Stress Corrosion Cracking Susceptibility of 304L Substrate and 308L Weld Metal Exposed to a Salt Spray
Abstract
:1. Introduction
2. Material and Experimental Procedures
2.1. Preparation of Samples
2.2. SCC and Weight-Loss Tests in a Salt Spray
2.3. Ferrite Determinations and Microstructural Observations
3. Results
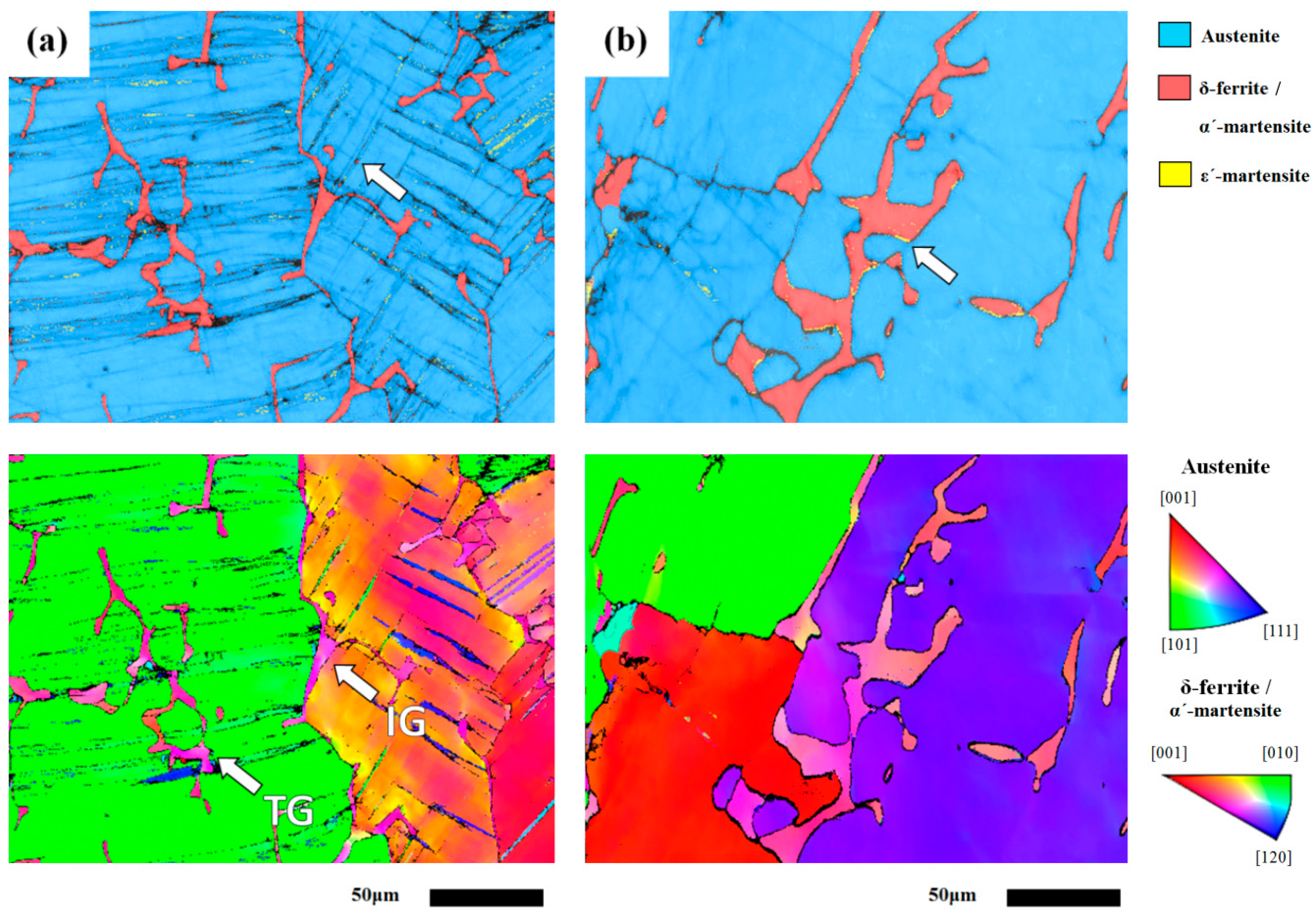
3.1. Microstructures
3.2. Electron Backscatter Diffraction (EBSD) Analysis
3.3. Weight-Loss Tests in a Salt Spray
3.4. U-Bend Tests in a Salt-Spray Environment
3.5. Surface Features of Weight Loss and U-Bend Specimens
4. Discussion
5. Conclusions
- The 308L deposit consisted of a certain amount of δ-ferrite distributed intra- and intergranularly in the solidified structure, as compared with the granular structure of the 304L substrate. With the same thickness reduction after cold rolling, the 308L deposit showed a lesser tendency of transformation of austenite to martensite. Those intragranular δ-ferrites in the 308L deposit might act as barriers to dislocation slips, resulting in less strain-hardening and martensite formation relative to the 304L substrate.
- The results of the weight-loss tests revealed that the sensitization treatment was very harmful to the corrosion resistance of the specimens in salt spray, irrespective of the groups of the 304L substrate and 308L deposit. The dissolution of the skeletal structures in all 308L deposits at various levels was responsible for the high weight loss of the specimens.
- None of the specimens of the 304L base metal or the as-welded 308L deposit of the U-bend exhibited visible cracks during the testing interval, indicating that these samples were resistant to SCC in a salt spray. Crack initiation within a short time, and rapid crack growth in the 308L-CR specimens after testing, could be attributed to the presence of high residual tensile stress. The ease of dissolution along the skeletal structure of the 308L-CRS specimens, which was confirmed by their high weight loss, accounted for the high SCC susceptibility. Moreover, assisted dissolution at the slip bands enhanced the SCC susceptibility for the specimens subjected to cold rolling. The increased trend of grain boundary corrosion in cold-rolled and sensitized specimens (304L-CRS) resulted in increasing SCC susceptibility. Therefore, the SCC susceptibility of the investigated specimens in a salt spray was associated with their weight-loss characteristics.
Acknowledgments
Author Contributions
Conflicts of Interest
List of Abbreviations
| SS | Stainless steel |
| SCC | Stress corrosion cracking |
| BWR | Boiling water reactor |
| FZ | Fusion zone |
| ER | Electrode rod |
| BM | Base metal |
| AW | As-welded |
| CR | Cold-rolled |
| CRS | Sensitization after cold rolling |
| MCL | Maximum crack length |
| TCL | Total crack length |
| OM | Optical microscope |
| SEM | Scanning electron microscope |
| EBSD | Electron backscatter diffraction |
| TEM | Transmission electron microscope |
| EDS | Energy dispersive spectroscopy |
| TG | Intra-granular |
| IG | Inter-granular |
| IPF | Inverse pole figure |
| RH | Relative humidity |
References
- Tani, J.; Mayuzumi, M.; Hara, N. Stress corrosion cracking of stainless-steel canister for concrete cask storage of spent fuel. J. Nucl. Mater. 2008, 379, 42–47. [Google Scholar] [CrossRef]
- Chiang, M.F.; Hsu, H.H.; Young, M.C.; Huang, J.Y. Mechanical degradation of cold-worked 304 stainless steel in salt spray environments. J. Nucl. Mater. 2012, 422, 58–68. [Google Scholar] [CrossRef]
- Mayuzumi, M.; Arai, T.; Hide, K. Chloride induced stress corrosion cracking of type 304 and 304L stainless steels in air. Zairyo-to-Kankyo 2003, 52, 166–170. [Google Scholar] [CrossRef]
- Mayuzumi, M.; Hayashibara, H.; Tani, J.; Arai, T. Failure propensity of austenitic stainless steels by chloride induced stress corrosion cracking in air. Zairyo-to-Kankyo 2006, 55, 20–24. [Google Scholar] [CrossRef]
- Prosek, T.; Iversen, A.; Taxén, C.; Thierry, D. Low-temperature stress corrosion cracking of stainless steels in the atmosphere in the presence of chloride deposits. Corrosion 2009, 65, 105–117. [Google Scholar] [CrossRef]
- Li, W.J.; Young, M.C.; Lai, C.L.; Kai, W.; Tsay, L.W. The effects of rolling and sensitization treatments on the stress corrosion cracking of 304L stainless steel in salt-spray environment. Corros. Sci. 2013, 68, 25–33. [Google Scholar] [CrossRef]
- Raman, K.R.; Siew, H.W. Stress corrosion cracking of an austenitic stainless steel in nitrite-containing chloride solutions. Materials 2014, 7, 7799–7808. [Google Scholar] [CrossRef]
- Alyousif, O.M.; Nishimura, R. The effect of test temperature on SCC behavior of austenitic stainless steels in boiling saturated magnesium chloride solution. Corros. Sci. 2006, 48, 4283–4293. [Google Scholar] [CrossRef]
- Alyousif, O.M.; Nishimura, R. The stress corrosion cracking behavior of austenitic stainless steels in boiling magnesium chloride solutions. Corros. Sci. 2007, 49, 3040–3051. [Google Scholar] [CrossRef]
- García, C.; Martín, F.; Tiedra, P.D.; Heredero, J.A.; Aparicio, M.L. Effects of prior cold work and sensitization heat treatment on chloride stress corrosion cracking in type 304 stainless steels. Corros. Sci. 2001, 43, 1519–1539. [Google Scholar] [CrossRef]
- García, C.; Martín, F.; Tiedra, P.D.; Alonso, S.; Aparicio, M.L. Stress corrosion cracking behavior of cold-worked and sensitized type 304 stainless steel using the slow strain rate test. Corrosion 2002, 58, 849–857. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Effect of surface machining and cold working on the ambient temperature chloride stress corrosion cracking susceptibility of AISI 304L stainless steel. Mater. Sci. Eng. A 2010, 527, 679–683. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nucl. Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- Zhu, L.K.; Yan, Y.; Qiao, L.J.; Volinsky, A.A. Stainless steel pitting and early-stage stress corrosion cracking under ultra-low elastic load. Corros. Sci. 2013, 77, 360–368. [Google Scholar] [CrossRef]
- Trethewey, K.R.; Wenman, M.; Chard-Tuckey, P.; Roebuck, B. Correlation of meso- and micro-scale hardness measurements with the pitting of plastically-deformed Type 304L stainless steel. Corros. Sci. 2008, 50, 1132–1141. [Google Scholar] [CrossRef]
- Kumar, B.R.; Singh, R.; Mahato, B.; De, P.K.; Bandyopadhyay, N.R.; Bhattacharya, D.K. Effect of texture on corrosion behavior of AISI 304L stainless steel. Mater. Charact. 2005, 54, 141–147. [Google Scholar] [CrossRef]
- Zhu, L.; Yan, Y.; Li, J.; Qiao, L.; Li, Z.; Volinsky, A.A. Stress corrosion cracking at low loads: Surface slip and crystallographic analysis. Corros. Sci. 2015, 100, 619–626. [Google Scholar] [CrossRef]
- Böhner, A.; Niendorf, T.; Amberger, D.; Höppel, H.W.; Göken, M.; Maier, H.J. Martensitic transformation in ultrafine-grained stainless steel AISI 304L under monotonic and cyclic loading. Metals 2012, 2, 56–64. [Google Scholar] [CrossRef]
- Bak, H.S.; Abro, A.M.; Lee, B.D. Effect of hydrogen and strain-induced martensite on mechanical properties of AISI 304 stainless steel. Metals 2016, 6, 169. [Google Scholar] [CrossRef]
- Acharyya, S.G.; Khandelwal, A.; Kain, V.; Kumar, A.; Samajdar, I. Surface working of 304L stainless steel: Impact on microstructure, electrochemical behavior and SCC resistance. Mater. Charact. 2012, 72, 68–76. [Google Scholar] [CrossRef]
- Kain, V.; Chandra, K.; Adhe, K.N.; De, P.K. Effect of cold work on low-temperature sensitization behaviour of austenitic stainless steels. J. Nucl. Mater. 2004, 334, 115–132. [Google Scholar]
- Parvathavarthini, N.; Dayal, R.K. Influence of chemical composition, prior deformation and prolonged thermal aging on the sensitization characteristics of austenitic stainless steels. J. Nucl. Mater. 2002, 305, 209–219. [Google Scholar] [CrossRef]
- Mannepalli, S.; Gupta, R.K.; Kumar, A.V.; Parvathavarthini, N.; Mudali, U.K. Influence of prior deformation on the sensitization kinetics of nitrogen alloyed 316L stainless steels. J. Mater. Eng. Perform. 2015, 24, 1848–1855. [Google Scholar] [CrossRef]
- Fang, Z.; Wu, Y.; Zhu, R. Stress corrosion cracking of type 304 stainless steel weldments in the active state. Corrosion 1994, 50, 171–175. [Google Scholar] [CrossRef]
- Pujar, M.G.; Dayal, R.K.; Gill, T.P.S.; Malhotra, S.N. Role of delta-ferrite in the dissolution of passive films on the austenitic stainless-steel weld metals. J. Mater. Sci. Lett. 1999, 18, 823–826. [Google Scholar] [CrossRef]
- De Tiedra, P.; Martín, Ó. Effect of welding on the stress corrosion cracking behaviour of prior cold worked AISI 316L stainless steel studied by using the slow strain rate test. Mater. Des. 2013, 49, 103–109. [Google Scholar] [CrossRef]
- Raja, K.S.; Rao, K.P. Effect of Applied potentials on room temperature stress corrosion cracking of austenitic stainless steel weldments. Corrosion 1992, 48, 634–640. [Google Scholar] [CrossRef]
- Rao, B.R.; Rao, K.P.; Iyer, K.J.L. Effect of chemical composition and ferrite content on room temperature SCC behavior of austenitic weld metals. Corrosion 1993, 49, 248–255. [Google Scholar] [CrossRef]
- Cai, J.B.; Yu, C.; Shiue, R.K.; Tsay, L.W. Stress corrosion cracking of austenitic weld deposits in a salt spray environment. J. Nucl. Mater. 2015, 465, 774–783. [Google Scholar] [CrossRef]
- Talonen, J.; Hänninen, H. Formation of shear bands and strain-induced martensite during plastic deformation of metastable austenitic stainless steels. Acta Mater. 2007, 55, 6108–6118. [Google Scholar] [CrossRef]
- Lai, C.L.; Tsay, L.W.; Kai, W.; Chen, C. The effects of cold rolling and sensitisation on hydrogen embrittlement of AISI 304L welds. Corros. Sci. 2010, 52, 1187–1193. [Google Scholar] [CrossRef]
- Jinlong, L.; Hongyun, L. Influence of tensile pre-strain and sensitization on martensite reversion mechanism in austenitic stainless steel. Mater. Charact. 2013, 77, 10–14. [Google Scholar] [CrossRef]
- Unnikrishnan, R.; Idury, K.S.N.S.; Ismail, T.P.; Bhadauria, A.; Shekhawat, S.K.; Khatirkar, R.K.; Sapate, S.G. Effect of heat input on the microstructure, residual stresses and corrosion resistance of 304L austenitic stainless steel weldments. Mater. Charact. 2014, 93, 10–23. [Google Scholar] [CrossRef]
- Lai, C.L.; Tsay, L.W.; Kai, W.; Chen, C. Notched tensile tests of cold-rolled 304L stainless steel in 40 wt. % 80 °C MgCl2 solution. Corros. Sci. 2009, 51, 380–386. [Google Scholar] [CrossRef]
- Tsay, L.W.; Lin, Y.J.; Chen, C. The effects of rolling temperature and sensitization treatment on the sulfide stress corrosion cracking of 304L stainless steel. Corros. Sci. 2012, 63, 267–274. [Google Scholar] [CrossRef]
- Han, G.; He, J.; Fukuyama, S.; Yokogawa, K. Effect of strain-induced martensite on hydrogen environment embrittlement of sensitized austenitic stainless steels at low temperatures. Acta Mater. 1998, 46, 4559–4570. [Google Scholar] [CrossRef]
- De Tiedra, P.; Martín, Ó.; López, M. Combined effect of resistance spot welding and post-welding sensitization on the degree of sensitization of AISI 304 stainless steel. Corros. Sci. 2011, 53, 2670–2675. [Google Scholar] [CrossRef]









| Material | Chemical Composition (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | S | P | Cr | Ni | Fe | |
| AISI 304L | 0.019 | 1.53 | 0.50 | 0.030 | 0.030 | 18.20 | 8.04 | bal. |
| ER 308L | 0.018 | 1.90 | 0.32 | 0.010 | 0.017 | 19.70 | 10.10 | bal. |
| Material | Testing Condition | ||
|---|---|---|---|
| Base Metal (BM)/As-Welded (AM) | Cold-Rolled (CR) | Sensitization after Cold Rolling (CRS) | |
| AISI 304L | 304L-BM | 304L-CR | 304L-CRS |
| ER 308L | 308L-AW | 308L-CR | 308L-CRS |
| Specimen | Vickers-Hardness (HV0.3) | ||
|---|---|---|---|
| BM/AW | CR | CRS | |
| 304L | 165 | 340 | 278 |
| 308L | 164 | 306 | 261 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-H.; Chen, T.-C.; Huang, R.-T.; Tsay, L.-W. Stress Corrosion Cracking Susceptibility of 304L Substrate and 308L Weld Metal Exposed to a Salt Spray. Materials 2017, 10, 187. https://doi.org/10.3390/ma10020187
Hsu C-H, Chen T-C, Huang R-T, Tsay L-W. Stress Corrosion Cracking Susceptibility of 304L Substrate and 308L Weld Metal Exposed to a Salt Spray. Materials. 2017; 10(2):187. https://doi.org/10.3390/ma10020187
Chicago/Turabian StyleHsu, Chia-Hao, Tai-Cheng Chen, Rong-Tan Huang, and Leu-Wen Tsay. 2017. "Stress Corrosion Cracking Susceptibility of 304L Substrate and 308L Weld Metal Exposed to a Salt Spray" Materials 10, no. 2: 187. https://doi.org/10.3390/ma10020187





