The Microstructure of Nanocrystalline TiB2 Films Prepared by Chemical Vapor Deposition
Abstract
:1. Introduction
2. Experimental Methods
3. Results and Discussion
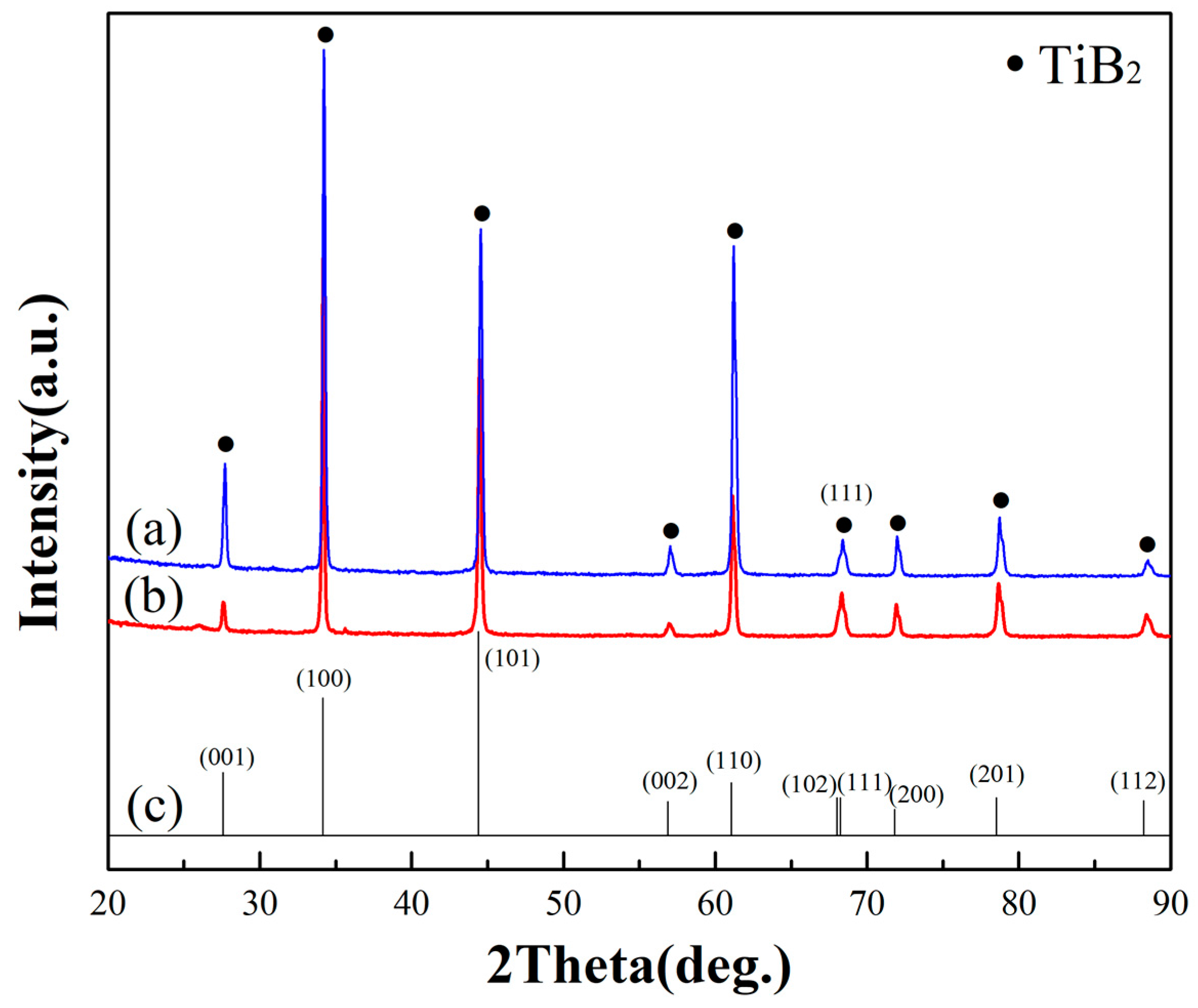
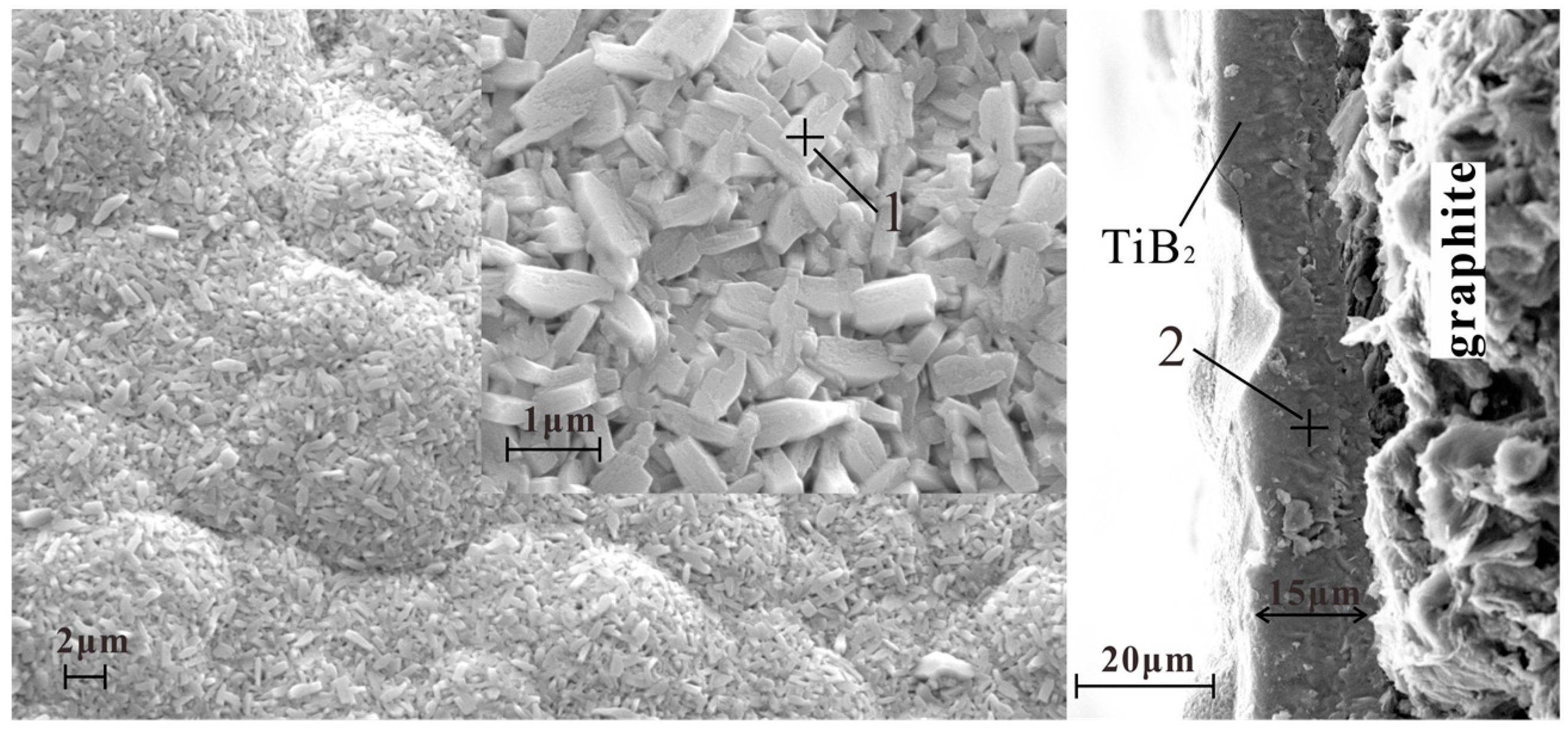
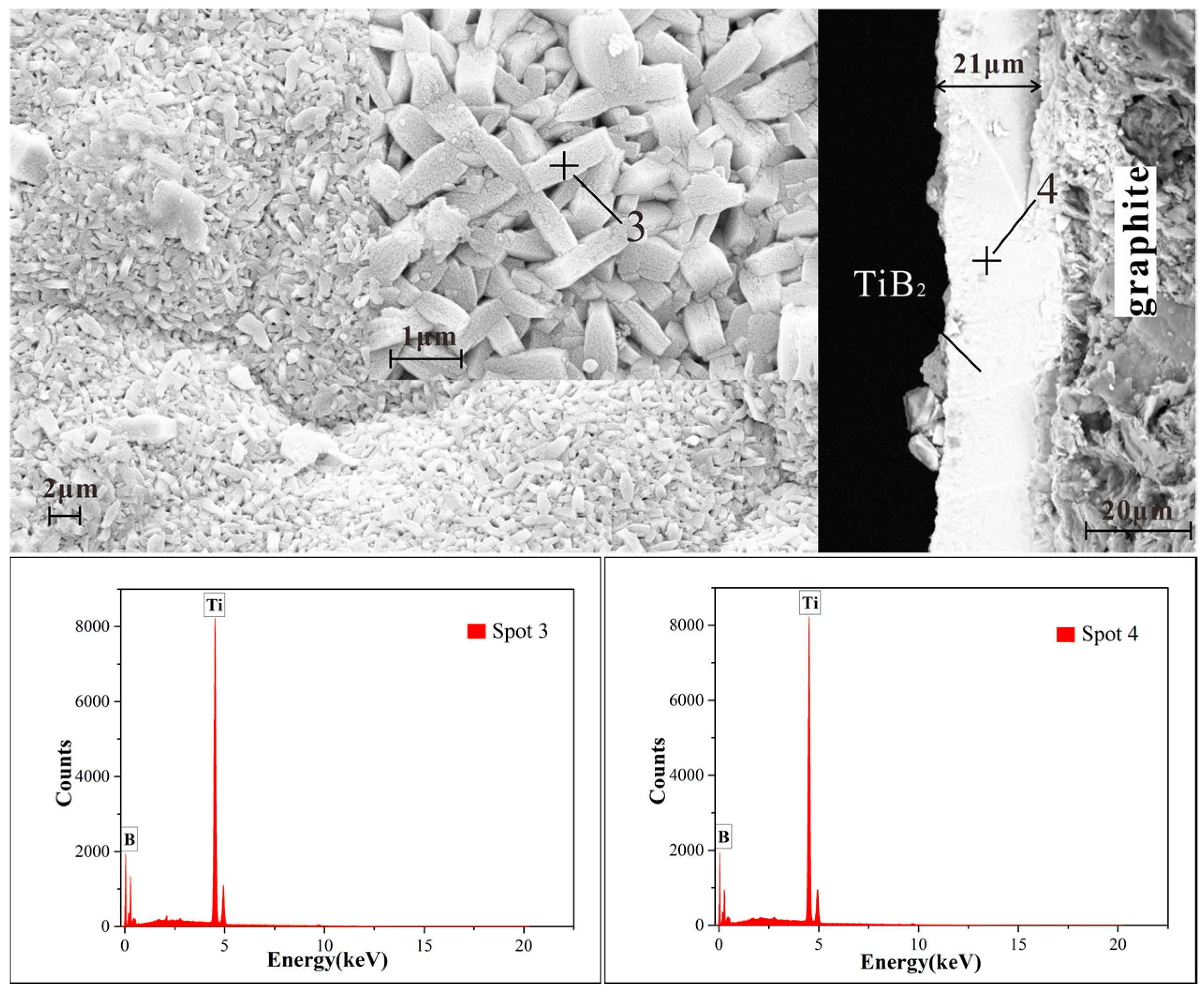
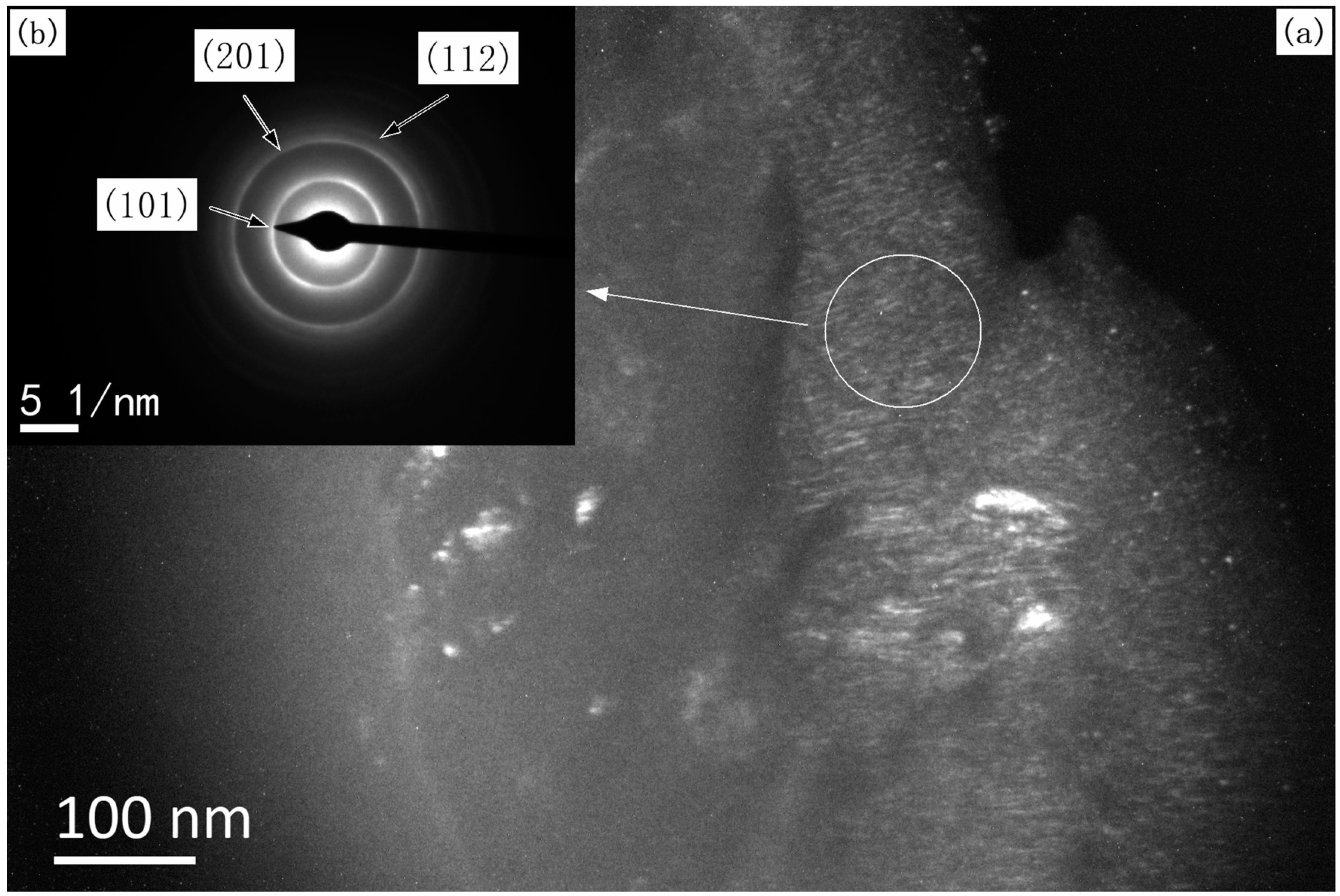
3.1. Structure, Morphology, and the Deposition Rate of Films
3.2. Grain Size of the Films
4. Conclusions
- TiB2 could be deposited at 1000 °C and 10 Pa by a CVD system. All deposits obtained under the condition of excessive hydrogen and different TiCl4/BCl3 gas flow ratios (1/2 and 1/1) were TiB2. Other impurity phases such as TiB were not found.
- These TiB2 films are nanocrystalline with a grain size in the range of 60 nm. All of the TiB2 films were typically columnar particles structure, and the columnar particles have a random orientation.
- X-ray diffraction indicated that all of the as-synthesized TiB2 films have a preferential orientation growth in the (100) direction.
- The TiCl4/BCl3 gas flow ratio has a certain impact on deposition rate and grain size, but these variations in the gas flow ratio of TiCl4/BCl3 did not appear to influence the preferred orientation of the deposits. The deposition rate is faster when using a greater TiCl4/BCl3 gas flow ratio, which in this case was 1/1. Meanwhile, when we provided a stoichiometric TiCl4/BCl3 gas ratio of 1/2, the grain size of the as-deposited TiB2 film was smaller.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schalk, N.; Keckes, J.; Czettl, C.; Burghammer, M.; Penoy, M.; Michotte, C.; Mitterer, C. Investigation of the origin of compressive residual stress in CVD TiB2 hard coatings using synchrotron X-ray nanodiffraction. Surf. Coat. Technol. 2014, 258, 121–126. [Google Scholar] [CrossRef]
- Raju, G.B.; Basu, B. Development of high temperature TiB2-based ceramics. Key Eng. Mater. 2009, 395, 89–124. [Google Scholar] [CrossRef]
- Shimada, S.; Takahashi, M.; Kiyono, H.; Tsujino, J. Coatings and microstructures of monolithic TiB2 films and double layer and composite TiCN/TiB2 films from alkoxide solutions by thermal plasma CVD. Thin Solid Films 2008, 516, 6616–6621. [Google Scholar] [CrossRef]
- Tang, W.-M.; Zheng, Z.-X.; Wu, Y.-C.; Wang, J.-M.; Lü, J.; Liu, J.-W. Synthesis of TiB2 nanocrystalline powder by mechanical alloying. Trans. Nonferrous Met. Soc. China 2006, 16, 613–617. [Google Scholar] [CrossRef]
- Berger, M.; Coronel, E.; Olsson, E. Microstructure of d.c. Magnetron sputtered TiB2 coatings. Surf. Coat. Technol. 2004, 185, 240–244. [Google Scholar] [CrossRef]
- Pfohl, C.; Bulak, A.; Rie, K.T. Development of titanium diboride coatings deposited by PACVD. Surf. Coat. Technol. 2000, 131, 141–146. [Google Scholar] [CrossRef]
- Martin, C.; Cales, B.; Vivier, P.; Mathieu, P. Electrical discharge machinable ceramic composites. Mater. Sci. Eng. A 1989, 109, 351–356. [Google Scholar] [CrossRef]
- Tennery, V.; Finch, C.; Yust, C.; Clark, G. Structure-property correlations for TiB2-based ceramics densified using active liquid metals. In Science of Hard Materials; Springer: Boston, MA, USA, 1983; pp. 891–909. ISBN 978-1-4684-4319-6. [Google Scholar]
- Ferber, M.K.; Becher, P.F.; Finch, C.B. Effect of microstructure on the properties of TiB2 ceramics. J. Am. Ceram. Soc. 1983, 66, C-2–C-3. [Google Scholar] [CrossRef]
- Subramanian, C.; Murthy, T.S.R.C.; Suri, A.K. Synthesis and consolidation of titanium diboride. Int. J. Refract. Met. Hard Mater. 2007, 25, 345–350. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, K.H.; Hong, S.C.; Lee, J.J. Low temperature deposition of TiB2 by inductively coupled plasma assisted CVD. Surf. Coat. Technol. 2007, 201, 5211–5215. [Google Scholar] [CrossRef]
- Elders, J.; van Voorst, J.D.W. Laser-induced chemical vapor deposition of titanium diboride. Appl. Surf. Sci. 1992, 54, 135–140. [Google Scholar] [CrossRef]
- Sun, L.; Gao, Y.; Xiao, B.; Li, Y.; Wang, G. Anisotropic elastic and thermal properties of titanium borides by first-principles calculations. J. Alloys Compd. 2013, 579, 457–467. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Wang, D.; Tu, G. Characterization of the structure of TiB2/TiC composites prepared via mechanical alloying and subsequent pressureless sintering. Powder Technol. 2012, 217, 340–346. [Google Scholar] [CrossRef]
- Vallauri, D.; Atías Adrián, I.C.; Chrysanthou, A. TiC–TiB2 composites: A review of phase relationships, processing and properties. J. Eur. Ceram. Soc. 2008, 28, 1697–1713. [Google Scholar] [CrossRef]
- Jeitschko, W.; Pöttgen, R.; Hoffman, R. Structural chemistry of hard materials. In Handbook of Ceramic Hard Materials, 1st ed.; Wiley-VCH: New York, NY, USA, 2008; pp. 10–11. ISBN 9783527618217. [Google Scholar]
- Batanov, G.M.; Berezhetskaya, N.K.; Borzosekov, V.D.; Iskhakova, L.D.; Kolik, L.V.; Konchekov, E.M.; Letunov, A.A.; Malakhov, D.V.; Milovich, F.O.; Obraztsova, E.A.; et al. Application of microwave discharge for the synthesis of TiB2 and bn nano- and microcrystals in a mixture of Ti-B powders in a nitrogen atmosphere. Plasma Phys. Rep. 2013, 39, 843–848. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, D.J. Synthesis of nano-titanium diboride powders by carbothermal reduction. J. Eur. Ceram. Soc. 2007, 27, 715–718. [Google Scholar] [CrossRef]
- Caputo, A.J. Chemical vapor deposition of erosion-resistant TiB2 coatings. J. Electrochem. Soc. 1985, 132. [Google Scholar] [CrossRef]
- Pierson, H.O.; Randich, E.; Mattox, D.M. The chemical vapor deposition of TiB2 on graphite. J. Less Common Met. 1979, 67, 381–388. [Google Scholar] [CrossRef]
- Takahashi, T.; Kamiya, H. Chemical vapor deposition of titanium diboride. J. Cryst. Growth 1974, 26, 203–209. [Google Scholar] [CrossRef]
- Williams, L.M. Plasma enhanced chemical vapor deposition of titanium diboride films. Appl. Phys. Lett. 1985, 46, 43–45. [Google Scholar] [CrossRef]
- Randich, E.; Gerlach, T.M. The calculation and use of chemical vapor deposition phase diagrams with applications to the Ti–B–Cl–H system between 1200 and 800 K. Thin Solid Films 1981, 75, 271–291. [Google Scholar] [CrossRef]
- Pierson, H.O. Fundamentals of Chemical Vapor Deposition. In Handbook of Chemical Vapor Deposition: Principles, Technology and Applications, 2nd ed.; William Andrew: New York, NY, USA, 1999; pp. 43–63. ISBN 0815517432. [Google Scholar]


| Substrate | Graphite |
|---|---|
| deposition temperature | 1000 (°C) |
| deposition time | 3 (h) |
| vacuum level | 10 (Pa) |
| temperature of H2 | 25 (°C) |
| pressure of H2 | 0.06 (MPa) |
| flow rate of H2 | 0.9 (m3/h) |
| temperature of TiCl4 | 135 (°C) |
| pressure of TiCl4 | 0.1 (MPa) |
| flow rate of TiCl4 | 0.055, 0.11 (m3/h) |
| temperature of BCl3 | 12 (°C) |
| pressure of BCl3 | 0.1 (MPa) |
| flow rate of BCl3 | 0.085 (m3/h) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Sun, S.; Tu, G.; Lu, S.; Li, K.; Zhu, X. The Microstructure of Nanocrystalline TiB2 Films Prepared by Chemical Vapor Deposition. Materials 2017, 10, 1425. https://doi.org/10.3390/ma10121425
Huang X, Sun S, Tu G, Lu S, Li K, Zhu X. The Microstructure of Nanocrystalline TiB2 Films Prepared by Chemical Vapor Deposition. Materials. 2017; 10(12):1425. https://doi.org/10.3390/ma10121425
Chicago/Turabian StyleHuang, Xiaoxiao, Shuchen Sun, Ganfeng Tu, Shuaidan Lu, Kuanhe Li, and Xiaoping Zhu. 2017. "The Microstructure of Nanocrystalline TiB2 Films Prepared by Chemical Vapor Deposition" Materials 10, no. 12: 1425. https://doi.org/10.3390/ma10121425




