Fretting Corrosion Behavior of Experimental Ti-20Cr Compared to Titanium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Specimens
2.2. Fretting Corrosion Test
2.3. Surface Morphology
2.4. Statistical Analysis
3. Results
3.1. Current Intensity
3.2. Repassivation Time
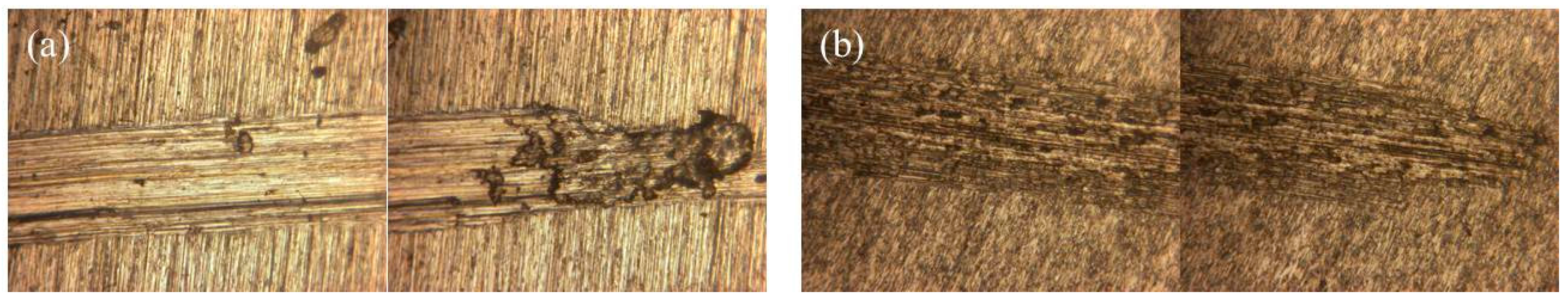
3.3. Surface Morphology
4. Discussion
5. Conclusions
- The maximum activation peaks in Ti-20Cr were lower compared to those in CP-Ti in the series of scratching speed; however, different kinetic behaviors were observed between the materials.
- The average activation peaks in Ti-20Cr were significantly lower compared to those in CP-Ti (p < 0.01), except at the 20 mm/s scratching speed.
- The scratching speed also influenced the repassivation time and a faster speed showed a shorter repassivation time.
- The profile depth of the scratched area as determined by topography observations was significantly lower in Ti-20Cr compared to CP-Ti (p < 0.05).
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Abraham, C.M. A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Barão, V.A.; Mathew, M.T.; Assunção, W.G.; Yuan, J.C.; Wimmer, M.A.; Sukotjo, C. Stability of cp-Ti and Ti-6Al-4V alloy for dental implants as a function of saliva pH—An electrochemical study. Clin. Oral Implants Res. 2012, 23, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Ohkubo, C.; Hanatani, S.; Hosoi, T. Present status of titanium removable dentures—A review of the literature. J. Oral Rehabil. 2008, 35, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.H.; Park, S.B.; Kim, H.I.; Kwon, Y.H. Corrosion-related changes on Ti-based orthodontic brackets in acetic NaF solutions: Surface morphology, microhardness, and element release. Dent. Mater. J. 2008, 27, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Ananth, H.; Kundapur, V.; Mohammed, H.S.; Anand, M.; Amarnath, G.S.; Mankar, S.A. Review on Biomaterials in Dental Implantology. Int. J. Biomed. Sci. 2015, 11, 113–120. [Google Scholar] [PubMed]
- Carlsson, G.E.; Omar, R. The future of complete dentures in oral rehabilitation. A critical review. J. Oral Rehabil. 2010, 37, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Papi, P.; Giardino, R.; Sassano, P.; Amodeo, G.; Pompa, G.; Cascone, P. Oral health related quality of life in cleft lip and palate patients rehabilitated with conventional prostheses or dental implants. J. Int. Soc. Prev. Community Dent. 2015, 5, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Sutton, A.J.; Rogers, P.M. Discoloration of a titanium alloy removable partial denture: A clinical report. J. Prosthodont. 2001, 10, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Gilbert, J.L. Potential and frequency effects on fretting corrosion of Ti6Al4V and CoCrMo surfaces. J. Biomed. Mater. Res. A 2013, 101, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Oda, Y. Discoloration and dissolution of titanium and titanium alloys with immersion in peroxide- or fluoride-containing solutions. Dent. Mater. J. 2008, 27, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, E. Biocorrosion properties of antibacterial Ti-10Cu sintered alloy in several simulated biological solutions. J. Mater. Sci. Mater. Med. 2015, 26, 142. [Google Scholar] [CrossRef] [PubMed]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Asami, K.; Oda, Y. Corrosion behavior and surface characterization of Ti-20Cr alloy in a solution containing fluoride. Dent. Mater. J. 2004, 23, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium-copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 4280–4287. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-C.; Wu, S.-C.; Wang, C.-F.; Ho, W.-F. Electrochemical behavior of Ti–Cr alloys in artificial saliva. J. Alloys Compd. 2009, 487, 439–444. [Google Scholar] [CrossRef]
- Takemoto, S.; Hattori, M.; Yoshinari, M.; Kawada, E.; Asami, K.; Oda, Y. Corrosion mechanism of Ti-Cr alloys in solution containing fluoride. Dent. Mater. 2009, 25, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Gittens, R.A.; Olivares-Navarrete, R.; Tannebaum, R.; Boyan, B.D.; Schwartz, Z. Electrical implications of corrosion for osseointegration of titanium implants. J. Dent. Res. 2011, 90, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Bhola, R.; Bhola, S.M.; Mishra, B.; Olsen, D.L. Corrosion in titanium dental implants/prostheses—A review. Trends Biomater. Artif. Organs 2011, 25, 34–46. [Google Scholar]
- Mathew, M.T.; Abbey, S.; Hallab, N.J.; Hall, D.J.; Sukotjo, C.; Wimmer, M.A. Influence of pH on the tribocorrosion behavior of CpTi in the oral environment: Synergistic interactions of wear and corrosion. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Barril, S.; Mischler, S.; Landolt, D. Electrochemical effects on the fretting corrosion behaviour of Ti6Al4V in 0.9% sodium chloride solution. Wear 2005, 259, 282–291. [Google Scholar] [CrossRef]
- Komotori, J.; Hisamori, N.; Ohmori, Y. The corrosion wear mechanisms of Ti6Al-4V alloy for different scratching rates. Wear 2007, 263, 412–418. [Google Scholar] [CrossRef]
- Hattori, M.; Takemoto, S.; Yoshinari, M.; Kawada, E.; Oda, Y. Effect of chromium content on mechanical properties of casting Ti-Cr alloys. Dent. Mater. J. 2010, 29, 570–574. [Google Scholar] [CrossRef] [PubMed]
- Koike, M.; Cai, Z.; Oda, Y.; Hattori, M.; Fujii, H.; Okabe, T. Corrosion behavior of cast Ti-6Al-4V alloyed with Cu. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 73, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Engelen, L.; Fontijn-Tekamp, A.; van der Bilt, A. The influence of product and oral characteristics on swallowing. Arch. Oral Biol. 2005, 50, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Chen, J. Food oral processing—A review. Food Hydrocoll. 2009, 23, 1–25. [Google Scholar] [CrossRef]
- Goldberg, J.R.; Gilbert, J.L. Electrochemical response of CoCrMo to high-speed fracture of its metal oxide using an electrochemical scratch test method. Biomed. Mater. Res. 1997, 37, 421–431. [Google Scholar] [CrossRef]
- Upadhyay, D.; Panchal, M.A.; Dubey, R.S.; Srivastava, V.K. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Özcan, M.; Hämmerle, C. Titanium as a reconstruction and implant materials in dentistry: Advantages and pitfalls. Materials 2012, 5, 1528–1545. [Google Scholar] [CrossRef] [Green Version]
- Tschernitschek, H.; Borchers, L.; Geurtsen, W. Nonalloyed titanium as a bioinert metal—A review. Quintessence Int. 2005, 36, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New developments of Ti-based alloys for biomedical application. Materials 2014, 7, 1709–1800. [Google Scholar] [CrossRef]
- Mischler, S.; Spiegel, A.; Stemp, M.; Landolt, D. Influence of passivity on the tribocorrosion of carbon steel in aqueous solutons. Wear 2001, 251, 1295–1307. [Google Scholar] [CrossRef]
- Corso, P.P., Jr.; German, R.M.; Simmons, H.D., Jr. Corrosion evaluation of gold-based dental alloys. J. Dent. Res. 1985, 64, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Hanawa, T.; Asami, K.; Asaoka, K. Repassivation of titanium and surface oxide film regenerated in simulated bioliquid. J. Biomed. Mater. Res. 1998, 40, 530–538. [Google Scholar] [CrossRef]
- Mischler, S. Tribochemical techniques and interpretation methods in tribocorrosion: A comparative evaluation. Tribol. Int. 2008, 41, 573–583. [Google Scholar] [CrossRef]







| Specimens | Scratching Speed | ||
|---|---|---|---|
| 10 mm/s | 20 mm/s | 40 mm/s | |
| Commercially pure titanium (CP-Ti) | 312 (31) a,A | 152 (23) b,A | 108 (26) b,A |
| Titanium alloys containing 20 mass% chromium (Ti-20Cr) | 378 (19) a,B | 173 (33) b,A | 80 (13) c,A |
| Specimens | Scratching Speed | ||
|---|---|---|---|
| 10 mm/s | 20 mm/s | 40 mm/s | |
| Commercially pure titanium (CP-Ti) | 9.20 (1.78) a,A | 7.56 (0.27) a,A | 7.33 (1.43) a,A |
| Titanium alloys containing 20 mass% chromium (Ti-20Cr) | 2.01 (0.30) a,B | 4.10 (1.51) a,B | 3.40 (0.46) a,B |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, T.; Schille, C.; Almadani, A.; Geis-Gerstorfer, J. Fretting Corrosion Behavior of Experimental Ti-20Cr Compared to Titanium. Materials 2017, 10, 194. https://doi.org/10.3390/ma10020194
Sawada T, Schille C, Almadani A, Geis-Gerstorfer J. Fretting Corrosion Behavior of Experimental Ti-20Cr Compared to Titanium. Materials. 2017; 10(2):194. https://doi.org/10.3390/ma10020194
Chicago/Turabian StyleSawada, Tomofumi, Christine Schille, Atif Almadani, and Jürgen Geis-Gerstorfer. 2017. "Fretting Corrosion Behavior of Experimental Ti-20Cr Compared to Titanium" Materials 10, no. 2: 194. https://doi.org/10.3390/ma10020194






