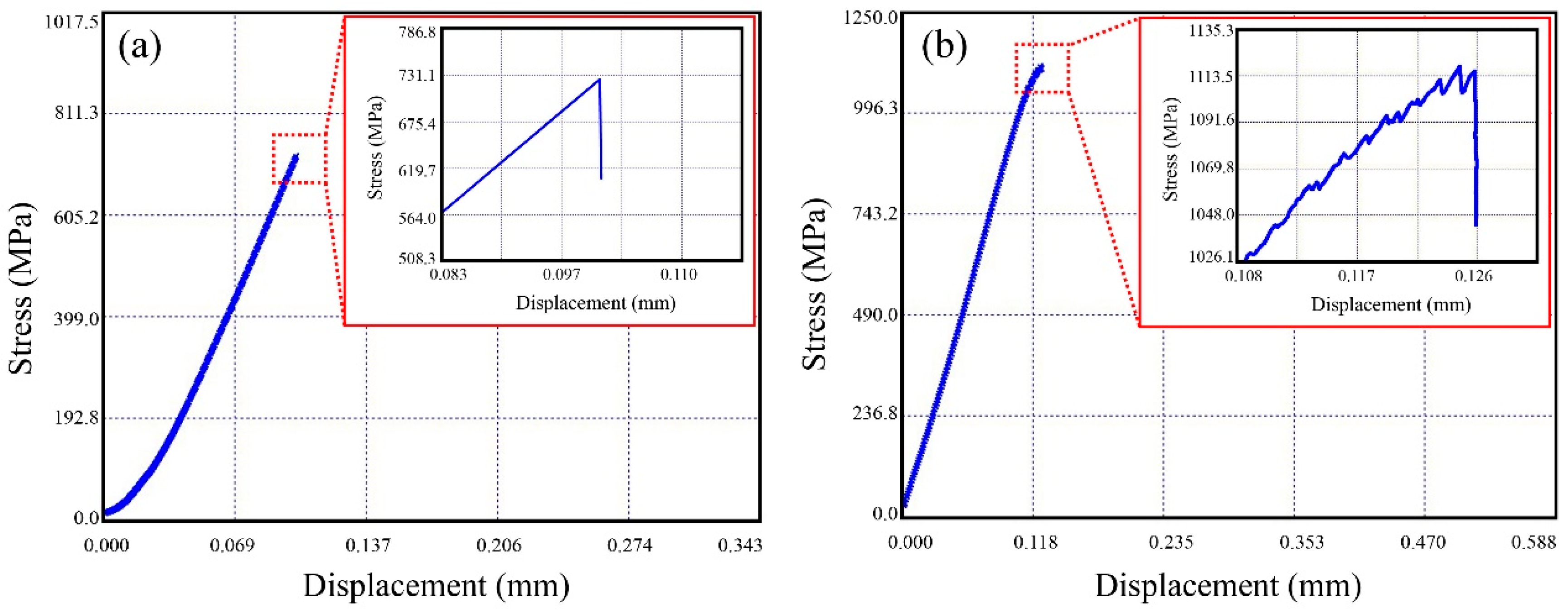
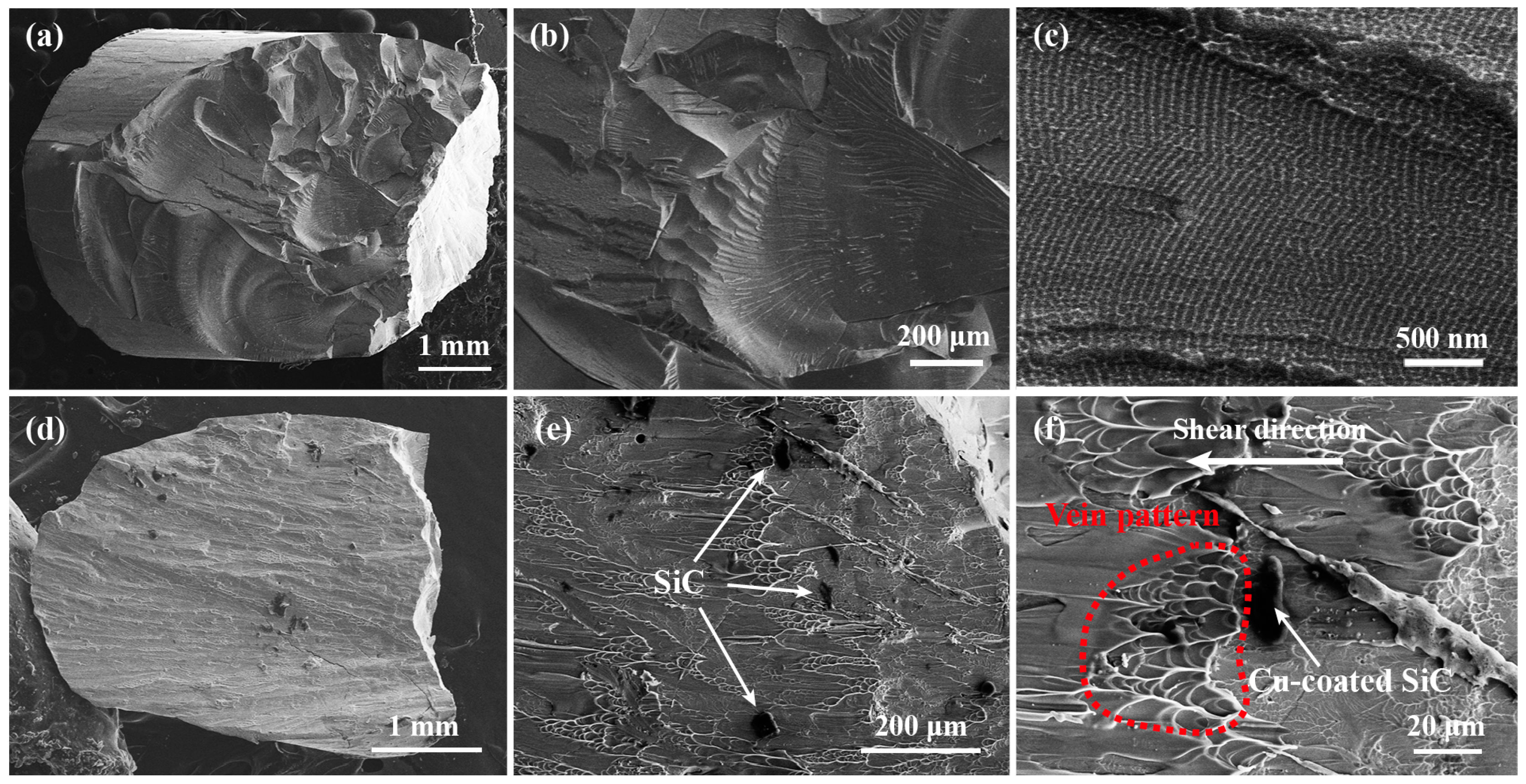
3.3. Mechanical Behavior of BMGMC
4. Discussion
4.1. Role of Electroless Plating
4.2. Toughening Mechanism
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Inoue, A.; Kato, A.; Zhang, T.; Kim, S.G.; Masumoto, T. Mg-Cu-Y amorphous alloys with high mechanical strengths produced by a metallic mold casting method. Mater. Trans. 1991, 32, 609–616. [Google Scholar] [CrossRef]
- Zberg, B.; Uggowitzer, P.J.; Loffler, J.F. MgZnCa glasses without clinically observable hydrogen evolution for biodegradable implants. Nat. Mater. 2009, 8, 887–891. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.F.; Greer, A.L. Metallic glasses as structural materials. Scr. Mater. 2006, 54, 321–326. [Google Scholar] [CrossRef]
- Xu, J.; Ramamurty, U.; Ma, E. The fracture toughness of bulk metallic glasses. JOM 2010, 62, 10–18. [Google Scholar] [CrossRef]
- Qiao, J.; Jia, H.; Liaw, P.K. Metallic glass matrix composites. Mater. Sci. Eng. R Rep. 2016, 100, 1–69. [Google Scholar] [CrossRef]
- Choi-Yim, H.; Johnson, W. Bulk metallic glass matrix composites. Appl. Phys. Lett. 1997, 71, 3808–3810. [Google Scholar] [CrossRef]
- Hofmann, D.C.; Suh, J.-Y.; Wiest, A.; Duan, G.; Lind, M.-L.; Demetriou, M.D.; Johnson, W.L. Designing metallic glass matrix composites with high toughness and tensile ductility. Nature 2008, 451, 1085–1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.H.; Wang, G.; Wang, R.J.; Zhao, D.Q.; Pan, M.X.; Wang, W.H. Super plastic bulk metallic glasses at room temperature. Science 2007, 315, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.G.; Zhang, H.F.; Wang, A.M.; Hu, Z.Q. Enhanced plasticity in Mg-based bulk metallic glass composite reinforced with ductile Nb particles. Appl. Phys. Lett. 2006, 89, 261904. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.; Wei, Y.; Guo, S.; Pan, F. Enhanced mechanical properties and corrosion resistance of a Mg-Zn-Ca bulk metallic glass composite by Fe particle addition. Mater. Lett. 2013, 91, 311–314. [Google Scholar] [CrossRef]
- Tang, Y.L.; Wu, R.F.; Jiao, Z.M.; Shi, X.H.; Wang, Z.H.; Qiao, J.W. Shear softening of Ta-containing metallic glass matrix composites upon dynamic loading. Mater. Sci. Eng. A 2017, 704, 322–328. [Google Scholar] [CrossRef]
- Conner, R.; Dandliker, R.; Scruggs, V.; Johnson, W. Dynamic deformation behavior of tungsten-fiber/metallic–glass matrix composites. Int. J. Impact Eng. 2000, 24, 435–444. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, H.; Wu, Y.; Liu, X.; Chen, H.; Yao, M.; Gault, B.; Ponge, D.; Raabe, D.; Hirata, A.; et al. Ultra strong steel via minimal lattice misfit and high-density nanoprecipitation. Nature 2017, 544, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cui, C.; Liu, S.; Zhao, L.; Li, N. Microstructure and mechanical properties of TC4 alloy modified and reinforced by TiB+TiN/Ti inoculants ribbons. Mater. Sci. Eng. A 2016, 663, 8–16. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Liu, S.; Liu, S.; Cui, S.; Wang, Q. Microstructure and mechanical properties of Ti6Al4V alloy modified and reinforced by in situ Ti5Si3/Ti composite ribbon inoculants. Metals 2017, 7, 267. [Google Scholar] [CrossRef]
- Li, N.; Cui, C.; Liu, S.; Zhao, L.; Liu, S. Fabrication of the Ti5Si3/Ti composite inoculants and its refining mechanism on pure titanium. Met. Mater. Int. 2017, 23, 397–404. [Google Scholar] [CrossRef]
- Han, Z.; Liu, X.; Zhao, S.; Shao, Y.; Li, J.; Yao, K. Microstructure, phase stability and mechanical properties of Nb–Ni–Ti–Co–Zr and Nb–Ni–Ti–Co–Zr–Hf high entropy alloys. Prog. Nat. Sci. Mater. Int. 2015, 25, 365–369. [Google Scholar] [CrossRef]
- Gong, P.; Jin, J.; Deng, L.; Wang, S.; Gu, J.; Yao, K.; Wang, X. Room temperature nanoindentation creep behavior of TiZrHfBeCu(Ni) high entropy bulk metallic glasses. Mater. Sci. Eng. A 2017, 688, 174–179. [Google Scholar] [CrossRef]
- Li, J.Q.; Wang, L.; Zhang, H.F.; Hu, Z.Q.; Cai, H.N. Synthesis and characterization of particulate reinforced Mg-based bulk metallic glass composites. Mater. Lett. 2007, 61, 2217–2221. [Google Scholar] [CrossRef]
- Wang, B.-P.; Wang, L.; Xue, Y.-F.; Wang, Y.-W.; Zhang, H.-F.; Fu, H.-M. Dynamic indentation response of porous SiC/Ti-based metallic glass composite. Trans. Nonferrous Met. Soc. China 2016, 26, 3154–3160. [Google Scholar] [CrossRef]
- Wang, B.P.; Wang, L.; Xue, Y.F.; Wang, S.Y.; Wang, Y.Q.; Zhang, H.F.; Fu, H.M. Strain rate-dependent compressive deformation and failure behavior of porous SiC/Ti-based metallic glass composite. Mater. Sci. Eng. A 2014, 609, 53–59. [Google Scholar] [CrossRef]
- Lin, H.-M.; Jeng, R.-R.; Lee, P.-Y. Microstructure and mechanical properties of vacuum hot-pressing SiC/Ti–Cu–Ni–Sn bulk metallic glass composites. Mater. Sci. Eng. A 2008, 493, 246–250. [Google Scholar] [CrossRef]
- Xie, G.; Louzguine-Luzgin, D.V.; Inoue, A. Characterization of interface between the particles in NiNbZrTiPt metallic glassy matrix composite containing SiC fabricated by spark plasma sintering. J. Alloyd Compd. 2009, 483, 239–242. [Google Scholar] [CrossRef]
- Xie, G.; Kimura, H.; Louzguine-Luzgin, D.V.; Men, H.; Inoue, A. SiC dispersed Fe-based glassy composite cores produced by spark plasma sintering and their high frequency magnetic properties. Intermetallics 2012, 20, 76–81. [Google Scholar] [CrossRef]
- Ma, H.; Shi, L.L.; Xu, J. Discovering inch-diameter metallic glasses in three-dimensional composition space. Appl. Phys. Lett. 2005, 87, 181915. [Google Scholar] [CrossRef]
- Zheng, Q.; Xu, J.; Ma, E. High glass-forming ability correlated with fragility of Mg-Cu(Ag)-Gd alloys. J. Appl. Phys. 2007, 102, 113519. [Google Scholar] [CrossRef]
- Wang, X.; Gong, P.; Yao, K.F. Mechanical behavior of bulk metallic glass prepared by copper mold casting with reversed pressure. J. Mater. Process. Technol. 2016, 237, 270–276. [Google Scholar] [CrossRef]
- Wang, X. Surface Crystallization in Mg-Based Bulk Metallic Glass during Copper Mold Casting. Adv. Mater. Sci. Eng. 2014, 2014, 798479. [Google Scholar] [CrossRef]
- Wang, S.G.; Sun, M.Y.; Song, Z.Q.; Xu, J. Cast defects induced sample-size dependency on compressive strength and fracture toughness of Mg-Cu-Ag-Gd bulk metallic glass. Intermetallics 2012, 29, 123–132. [Google Scholar] [CrossRef]
- Qiao, J.W.; Zhang, Y.; Liaw, P.K. Serrated flow kinetics in a Zr-based bulk metallic glass. Intermetallics 2010, 18, 2057–2064. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.P.; Chen, S.Y.; Xie, X.; Liaw, P.K.; Dahmen, K.A.; Qiao, J.W.; Wang, Y.L. Serration and noise behaviors in materials. Prog. Mater. Sci. 2017, 90, 358–460. [Google Scholar] [CrossRef]
- Park, E.S.; Kyeong, J.S.; Kim, D.H. Enhanced glass forming ability and plasticity in Mg-based bulk metallic glasses. Mater. Sci. Eng. A 2007, 449, 225–229. [Google Scholar] [CrossRef]
- Chen, H.M.; Lee, C.J.; Huang, J.C.; Li, T.H.; Jang, J.S.C. Flow serration and shear-band propagation in porous Mo particles reinforced Mg-based bulk metallic glass composites. Intermetallics 2010, 18, 1240–1243. [Google Scholar] [CrossRef]
- Zhao, J.X.; Qu, R.T.; Wu, F.F.; Zhang, Z.F.; Shen, B.L.; Stoica, M.; Eckert, J. Fracture mechanism of some brittle metallic glasses. J. Appl. Phys. 2009, 105, 103519. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Gong, P.; Yao, K.F. Effect of thermal cycling on the mechanical properties of Zr41Ti14Cu12.5Ni10Be22.5 alloy. Sci. China Phys. Mech. Astron. 2012, 55, 2357–2361. [Google Scholar] [CrossRef]
- Gong, P.; Wang, X.; Shao, Y.; Chen, N.; Liu, X.; Yao, K.F. A Ti-Zr-Be-Fe-Cu bulk metallic glass with superior glass-forming ability and high specific strength. Intermetallics 2013, 43, 177–181. [Google Scholar] [CrossRef]
- Wang, X.; Shao, Y.; Gong, P.; Yao, K.F. The effect of simulated thermal cycling on thermal and mechanical stability of a Ti-based bulk metallic glass. J. Alloys Compd. 2013, 575, 449–454. [Google Scholar] [CrossRef]
- Deibler, L.A.; Lewandowski, J.J. Model experiments to mimic fracture surface features in metallic glasses. Mater. Sci. Eng. A 2010, 527, 2207–2213. [Google Scholar] [CrossRef]
- Qu, R.T.; Stoica, M.; Eckert, J.; Zhang, Z.F. Tensile fracture morphologies of bulk metallic glass. J. Appl. Phys. 2010, 108, 42. [Google Scholar] [CrossRef]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Fracture of brittle metallic glasses: Brittleness or plasticity. Phys. Rev. Lett. 2005, 94, 125510. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Shi, L.L.; Xu, J. Mg-based bulk metallic glasses: Elastic properties and their correlations with toughness and glass transition temperature. J. Mater. Res. 2011, 26, 923–933. [Google Scholar] [CrossRef]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Periodic corrugation on dynamic fracture surface in brittle bulk metallic glass. Appl. Phys. Lett. 2006, 89, 181911. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhao, L.; Hu, X.; Cheng, Y.; Liu, S.; Chen, P.; Cui, C. Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles. Materials 2017, 10, 1371. https://doi.org/10.3390/ma10121371
Wang X, Zhao L, Hu X, Cheng Y, Liu S, Chen P, Cui C. Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles. Materials. 2017; 10(12):1371. https://doi.org/10.3390/ma10121371
Chicago/Turabian StyleWang, Xin, Lichen Zhao, Ximei Hu, Yongjian Cheng, Shuiqing Liu, Peng Chen, and Chunxiang Cui. 2017. "Fabrication and Mechanical Behavior of Ex Situ Mg-Based Bulk Metallic Glass Matrix Composite Reinforced with Electroless Cu-Coated SiC Particles" Materials 10, no. 12: 1371. https://doi.org/10.3390/ma10121371