A Study of the Curing and Flammability Properties of Bisphenol A Epoxy Diacrylate Resin Utilizing a Novel Flame Retardant Monomer, bis[di-acryloyloxyethyl]-p-tert-butyl-phenyl Phosphate
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Measurements
3. Results and Discussion
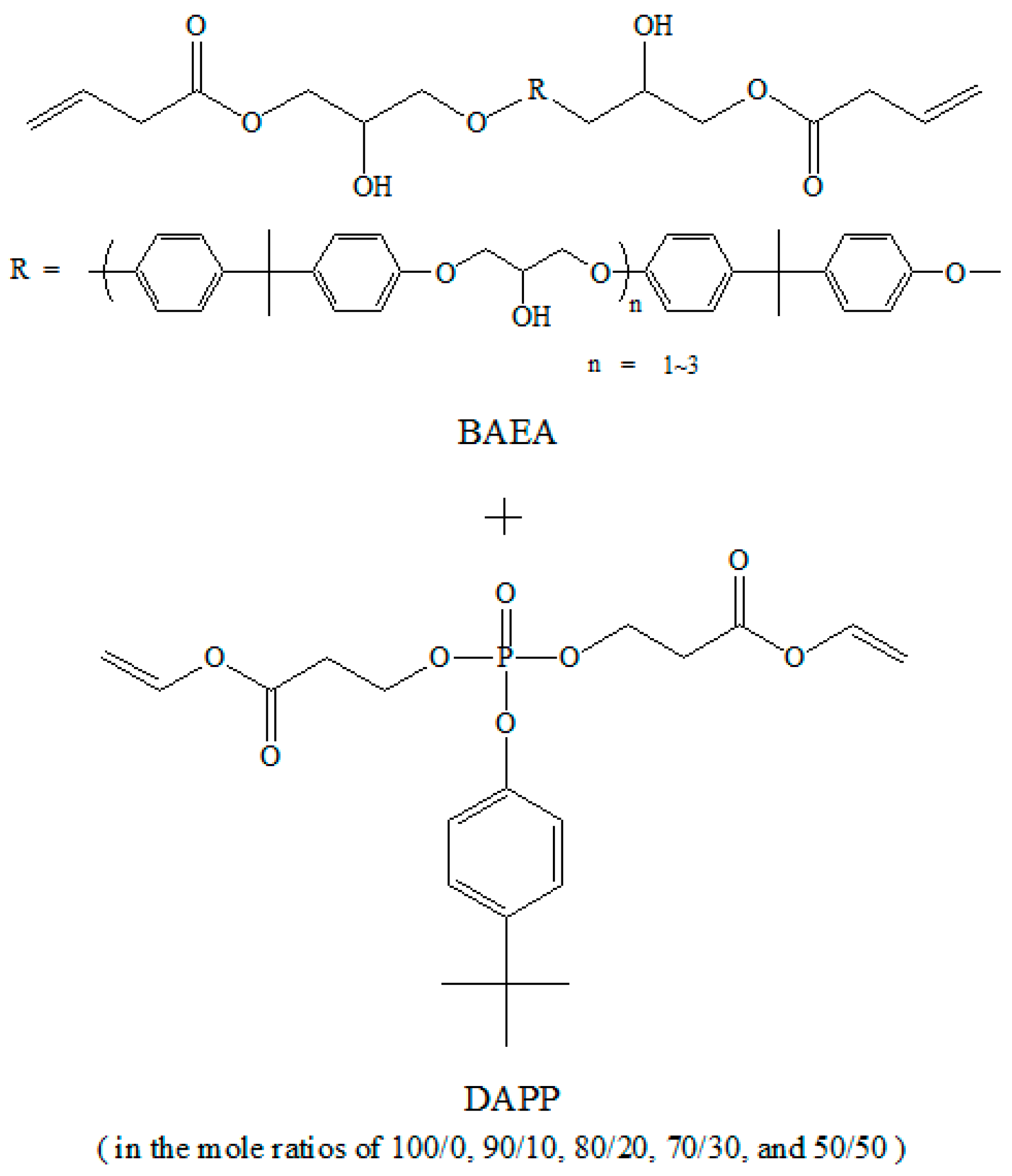
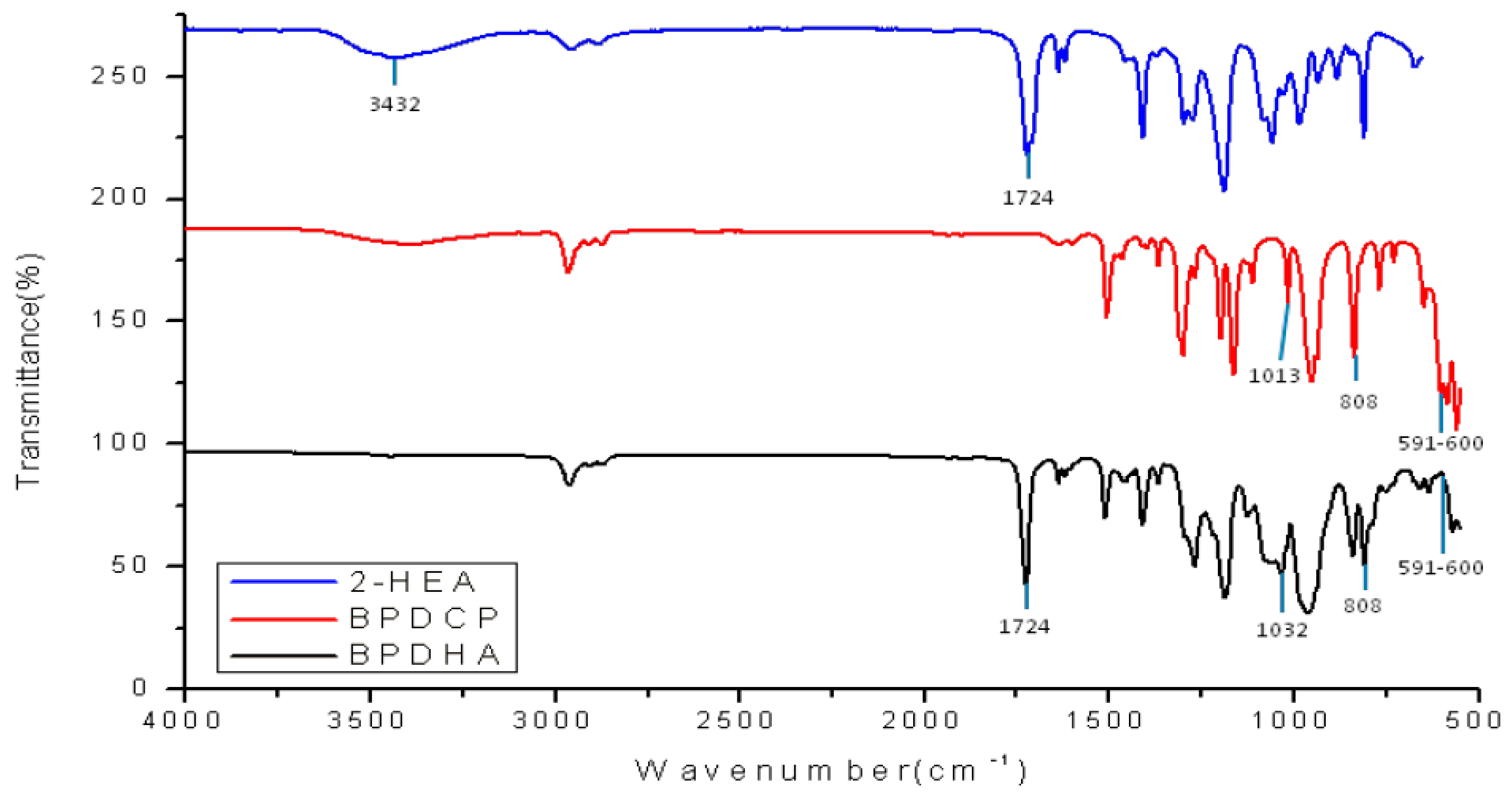
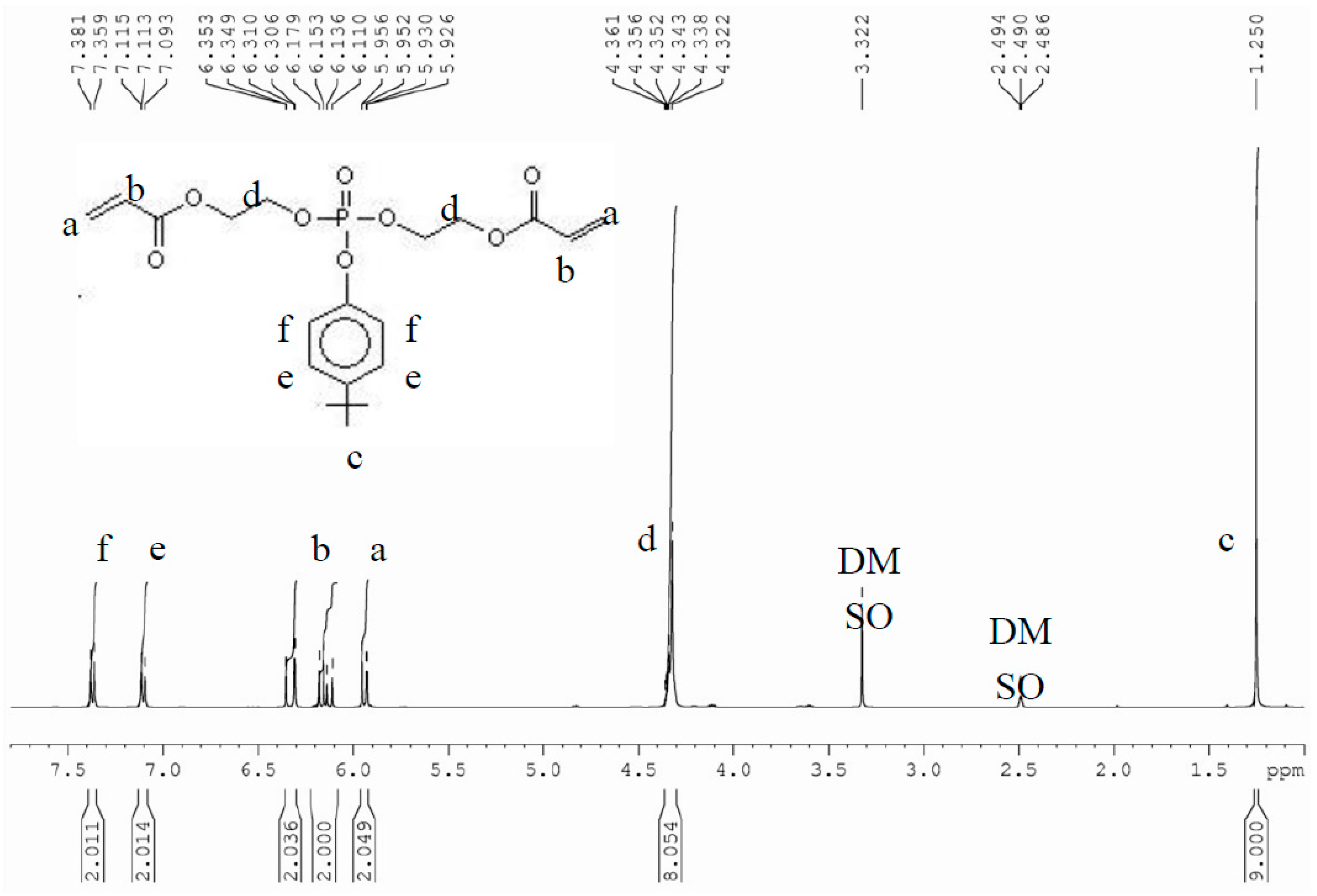
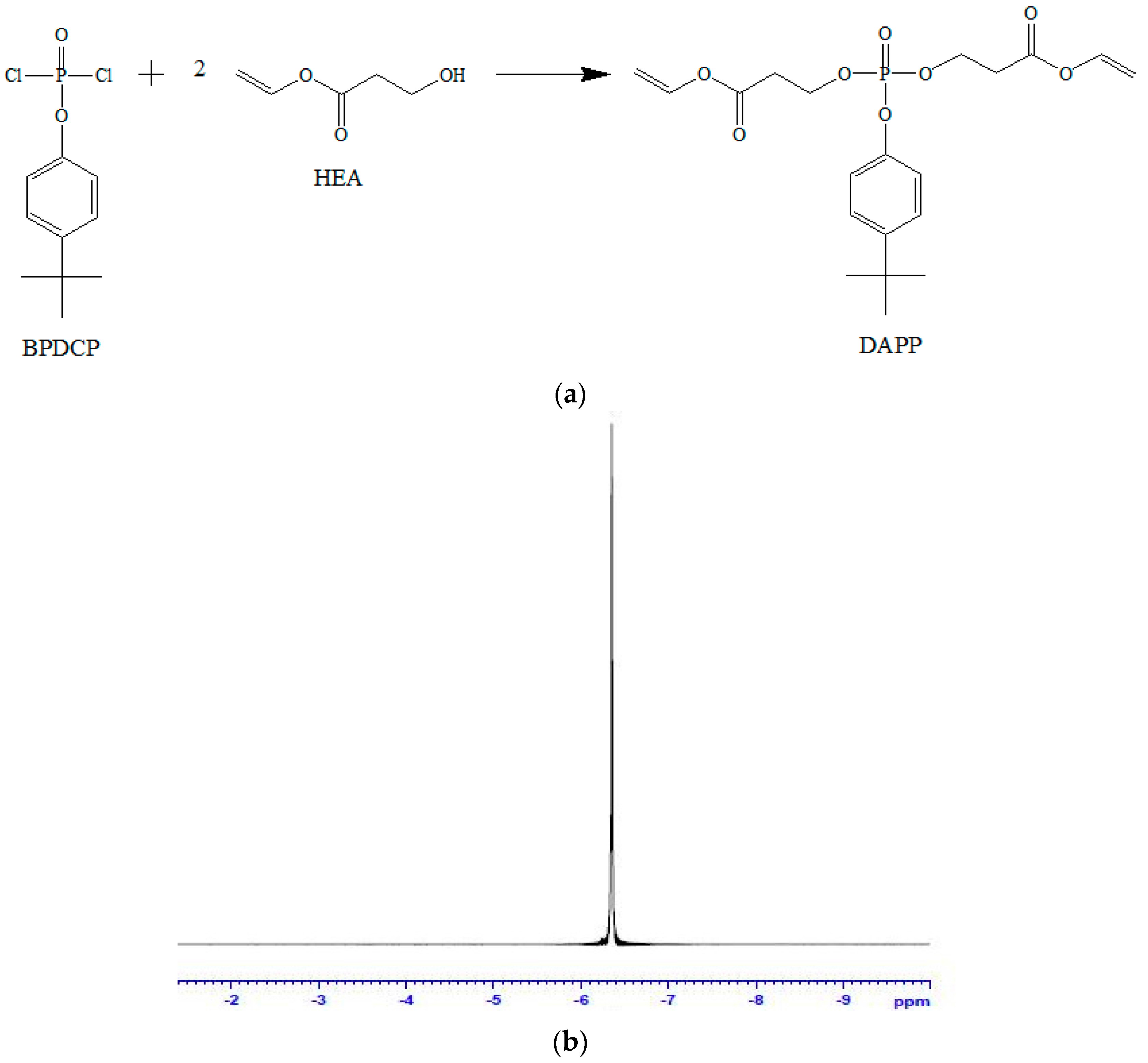
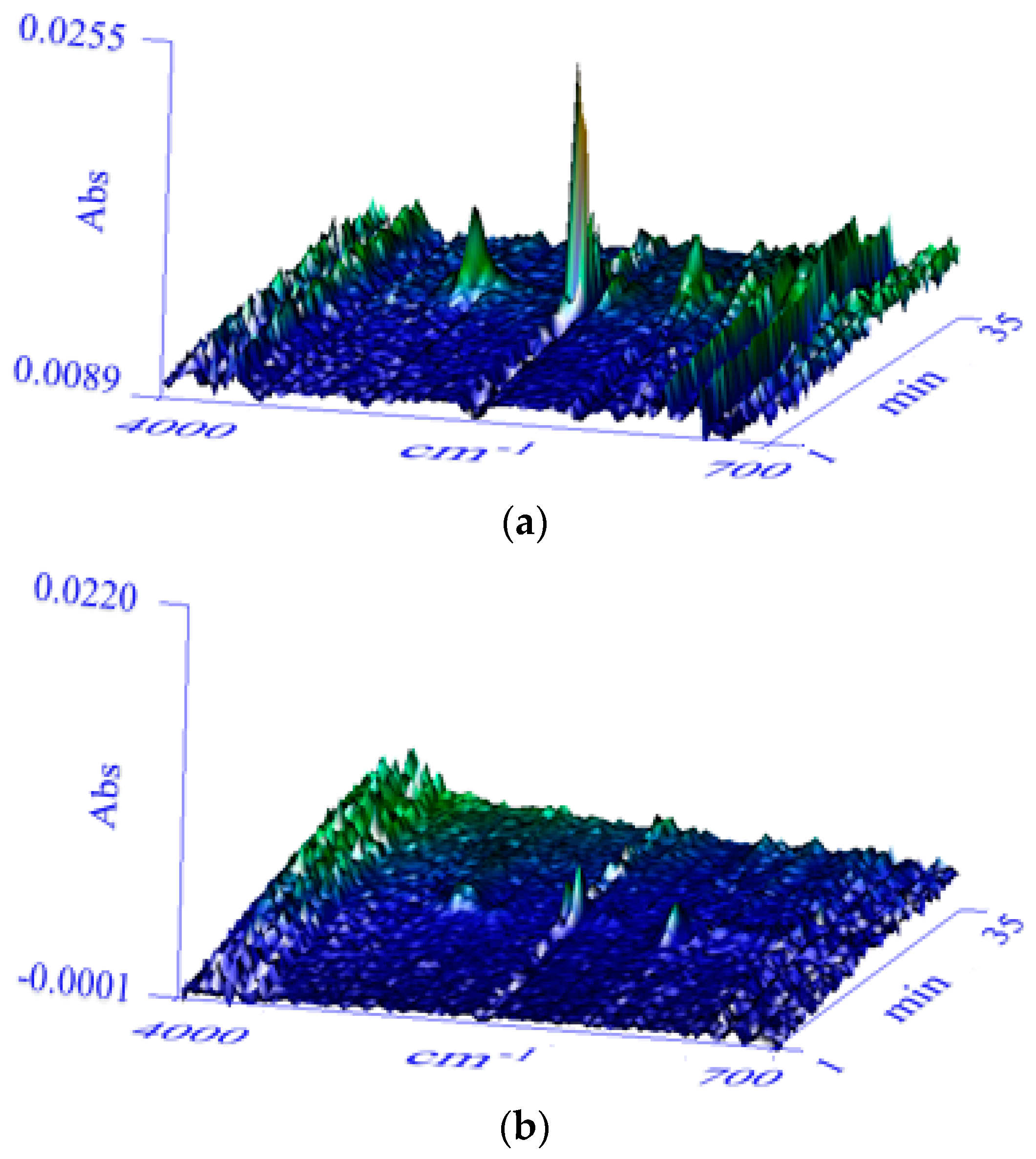
3.1. Confirmation of Synthesis
3.2. UV Curing Kinetics
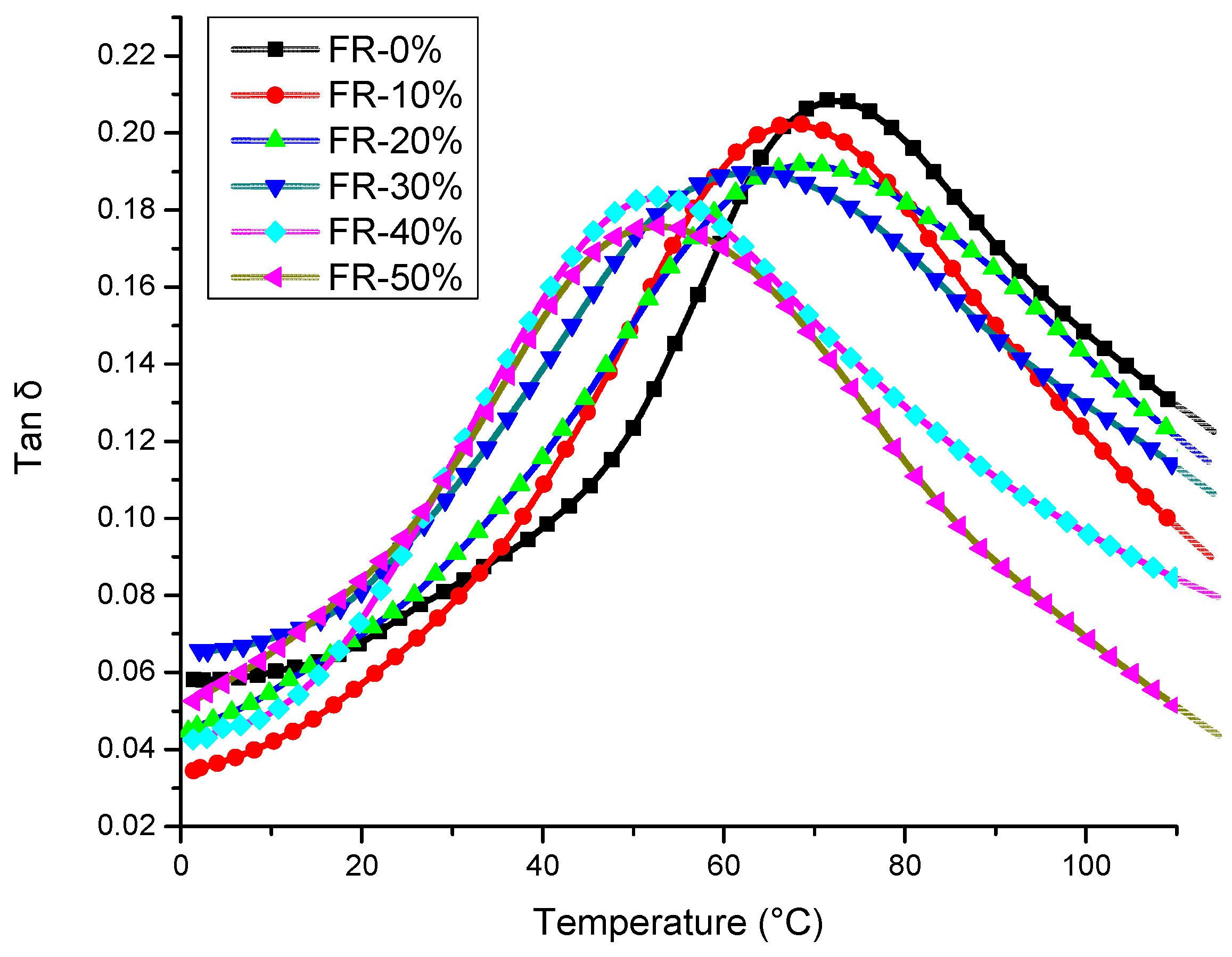
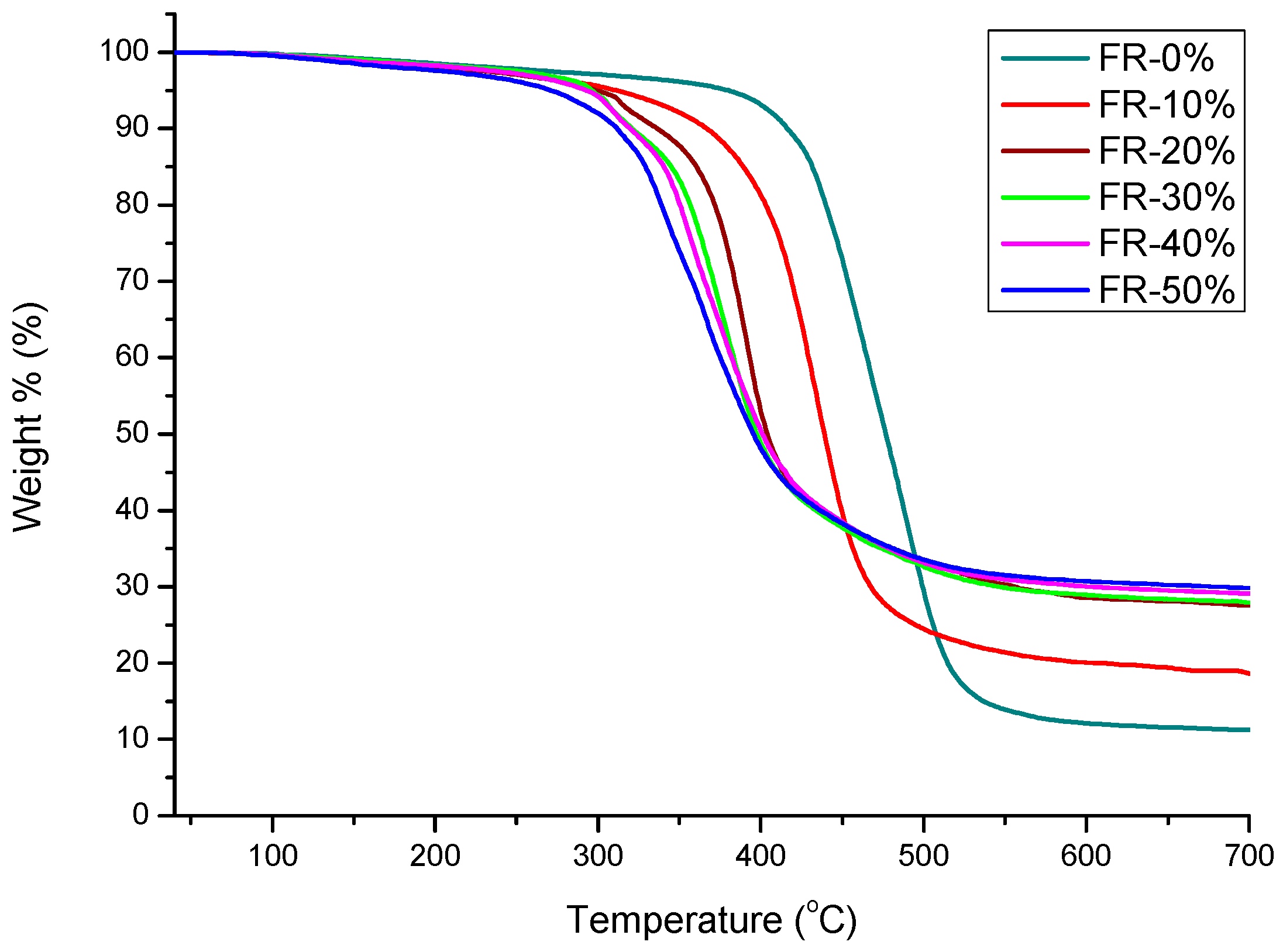
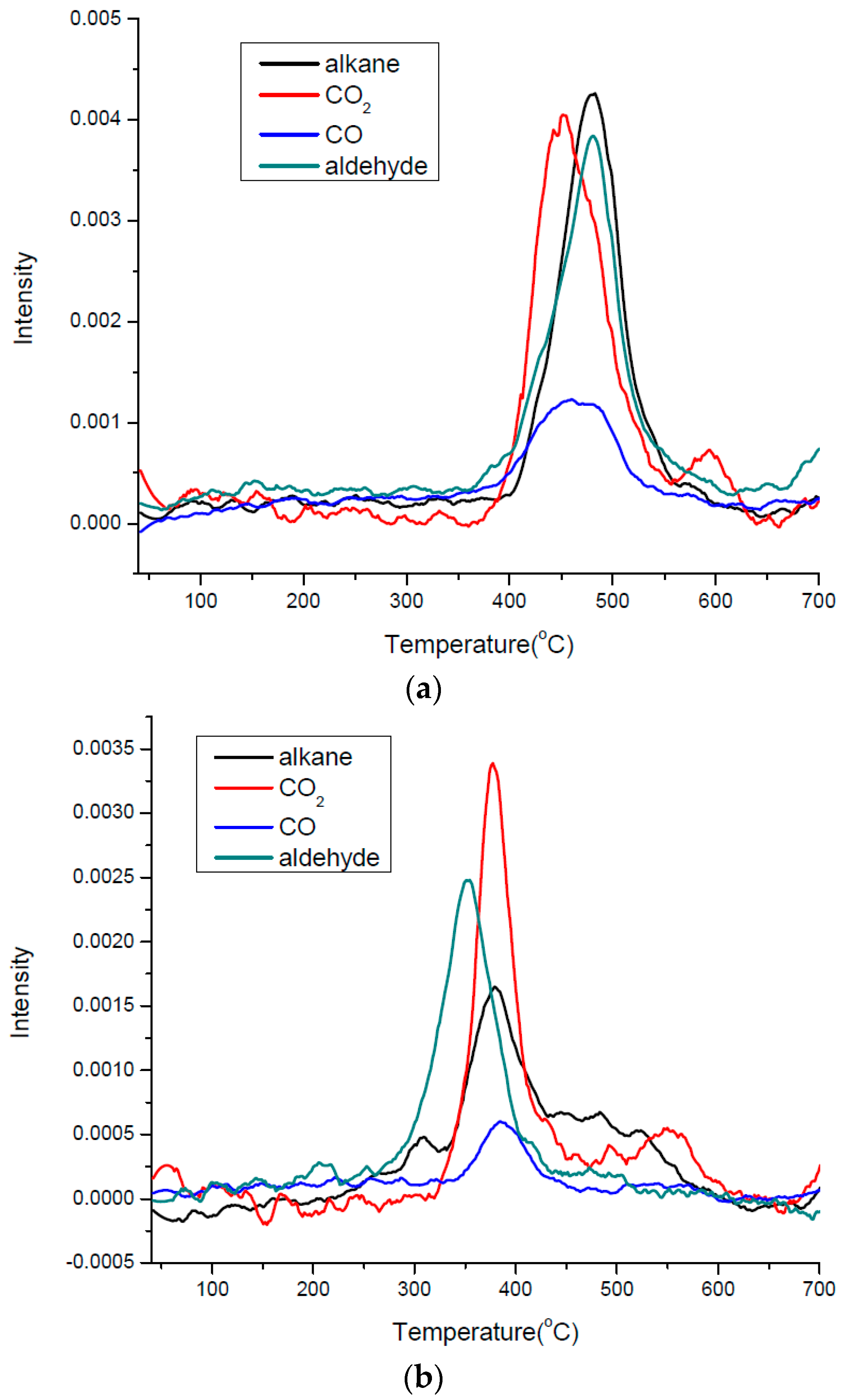
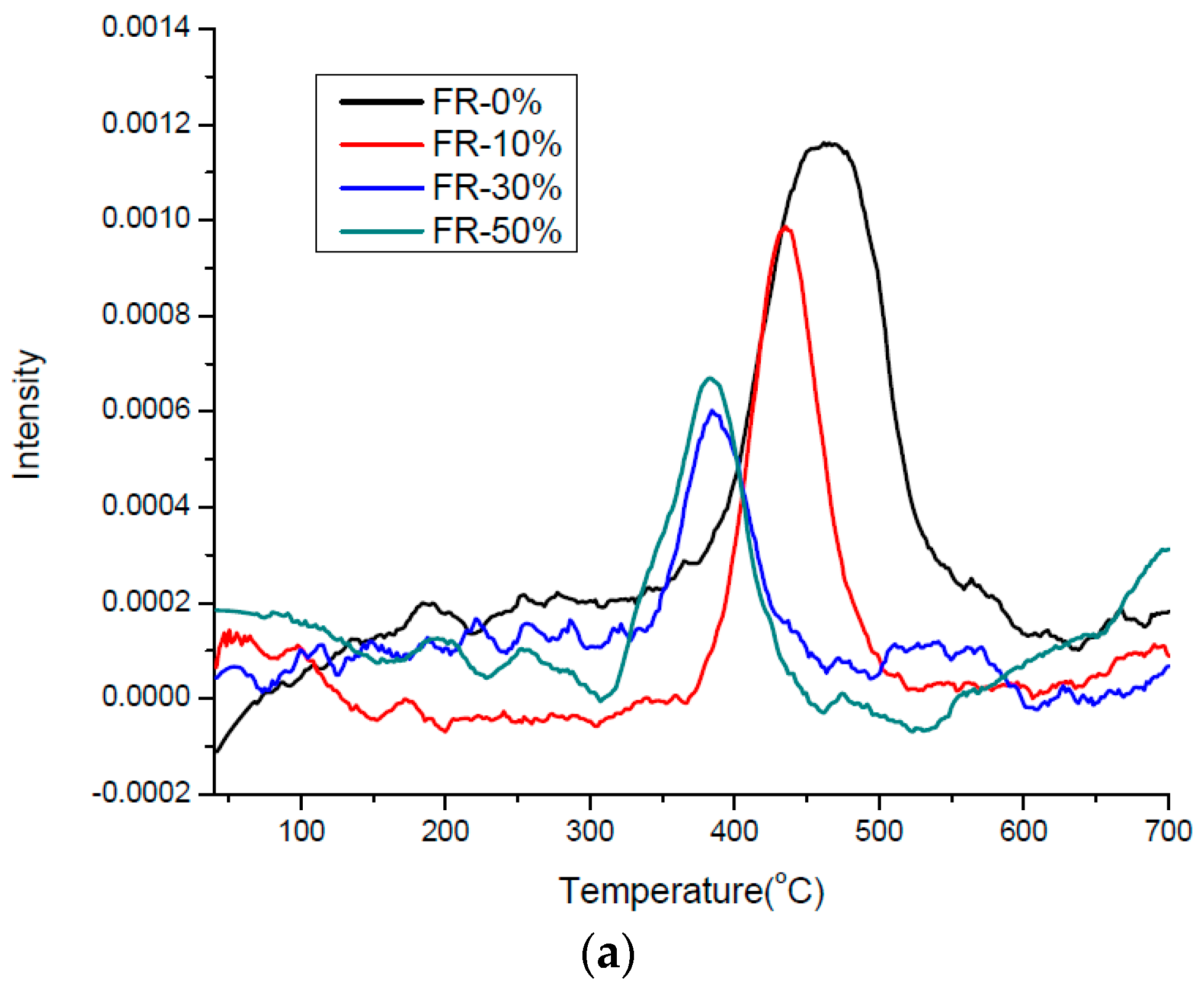
3.3. Thermal Effects and Characterization of Pyrolysis
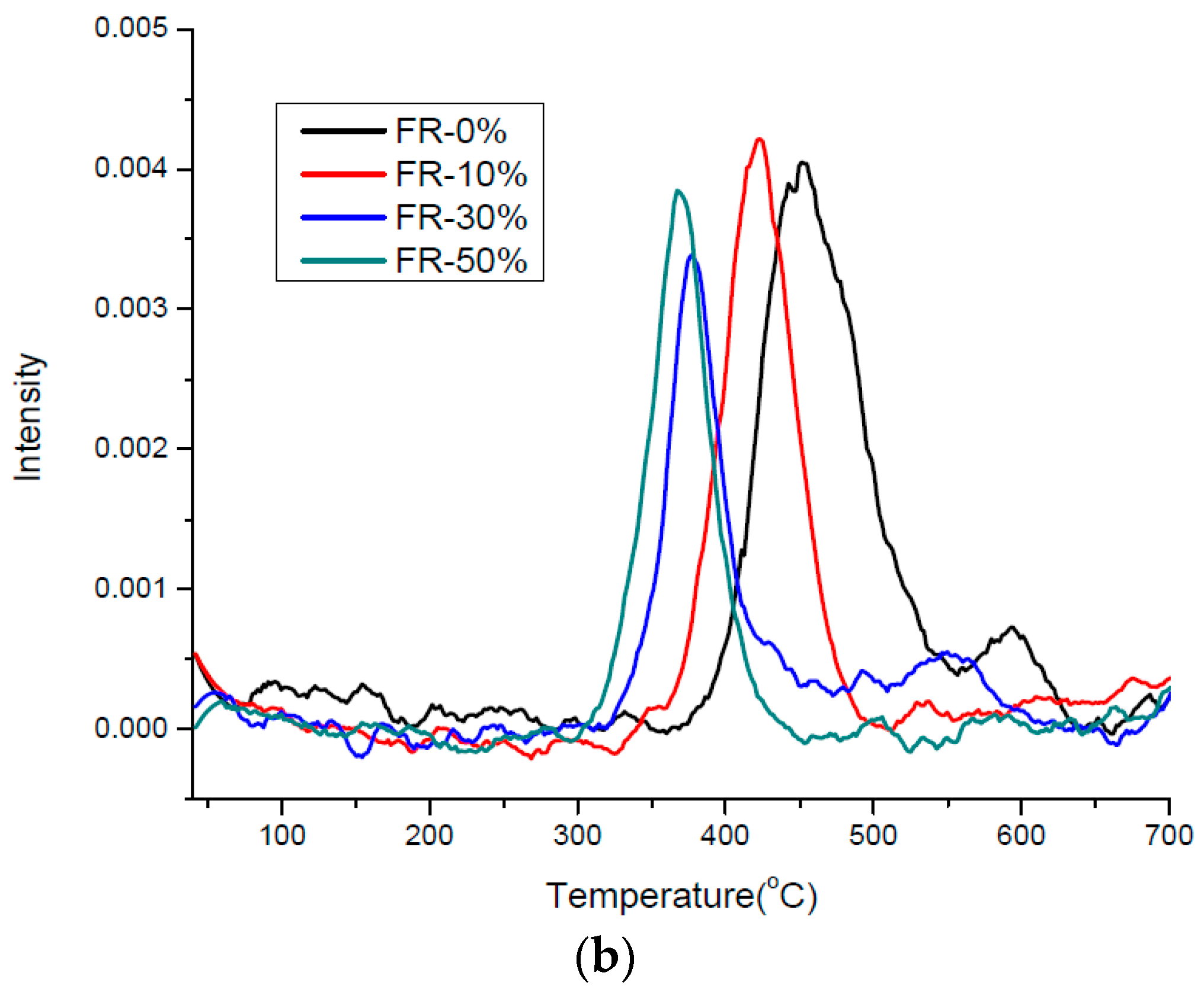
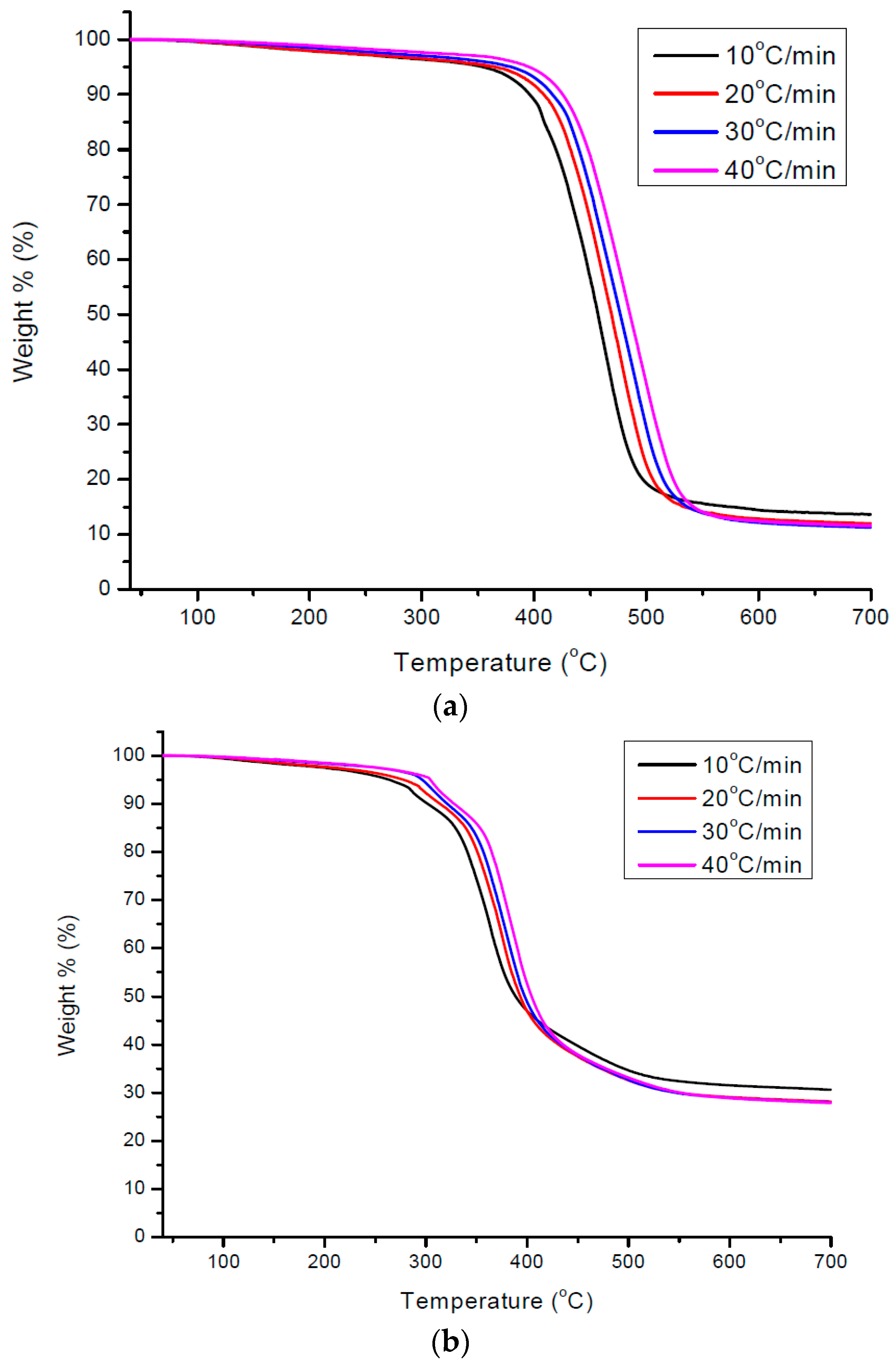
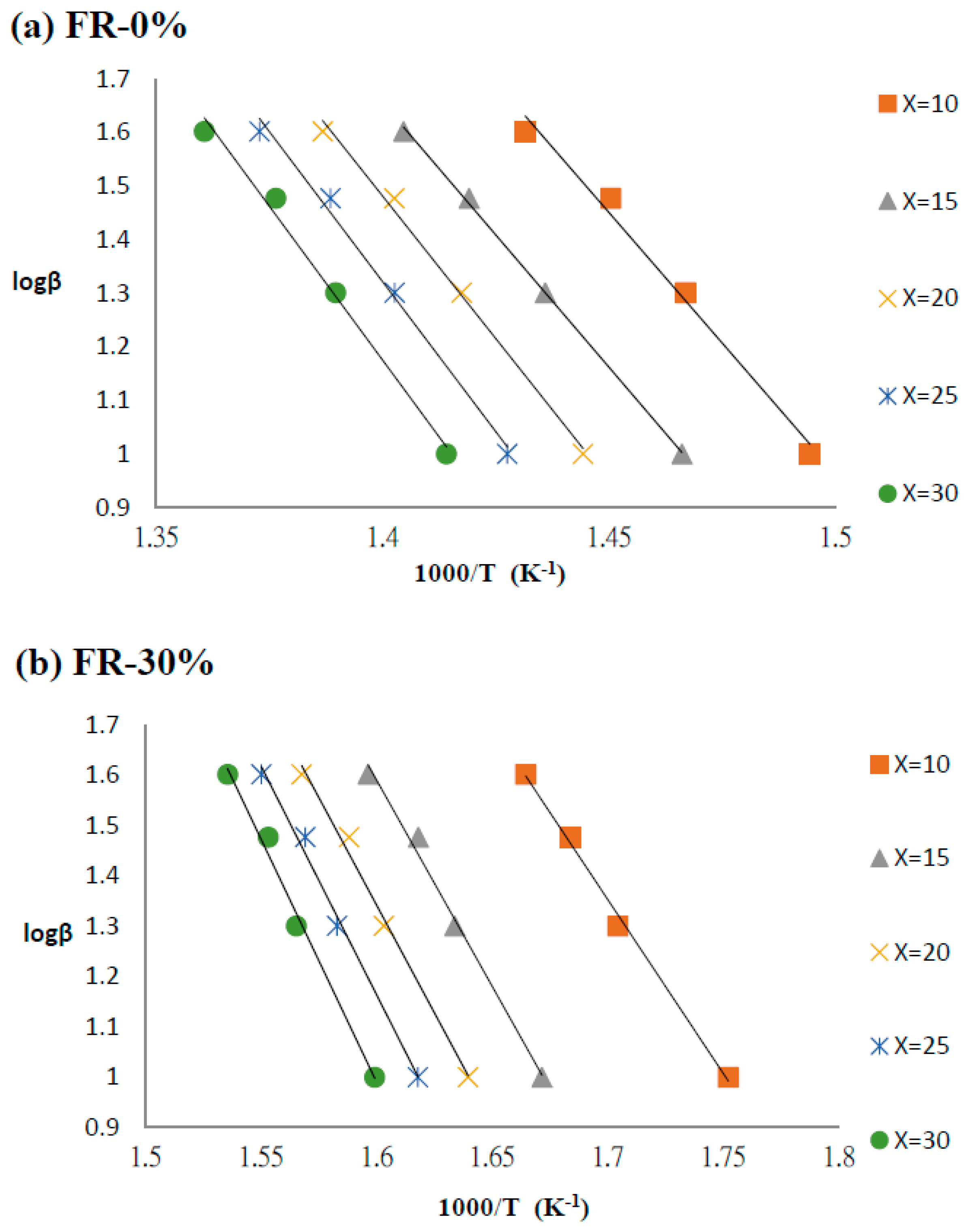
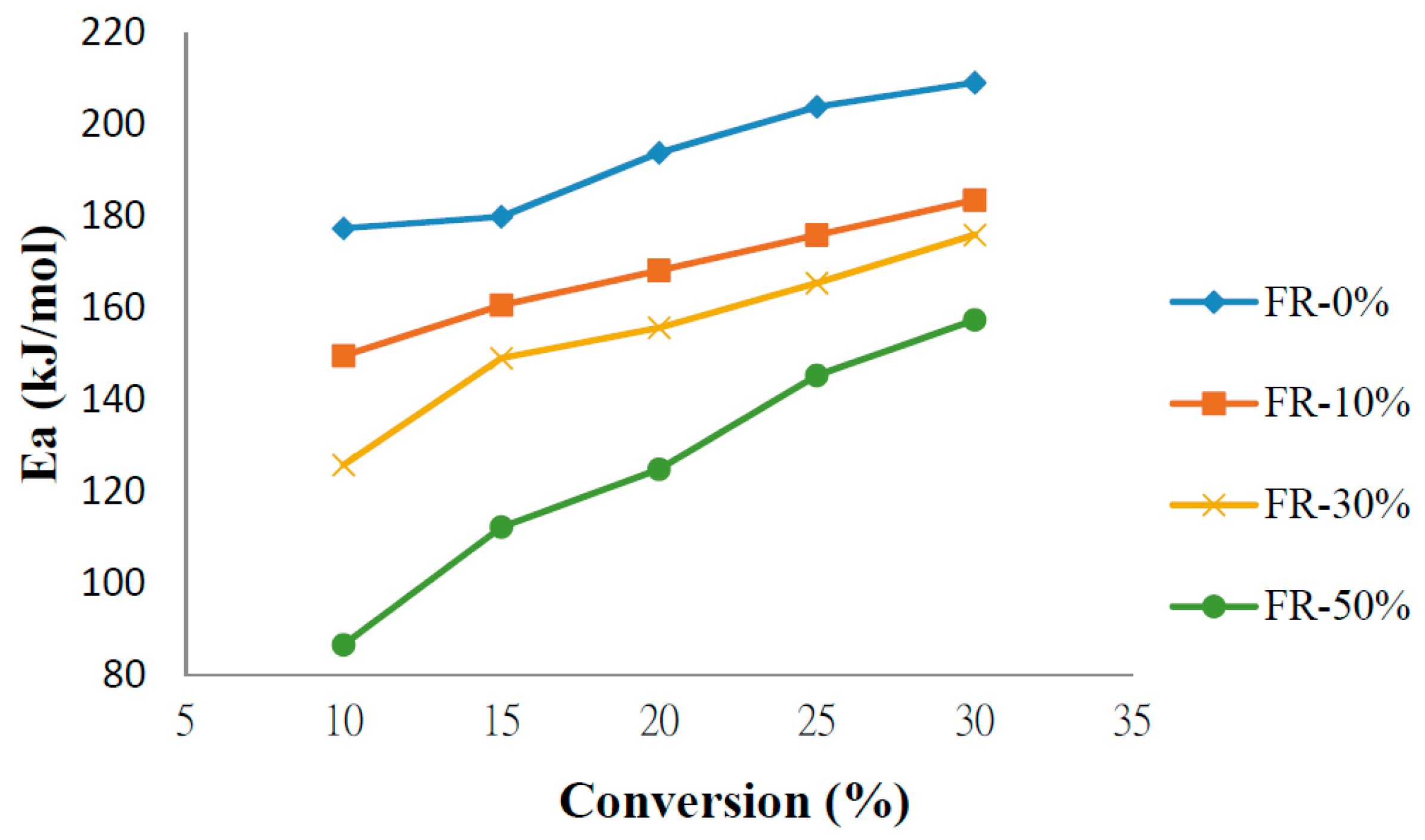
3.4. Kinetics of Pyrolysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chang, S.J.; Chang, F.C. Characterizations for blends of phosphorus-containing copolyester with poly(ethylene terephthalate). Polym. Eng. Sci. 1998, 38, 1471–1481. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. Thermal degradation behavior of hyperbranched polyphosphate acrylate/tri (acryloyloxyethyl) phosphate as an intumescent flame retardant system. Polym. Degrad. Stab. 2007, 92, 1193–1198. [Google Scholar] [CrossRef]
- Chen, X.; Hu, Y.; Jiao, C.; Song, L. Thermal and UV-curing behavior of phosphate diacrylate used for flame retardant coatings. Prog. Org. Coat. 2007, 59, 318–323. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. Synthesis and properties of poly(bisphenol A acryloxyethyl phosphate) as a UV curable flame retardant oligomer. Eur. Polym. J. 2006, 45, 1506–1515. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. UV curing behavior of hyperbranched polyphosphate acrylate/di(hydroxylpropyl methacrylate) piperazine and properties of the cured film. Prog. Org. Coat. 2007, 59, 312–317. [Google Scholar] [CrossRef]
- Xing, W.; Hu, Y.; Song, L.; Chen, X.; Zhang, P.; Ni, J. Thermal degradation and combustion of a novel UV curable coating containing phosphorus. Polym. Degrad. Stab. 2009, 94, 1176–1182. [Google Scholar] [CrossRef]
- Zhu, S.W.; Shi, W.F. Synthesis and photopolymerization of hyperbranched polyurethane acrylates applied to UV curable flame retardant coatings. Polym. Int. 2002, 51, 223–227. [Google Scholar] [CrossRef]
- Liang, H.; Asif, A.; Shi, W. Photopolymerization and thermal behavior of phosphate diacrylate and triacrylate used as reactive-type flame-retardant monomers in ultraviolet-curable resins. J. Appl. Polym. Sci. 2005, 97, 185–194. [Google Scholar] [CrossRef]
- Liang, H.; Asif, A.; Shi, W. Thermal degradation and flame retardancy of a novel methacrylated phenolic melamine used for UV curable flame retardant coatings. Polym. Degrad. Stab. 2005, 87, 495–501. [Google Scholar] [CrossRef]
- Cheng, X.; Shi, W. UV-curing behavior and properties of tri/di (acryloyloxyethyloxy) phenyl silane used for flame-retardant coatings. Prog. Org. Coat. 2010, 69, 252–259. [Google Scholar] [CrossRef]
- Huang, Z.; Shi, W. Thermal behavior and degradation mechanism of poly(bisphenyl acryloxyethyl phosphate) as a UV curable flame-retardant oligomer. Polym. Degrad. Stab. 2006, 91, 1674–1684. [Google Scholar] [CrossRef]
- Xing, W.; Song, L.; Jie, G.; Wang, X. Preparation, flame retardancy and thermal behavior of a novel UV-curable coating containing phosphorus and nitrogen. Mater. Chem. Phys. 2010, 123, 481–486. [Google Scholar] [CrossRef]
- Wang, X.; Wang, B.; Xing, W.; Tang, G.; Zhan, J.; Yang, W. Flame retardancy and thermal property of novel UV-curable epoxy acrylate coatings modified by melamine-based hyperbranched polyphosphonate acrylate. Prog. Org. Coat. 2014, 77, 94–100. [Google Scholar] [CrossRef]
- Cook, W.D. Thermal aspects of the kinetics of dimethacrylate photopolymerization. Polymer 1992, 33, 2152–2161. [Google Scholar] [CrossRef]
- Young, J.S.; Bowman, C.N. Effect of Polymerization Temperature and Cross-Linker Concentration on Reaction Diffusion Controlled Termination. Macromolecules 1999, 32, 6073–6081. [Google Scholar] [CrossRef]
- Cook, W.D. Photopolymerization kinetics of oligo(ethylene oxide) and oligo(methylene) oxide dimethacrylates. J. Polym. Sci. Part. A Polym. Chem. 1993, 31, 1053–1067. [Google Scholar] [CrossRef]
- Rwei, S.P.; Chen, J.D.; Su, C.M. Kinetics of UV-curing of waterborne polyurethane acrylate dendrimer. Polym. Bull. 2013, 70, 1019–1035. [Google Scholar] [CrossRef]
- Lindholm, J.; Brink, A.; Hupa, M. Cone Calorimeter—A Tool for Measuring Heat Release Rate; Åbo Akademi Process Chemistry Centre: Turku, Finland, 2009. [Google Scholar]
- Schartel, B.; Hull, T.R. Development of fire-retarded materials—Interpretation of cone calorimeter data. Fire Mater. 2007, 31, 327–354. [Google Scholar] [CrossRef]
- Huggett, C. Estimation of rate of heat release by means of oxygen consumption measurements. Fire Mater. 1980, 4, 61–65. [Google Scholar] [CrossRef]
- Method of Test for Heat Release Rate for Building Materials—Part 1: Cone Calorimeter Method; CNS, M.O.E.A., R.O.C.: Taipei, Taiwan, 2013; CNS 14705-1.
- Nair, C.P.R.; Clouet, G.; Guilbert, Y. Flame and thermal resistance of phosphorus-functionalized poly(methyl methacrylate) and polystyrene. Polym. Degrad. Stab. 1989, 26, 305–331. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Kamal, M.R. Thermoset characterization for moldability analysis. Polym. Eng. Sci. 1974, 14, 231–239. [Google Scholar] [CrossRef]
- Sourour, S.; Kamal, M.R. Differential scanning calorimetry of epoxy cure: Isothermal cure kinetics. Thermochim. Acta 1976, 14, 41–59. [Google Scholar] [CrossRef]
- Schartel B, B. Phosphorus-based flame retardancy mechanisms—Old hat or a starting point for future development? Materials 2010, 3, 4710–4745. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. FLAME RESISTANCE OF CHEMICAL FIBERS. Appl. Polym. Symp. 1977, 269–292. [Google Scholar]
- Rwei, S.P.; Cheng, C.Y.; Liou, G.S.; Cheng, K.C. Curing and pyrolysis of cresol novolac epoxy resins containing BABODOPN. Polym. Eng. Sci. 2005, 45, 478–486. [Google Scholar] [CrossRef]
- Rwei, S.P.; Kao, S.C.; Liou, G.S.; Cheng, K.C.; Guo, W. Curing and pyrolysis of epoxy resins containing 2-(6-oxido-6H-dibenz(c,e)(1,2)oxaphosphorin-6-yl)-1,4-naphthalenediol or bisphenol S. Colloid Polym. Sci. 2003, 281, 407–415. [Google Scholar] [CrossRef]
- Rwei, S.P.; Liu, A.Y.; Liou, G.S.; Cheng, K.C.; Guo, W. Curing and Pyrolysis of Epoxy Resins Containing ODOPN [2-(6-oxido-6H-dibenz(c,e) (1,2) oxaphosphorin-6-yl)-1,4-naphthalenediol] or Bisphenol-S. Polym. Eng. Sci. 2004, 44, 376–387. [Google Scholar] [CrossRef]
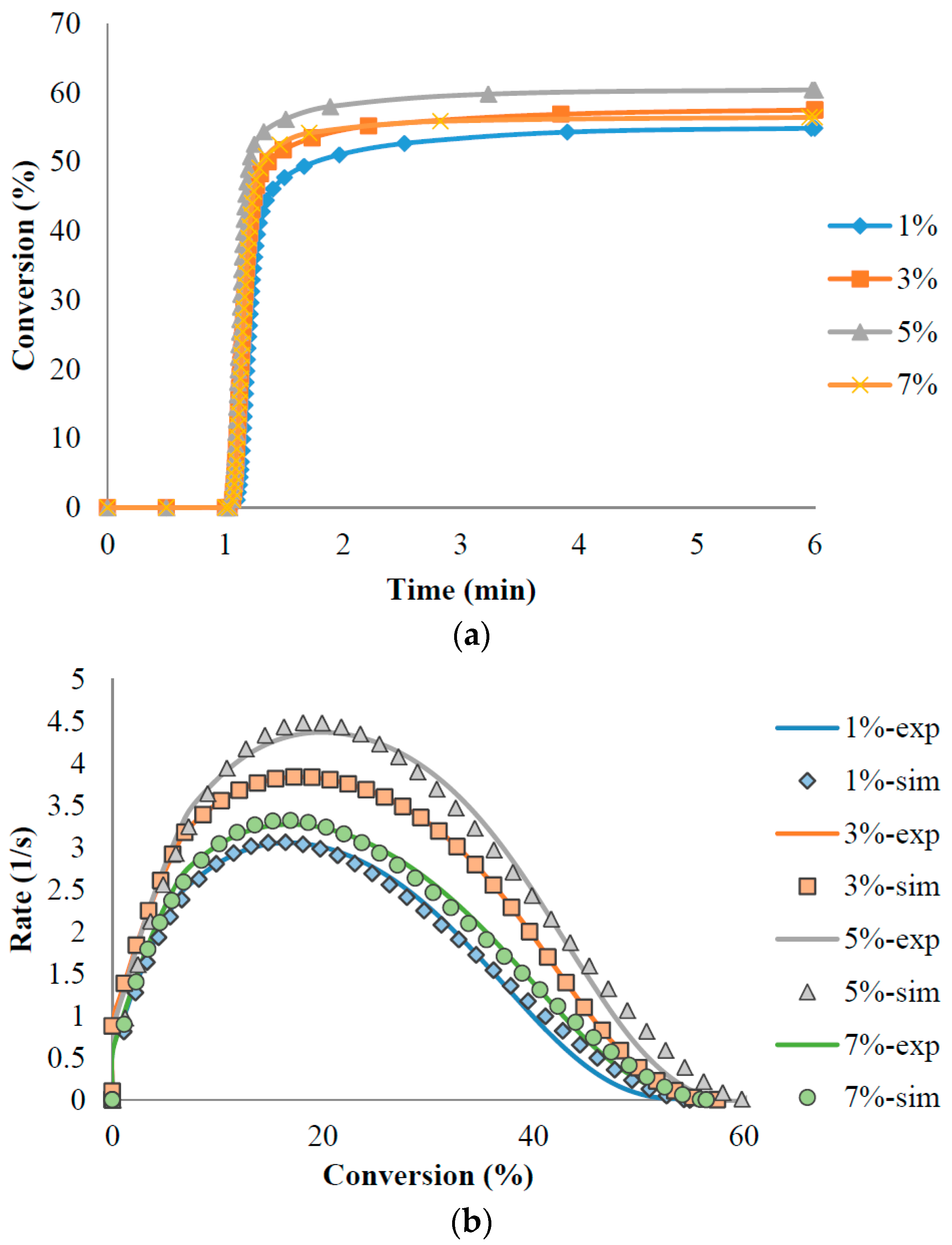
| Photoinitiator | ΔH (J/g) | k (L/min) | m | n | Conversion (α) |
|---|---|---|---|---|---|
| 1 wt % | 216.51 | 13.51 | 0.70 | 1.62 | 54.85 |
| 3 wt % | 229.10 | 16.41 | 0.70 | 1.64 | 57.47 |
| 5 wt % | 232.85 | 18.61 | 0.70 | 1.63 | 60.41 |
| 7 wt % | 224.97 | 14.24 | 0.70 | 1.65 | 56.43 |
| Sample | P (wt %) | Nitrogen Atmosphere | Air Atmosphere | ||||
|---|---|---|---|---|---|---|---|
| Tg (°C) | Td (°C) | 700 °C Residue (wt %) | Td (°C) | 700 °C Residue (wt %) | LOI | ||
| FR-0% | 0 | 72.52 | 380.16 | 11.58 | 370.87 | 0.35 | 22.0 ± 0.5 |
| FR-10% | 0.72 | 69.72 | 311.28 | 18.63 | 335.73 | 3.60 | 23.0 ± 0.5 |
| FR-20% | 1.44 | 69.00 | 299.02 | 27.57 | 301.61 | 17.34 | 24.5 ± 0.5 |
| FR-30% | 2.15 | 62.00 | 286.01 | 28.20 | 297.77 | 21.76 | 26.0 ± 1 |
| FR-40% | 2.87 | 52.67 | 282.68 | 29.62 | 296.49 | 23.85 | 26.5 ± 1 |
| FR-50% | 3.59 | 51.57 | 271.02 | 29.82 | 284.35 | 27.09 | 26.5 ± 1 |
| Sample | Phosphorus (wt %) | HRR (kw/m2) | THE (MJ/m2) | EHC (MJ/m2) | SEA (m2/kg) |
|---|---|---|---|---|---|
| FR-0% | 0 | 378.88 | 54.86 | 29.77 | 1203.58 |
| FR-10% | 0.72 | 175.89 | 48.73 | 28.75 | 619.35 |
| FR-30% | 2.15 | 156.43 | 34.50 | 26.76 | 471.91 |
| FR-50% | 3.59 | 155.25 | 33.58 | 25.01 | 414.65 |
| Sample | FR-0% | FR-10% | FR-30% | FR-50% | |
|---|---|---|---|---|---|
| Conversion Ratio | |||||
| X = 10 | 177.4291 | 149.6557 | 125.8781 | 86.66933 | |
| X = 15 | 179.9557 | 160.6835 | 149.1551 | 112.3091 | |
| X = 20 | 193.9051 | 168.2453 | 155.7831 | 124.9224 | |
| X = 25 | 203.9353 | 175.9927 | 165.5293 | 145.3633 | |
| X = 30 | 209.2144 | 183.6201 | 176.0255 | 157.4742 | |
| Average | 192.89 ± 16.33 | 167.64 ± 17.98 | 154.47 ± 21.56 | 125.3477 ± 32.12 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rwei, S.-P.; Chen, Y.-M.; Chiang, W.-Y.; Ting, Y.-T. A Study of the Curing and Flammability Properties of Bisphenol A Epoxy Diacrylate Resin Utilizing a Novel Flame Retardant Monomer, bis[di-acryloyloxyethyl]-p-tert-butyl-phenyl Phosphate. Materials 2017, 10, 202. https://doi.org/10.3390/ma10020202
Rwei S-P, Chen Y-M, Chiang W-Y, Ting Y-T. A Study of the Curing and Flammability Properties of Bisphenol A Epoxy Diacrylate Resin Utilizing a Novel Flame Retardant Monomer, bis[di-acryloyloxyethyl]-p-tert-butyl-phenyl Phosphate. Materials. 2017; 10(2):202. https://doi.org/10.3390/ma10020202
Chicago/Turabian StyleRwei, Syang-Peng, Yu-Ming Chen, Whe-Yi Chiang, and Yi-Tien Ting. 2017. "A Study of the Curing and Flammability Properties of Bisphenol A Epoxy Diacrylate Resin Utilizing a Novel Flame Retardant Monomer, bis[di-acryloyloxyethyl]-p-tert-butyl-phenyl Phosphate" Materials 10, no. 2: 202. https://doi.org/10.3390/ma10020202






