Tunable Luminescence in Sr2MgSi2O7:Tb3+, Eu3+Phosphors Based on Energy Transfer
Abstract
:1. Introduction
2. Results
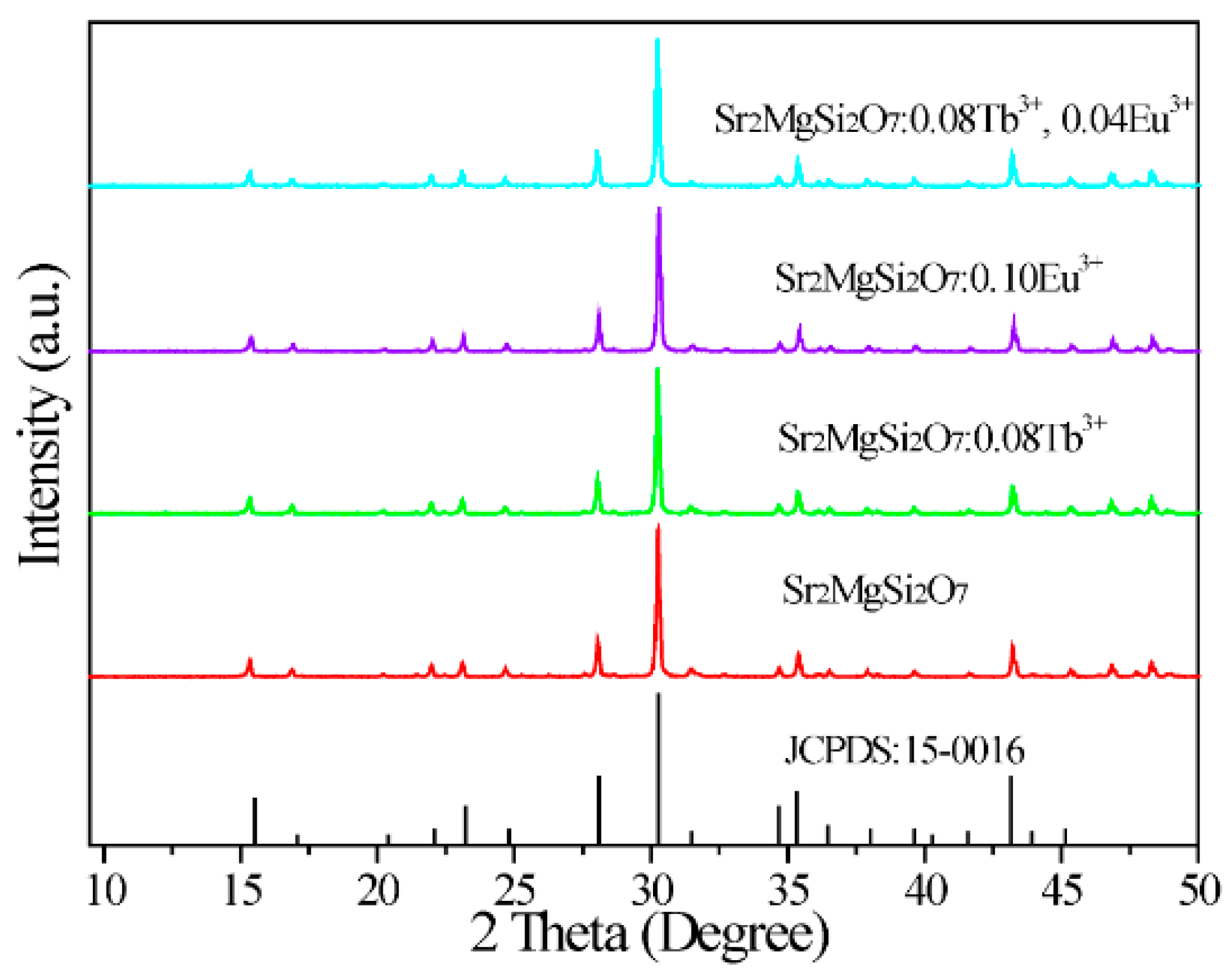
2.1.Phase and Structure Analysis
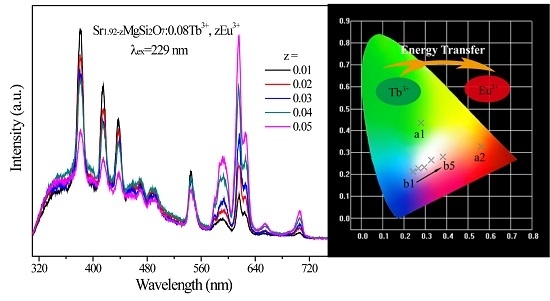
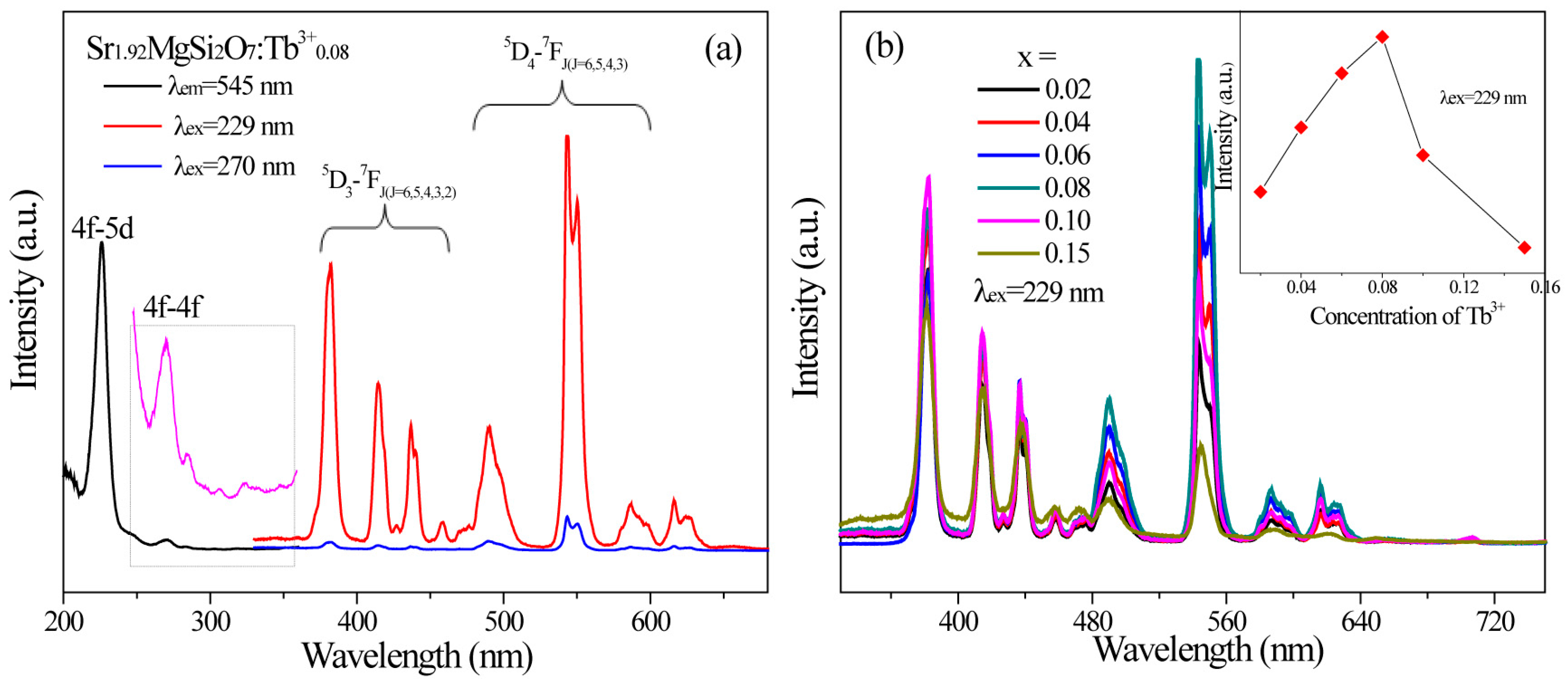
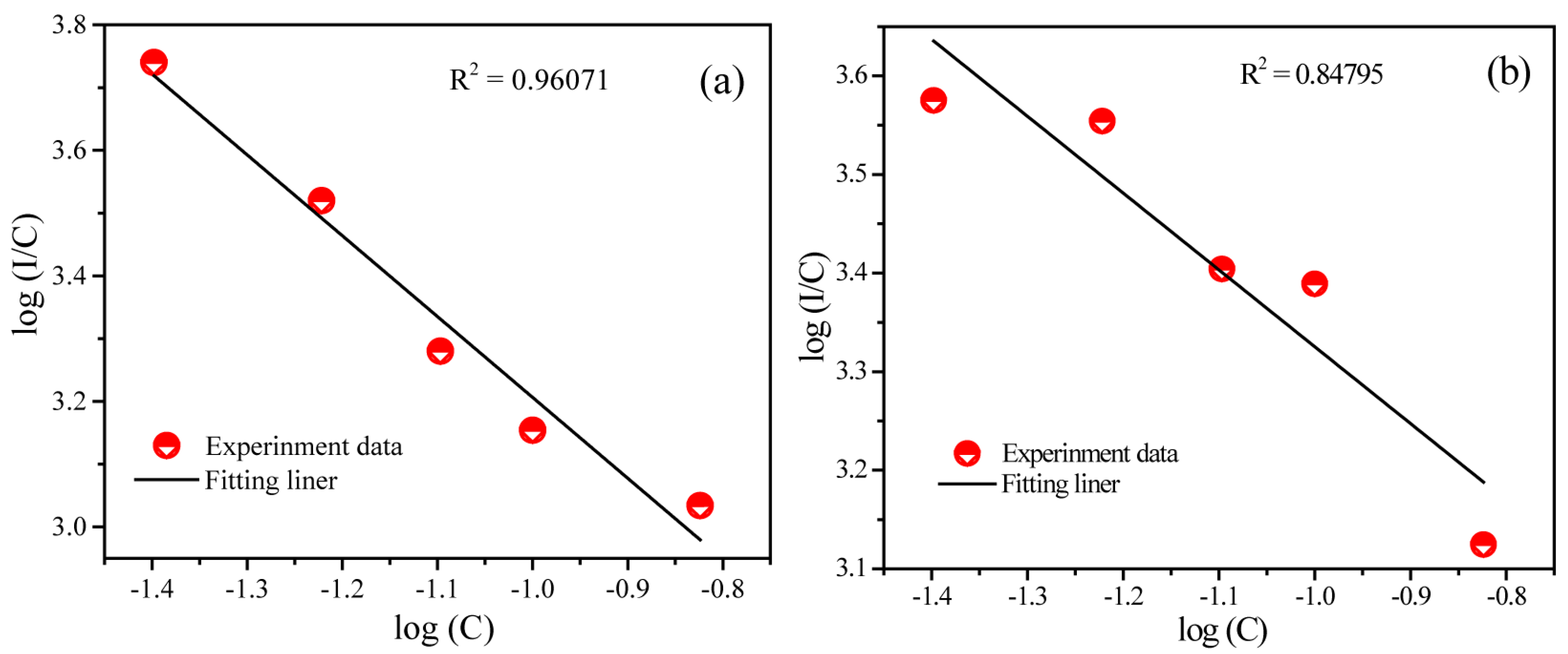
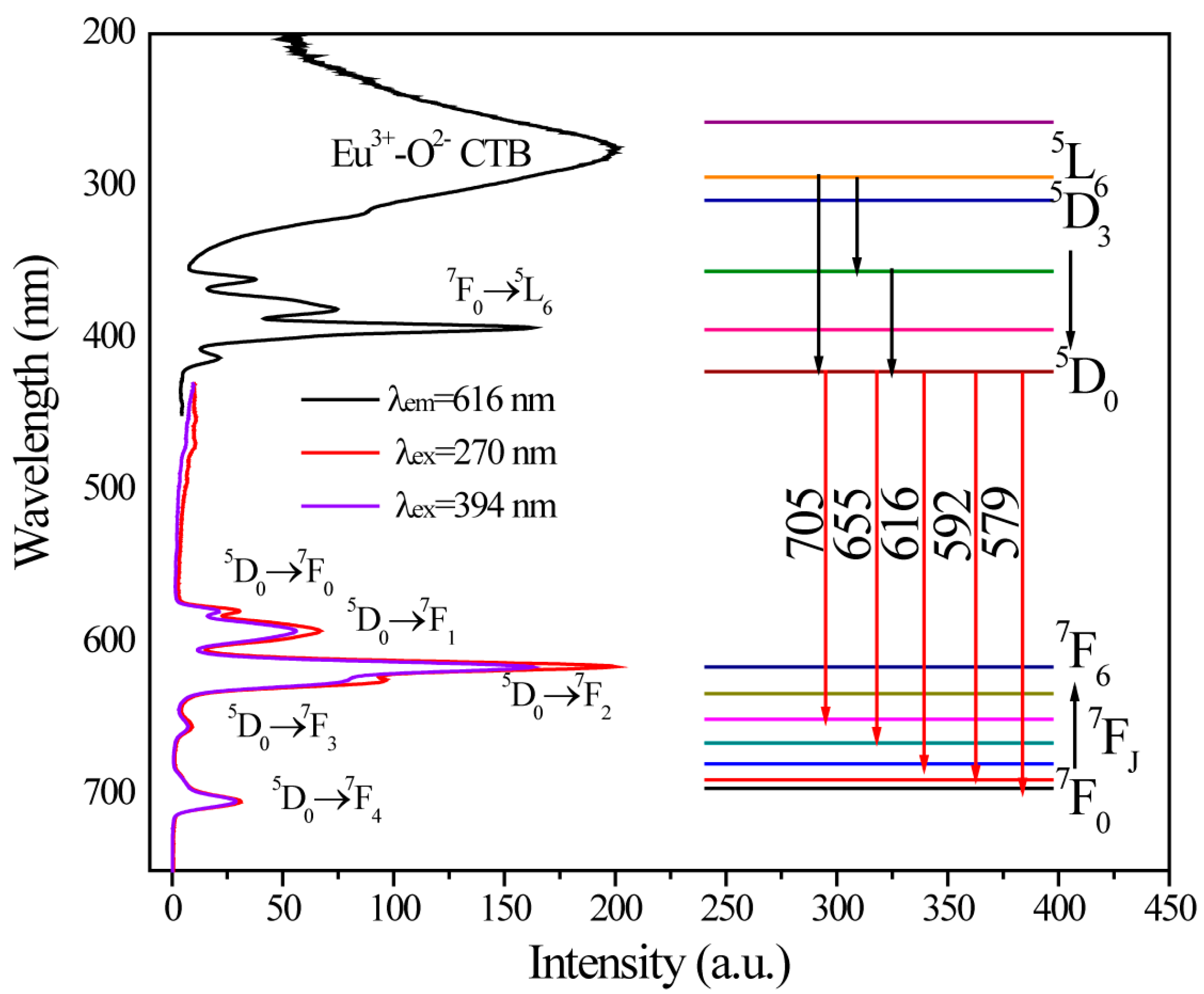
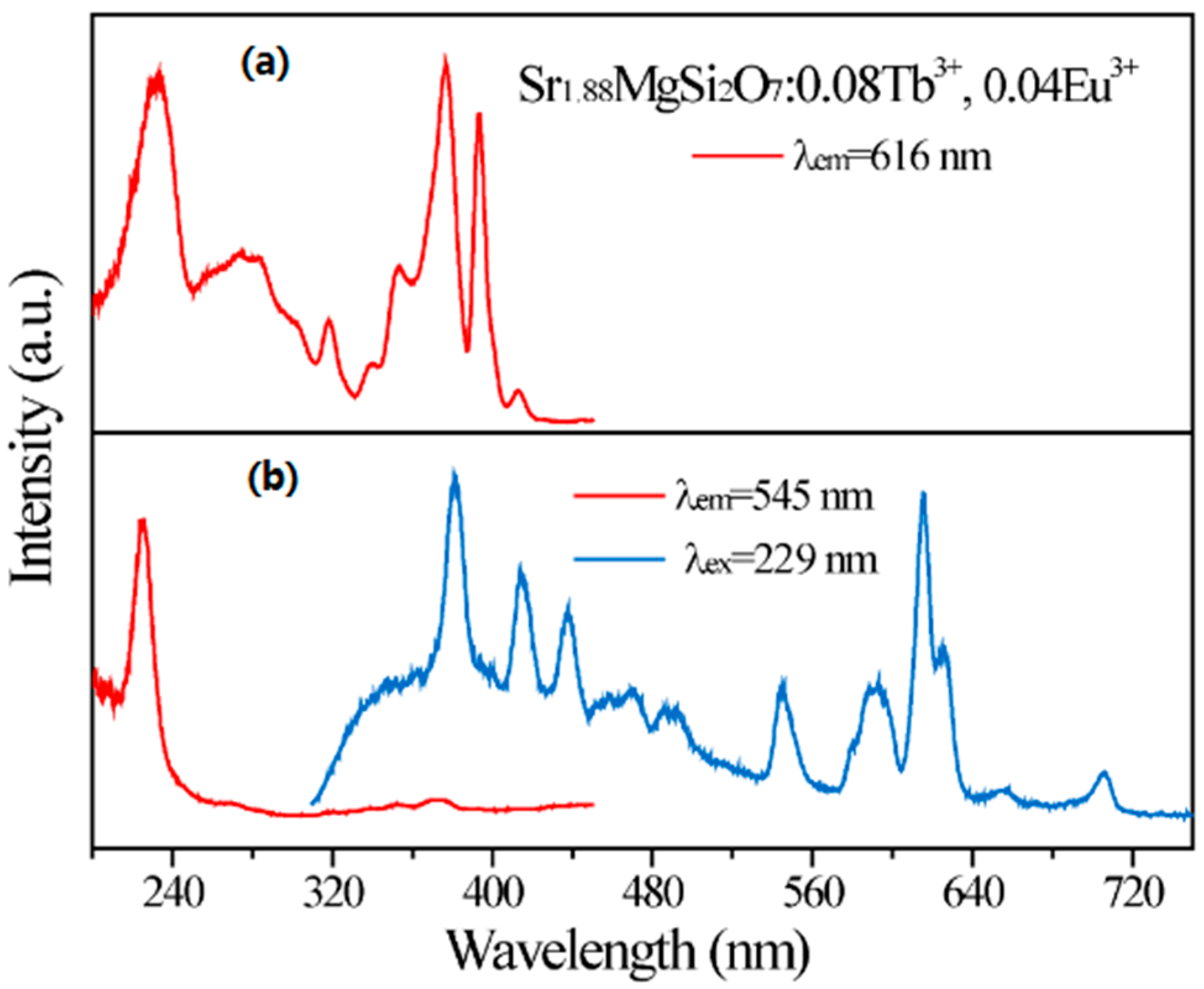
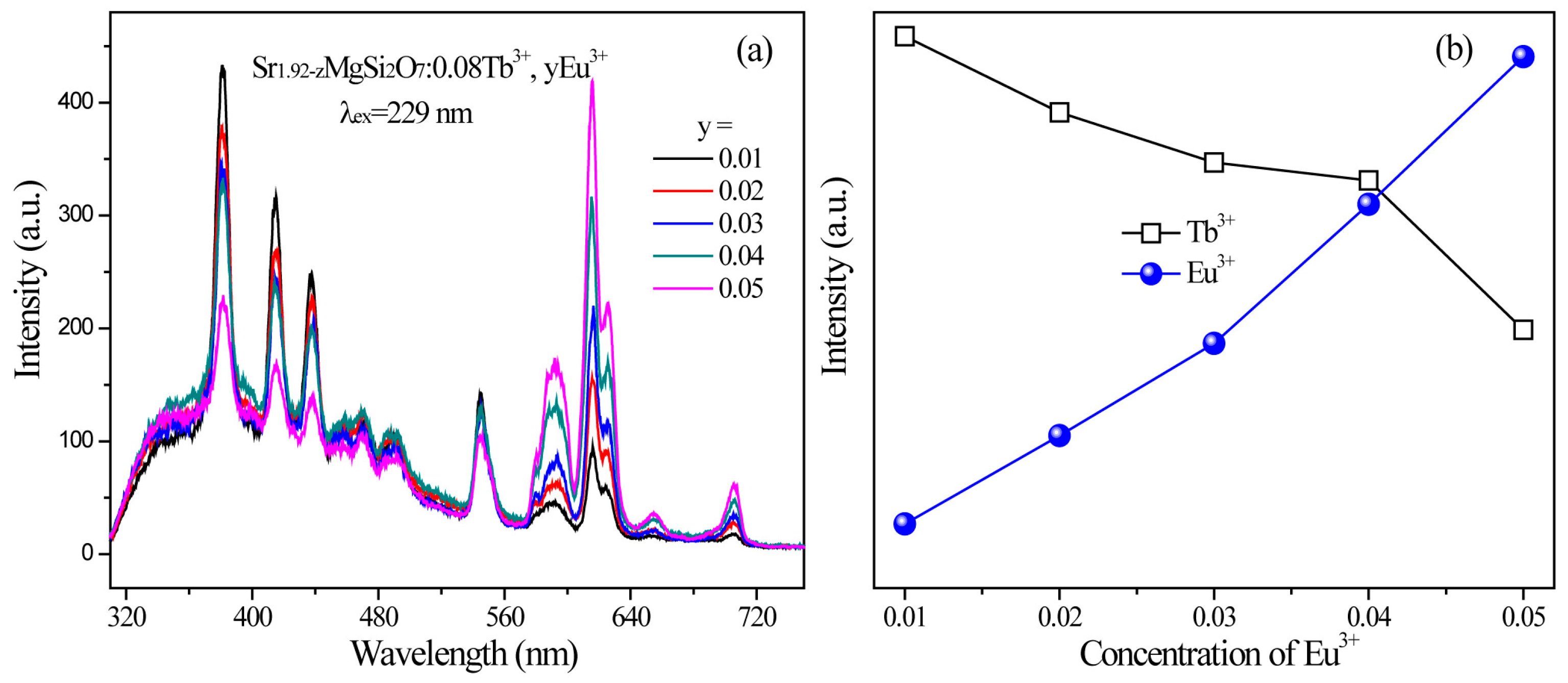
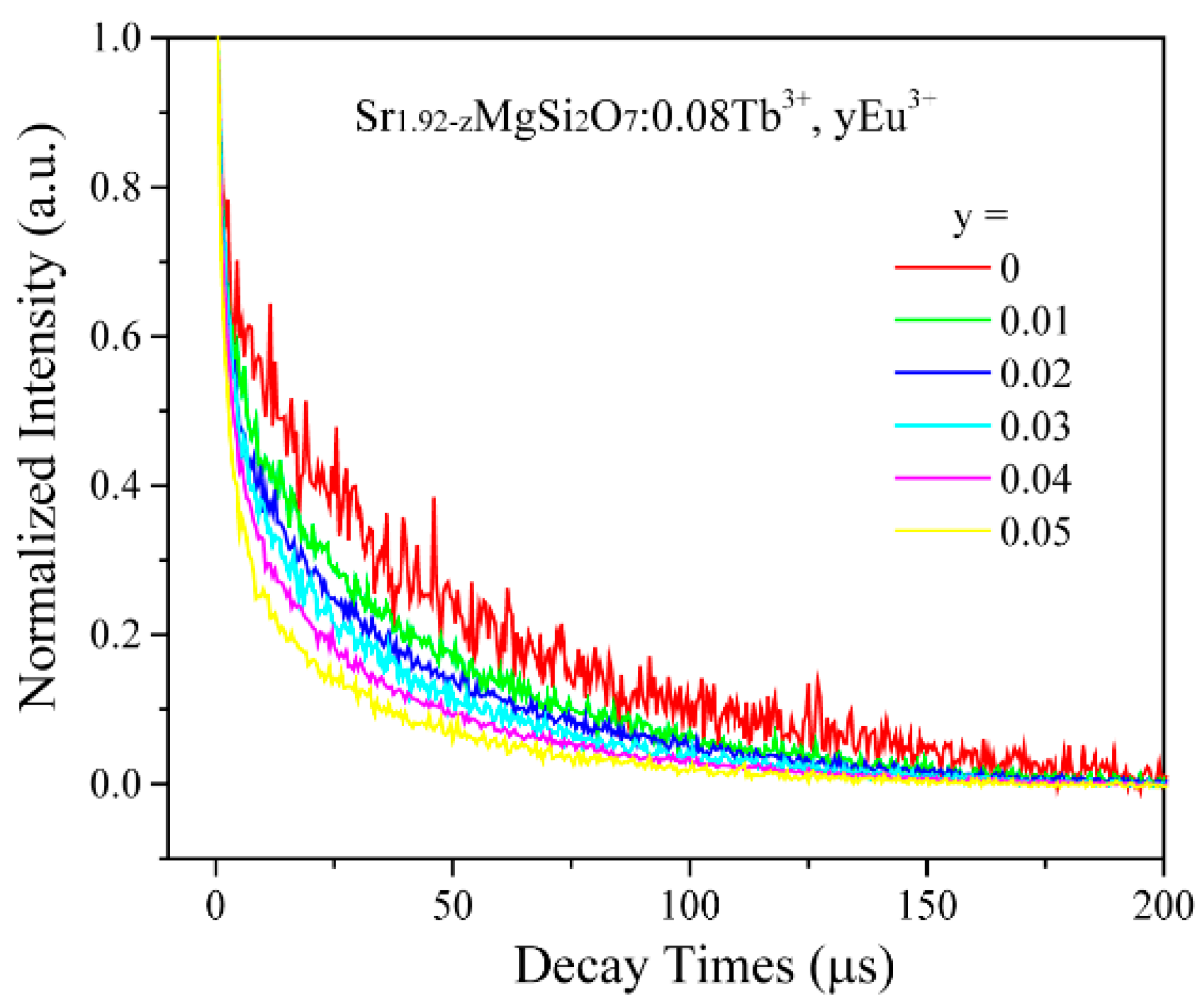
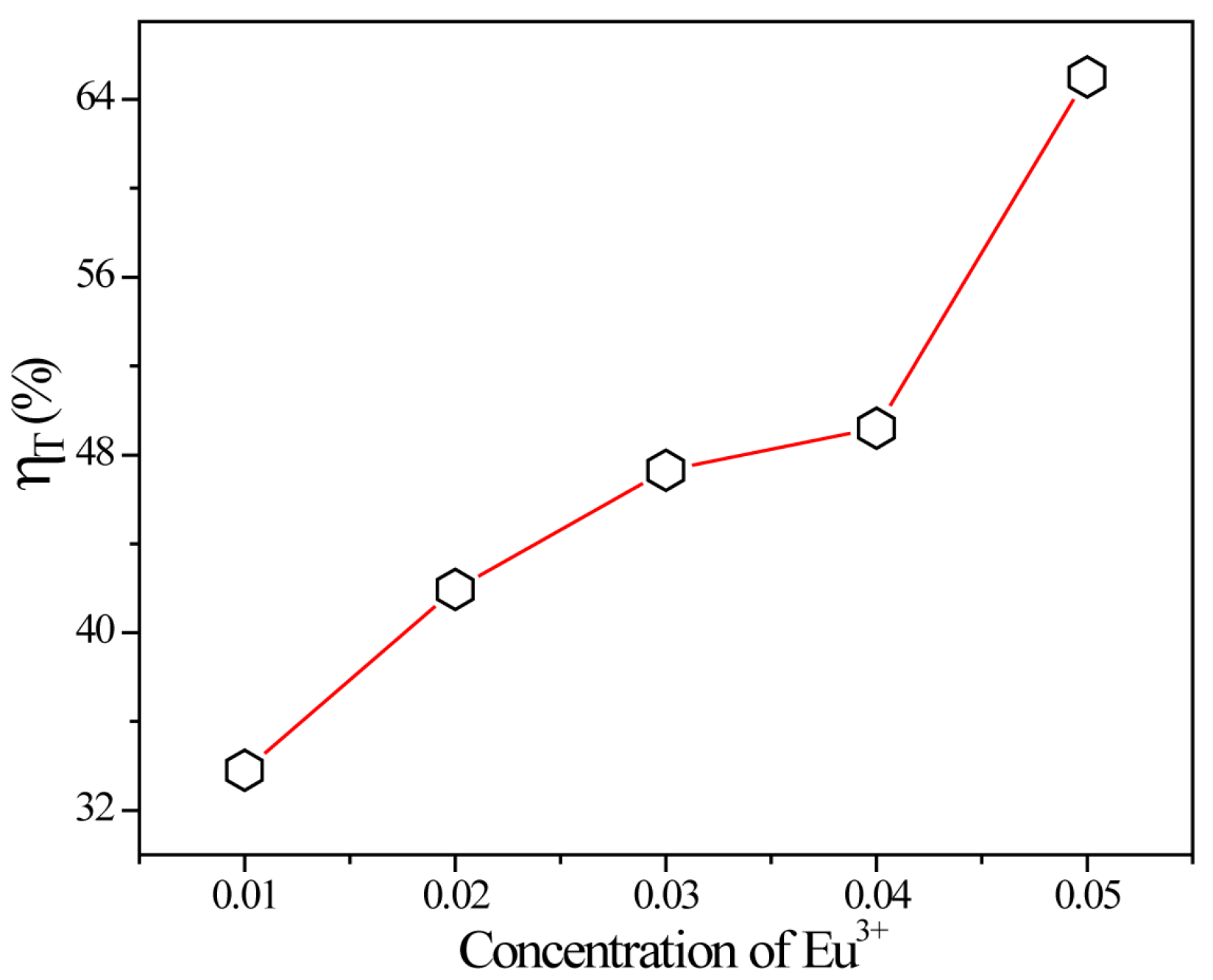
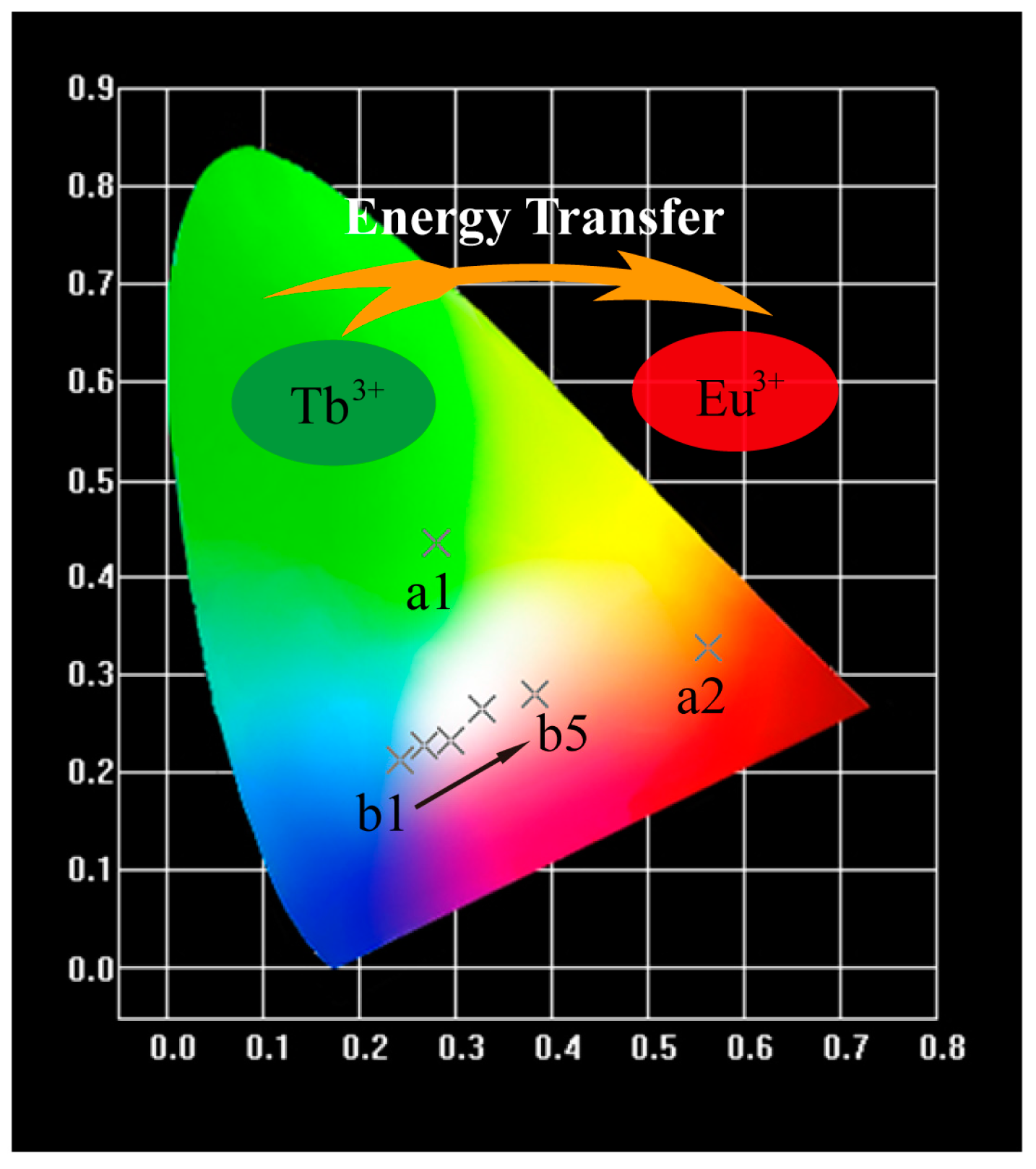
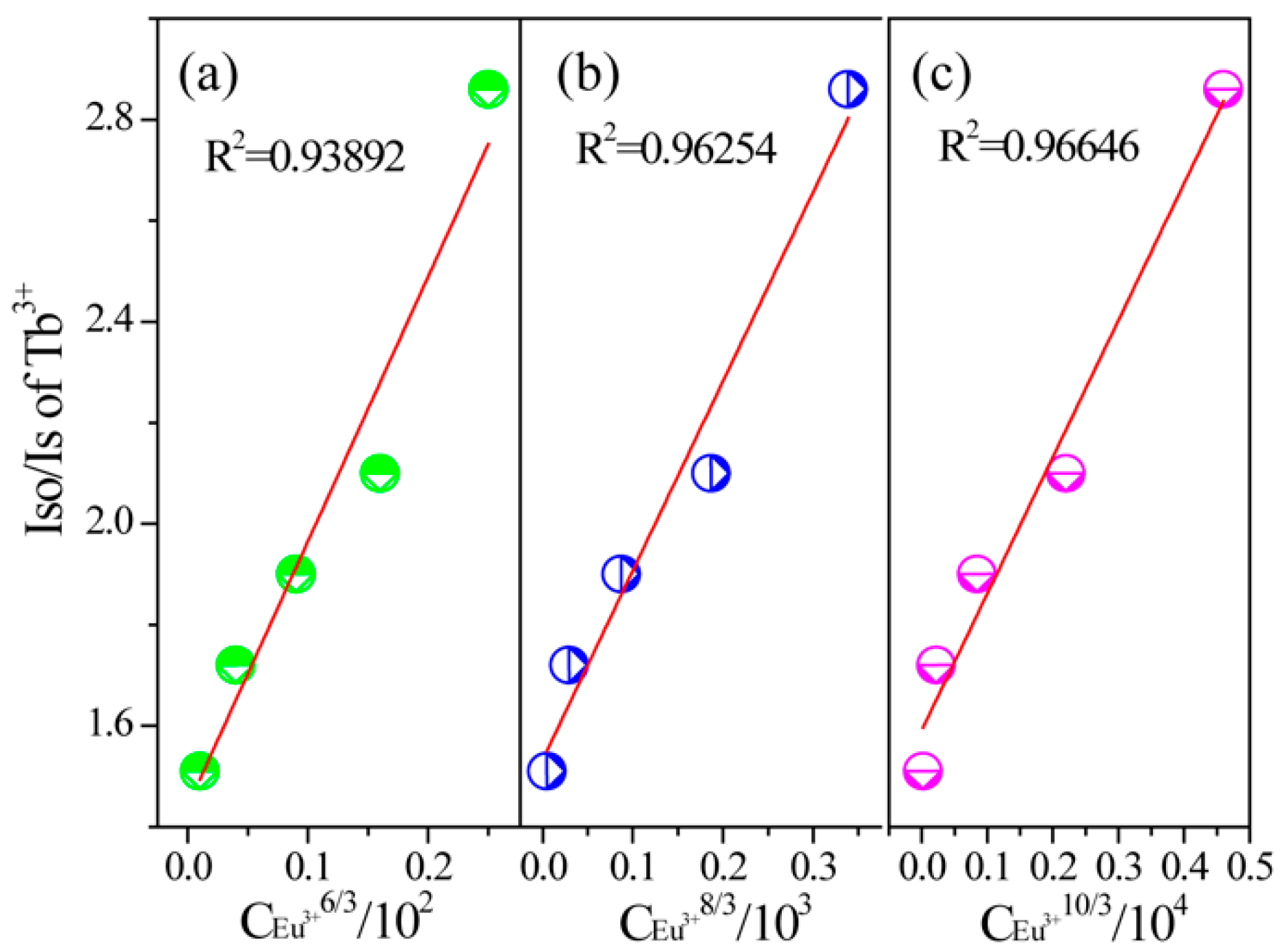
2.2. Photoluminescence and Energy Transfer
3. Materials and Methods
3.1. Sample Preparation
3.2. Measurements and Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, W.; Wang, J.; Zhang, H.; Liu, Y.; Lei, B.; Zhuang, J.; Cui, J.; Peng, M.; Zhu, Y. Tunable emissioncolor and mixed valence state via the modified activator site in the AlN-doped Sr3SiO5:Euphosphor. RSC Adv. 2016, 6, 33076–33082. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, W. Tunable Blue-Red Emission and Energy-Transfer Properties of Mg3(PO4)2:Eu2+, Mn2+ Phosphors. Eur. J. Inorg. Chem. 2015, 2015, 3940–3948. [Google Scholar] [CrossRef]
- Ran, W.G.; Wang, L.L.; Liang, H.B.; Guo, Y.; Kang, W.K.; Qu, D.; Shi, J.S.; Su, L.H. Luminescence properties and energy transfer of CdWO4:Sm3+,Bi3+,M+ (M = Li, Na, K) phosphors for white LEDs. Ceram. Int. 2015, 41, 4301–4307. [Google Scholar]
- Yang, P.; Yu, X.; Yu, H.; Jiang, T.; Xu, X.; Yang, Z.; Zhou, D.; Song, Z.; Yang, Y.; Zhao, Z.; et al. Ca2Al2SiO7:Bi3+, Eu3+, Tb3+: A potential single-phased tunable-color-emitting phosphor. J. Lumin. 2013, 135, 206–210. [Google Scholar] [CrossRef]
- Wang, L.; Noh, H.M.; Moon, B.K.; Park, S.H.; Kim, K.H.; Shi, J.S.; Jeong, J.H. Dual-Mode Luminescence with Broad Near UV and Blue Excitation Band from Sr2CaMoO6:Sm3+ Phosphor for White LEDs. J. Phys. Chem. C 2015, 119, 15517–15525. [Google Scholar] [CrossRef]
- Li, G.; Tian, Y.; Zhao, Y.; Lin, J. Recent progress in luminescence tuning of Ce3+ and Eu2+ activated phosphors for pc-WLEDs. Chem. Soc. Rev. 2015, 44, 8688–8713. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jia, Y.; Pang, R.; Li, H.; Ma, T.; Li, D.; Fu, J.; Zhang, S.; Jiang, L.; Li, C. Sr9Mg1.5(PO4)7:Eu2+: A Novel Broadband Orange-Yellow-Emitting Phosphor for Blue Light-Excited Warm White LEDs. Appl. Mater. Interface 2015, 7, 25219–25226. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lian, H.; Shang, M.; Lin, J. A novel greenish yellow-orange red Ba3Y4O9:Bi3+, Eu3+ phosphor with efficient energy transfer for UV-LEDs. Dalton. Trans. 2015, 44, 20542–20550. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Wang, L.; Molokeev, M.S.; Hirosaki, N.; Huang, Z.; Xia, Z.; Kate, O.M.; Liu, L.; Xie, R. New garnet structure phosphors, Lu3−xYxMgAl3SiO12:Ce3+ (x = 0–3), developed by solid solution design. J. Mater. Chem. C 2016, 4, 2359–2366. [Google Scholar] [CrossRef]
- Wang, C.; Xin, S.; Wang, X.; Zhu, G.; Wu, Q.; Wang, Y. Double substitution induced tunable photoluminescence in the Sr2Si5N8:Eu phosphor lattice. New J. Chem. 2015, 39, 6958–6964. [Google Scholar]
- Huang, C.H.; Chen, Y.C.; Kuo, T.W.; Chen, T.M. Novel green-emitting Na2CaPO4F:Eu2+ phosphors for near-ultraviolet white light-emitting diodes. J. Lumin. 2011, 131, 1346–1349. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Z.; Wang, Y. Ce3+and Tb3+singly- and co-doped MgGd4Si3O13 for ultraviolet light emitting diodes and field emission displays. J. Mater. Chem. C 2015, 3, 3676–3683. [Google Scholar] [CrossRef]
- Zhou, H.; Jin, Y.; Jiang, M.; Wang, Q.; Jiang, X. A single-phased tunable emission phosphor MgY2Si3O10:Eu3+, Bi3+ with efficient energy transfer for white LEDs. Dalton. Trans. 2015, 44, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Y.; Fang, M.; Huang, Z. Luminescence properties and energy transfer of Eu/Mn coactivated Mg2Al4Si5O18 as a potential phosphor for white-light LEDs. Inorg. Chem. 2014, 53, 11396–11403. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhou, L.; Pang, Q.; Shi, J.; Gong, M. Tunable Luminescence and Ce3+→Tb3+→Eu3+ EnergyTransfer of Broadband-Excited and Narrow Line Red Emitting Y2SiO5:Ce3+, Tb3+, Eu3+ Phosphor. J. Phys. Chem. C 2014, 118, 7591–7598. [Google Scholar] [CrossRef]
- Li, K.; Fan, J.; Shang, M.; Lian, H.; Lin, J. Sr2Y8(SiO4)6O2:Bi3+/Eu3+: A single-component white-emitting phosphor via energy transfer for UV w-LEDs. J. Mater. Chem. C 2015, 3, 9989–9998. [Google Scholar] [CrossRef]
- Kim, D.; Park, D.; Oh, N.; Kim, J.; Jeong, E.D.; Kim, S.J.; Kim, S.; Park, J.C. Luminescent properties of rare earth fully activated apatites, LiRE9(SiO4)6O2 (RE = Ce, Eu, and Tb): Site selective crystal field effect. Inorg. Chem. 2015, 54, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Fan, J.; Mi, X.; Zhang, Y.; Lian, H.; Shang, M.; Lin, J. Tunable-color luminescence via energy transfer in NaCa13/18Mg5/18PO4:A (A = Eu2+/Tb3+/Mn2+, Dy3+) phosphors for solid state lighting. Inorg. Chem. 2014, 53, 12141–12150. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ran, W.; Lv, Z.; Ren, C.; Jiang, H.; Li, W.; Shi, J. A strategy for developing deep-UV phosphor: Sr3AlO4F:Tb3+/Sm3+. J. Mater. Sci. Mater. Electron. 2015, 26, 9614–9623. [Google Scholar] [CrossRef]
- Wang, B.; Lin, H.; Xu, J.; Chen, H.; Wang, Y. CaMg2Al16O27:Mn4+ based red phosphor: A potential color converter for high-powered warm W-LED. Appl. Mater. Int. 2014, 6, 22905–22913. [Google Scholar] [CrossRef] [PubMed]
- Maheshwary, M.; Singh, B.P.; Singh, R.A. Color tuning in thermally stable Sm3+-activated CaWO4 nanophosphors. New. J. Chem. 2015, 39, 4494–4507. [Google Scholar] [CrossRef]
- Blasse, G. Energy Transfer in Oxidic Phosphors. Phys. Lett. 1968, 28, 444–445. [Google Scholar] [CrossRef]
- Li, K.; Shang, M.; Zhang, Y.; Fan, J.; Lian, H.; Lin, J. Photoluminescence properties of single-component white-emitting Ca9Bi(PO4)7:Ce3+, Tb3+, Mn2+ phosphors for UV LEDs. J. Mater. Chem. C 2015, 3, 7096–7104. [Google Scholar] [CrossRef]
- Huang, C.H.; Chiu, Y.C.; Yeh, Y.T.; Chan, T.S.; Chen, T.M. Eu2+ activated Sr8ZnSc(PO4)7: A novel near-ultraviolet converting yellow-emitting phosphor for white light-emitting diodes. Appl. Mater. Interface 2012, 4, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Uitert, I.G.V. Characterization of Energy Transfer Interactions between Rare Earth Ions. J. Electrochem. Soc. Solid State Sci. 1967, 114, 1048–1053. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Y.; Mei, L.; Liu, H.; Fang, M.; Huang, Z. Crystal structure and temperature-dependent luminescence characteristics of KMg4(PO4)3:Eu2+ phosphor for white light-emitting diodes. Sci. Rep. 2015, 5, 9673. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Q.; Jin, Y. Temperature dependence of energy transfer in tunable white light-emitting phosphor BaY2Si3O10:Bi3+, Eu3+ for near UV LEDs. J. Mater. Chem. C 2015, 3, 11151–11162. [Google Scholar] [CrossRef]
- Xu, M.; Wang, L.; Jia, D.; Zhao, H.; Setlur, A. Tuning the Color Emission of Sr2P2O7:Tb3+, Eu3+ Phosphors Based on Energy Transfer. J. Am. Ceram. Soc. 2015, 98, 1536–1541. [Google Scholar] [CrossRef]
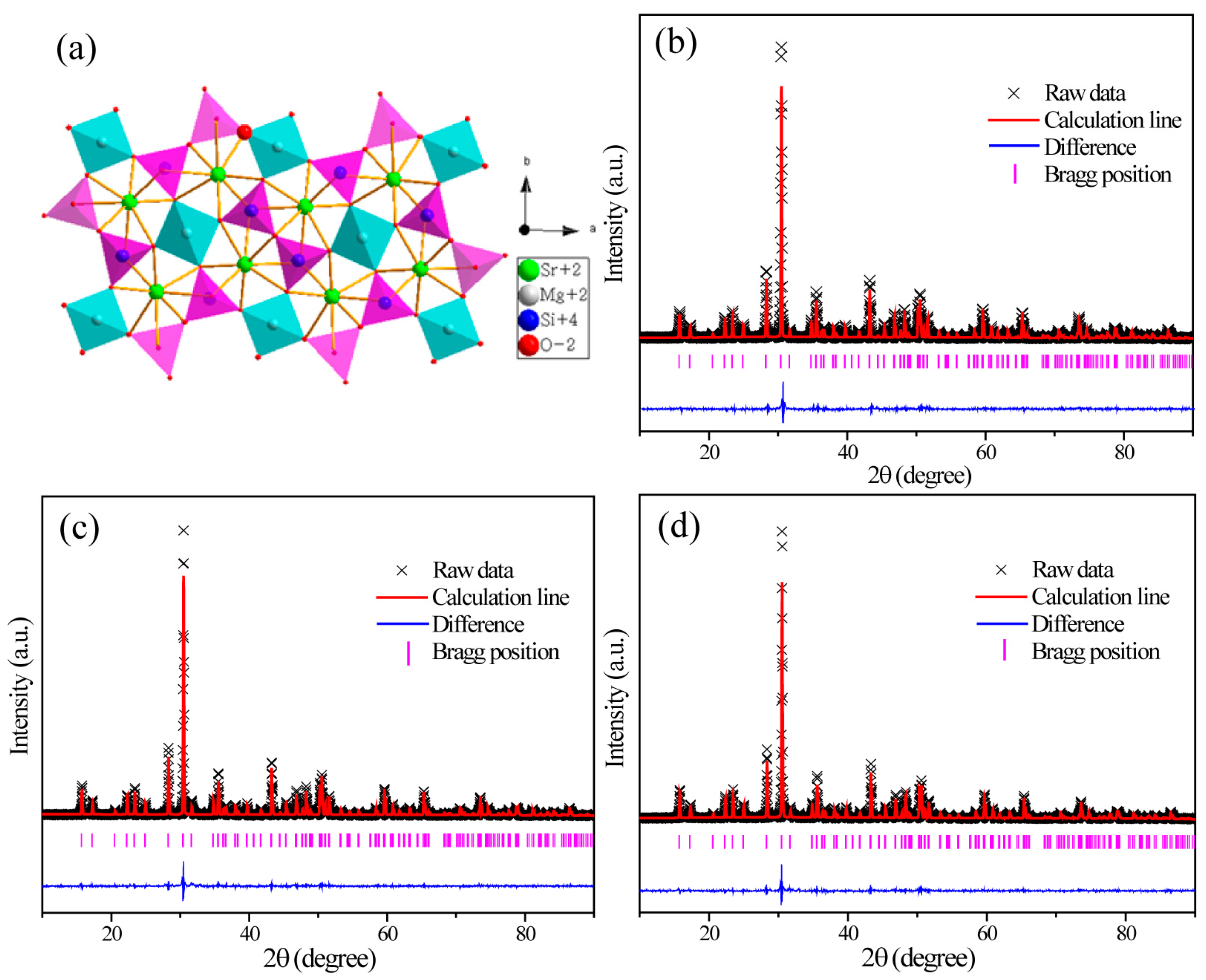
| Formula | SMSO | SMSO:0.08Tb | SMSO:0.10Eu | SMSO:0.08Tb, 0.04Eu |
|---|---|---|---|---|
| Crystal System | Tetragonal | Tetragonal | Tetragonal | Tetragonal |
| Space Group | P-421m (113) | P-421m (113) | P-421m (113) | P-421m (113) |
| a/Å | 8.01 | 8.0111 | 8.0112 | 8.0108 |
| b/Å | 8.01 | 8.0111 | 8.0112 | 8.0108 |
| c/Å | 5.16 | 5.1667 | 5.1657 | 5.1650 |
| v/Å3 | 331.34 | 331.58 | 331.53 | 331.45 |
| Z | 2 | 2 | 2 | 2 |
| Radiation Type | - | Cu−Kα | Cu−Kα | Cu−Kα |
| Wavelength/Å | - | 1.5405 | 1.5405 | 1.5405 |
| Profile Range/° | - | 10°−90° | 10°−90° | 10°−90° |
| Rp/% | - | 8.75 | 8.46 | 7.97 |
| Rwp/% | - | 11.95 | 11.51 | 10.9 |
| χ2 | - | 3.552 | 3.721 | 4.157 |
| Sample No. | SMSO:xTb3+, yEu3+ | CIE (x, y) | CCT (K) |
|---|---|---|---|
| a1 | x = 0.08, y = 0 | (0.2796, 0.4359) | 7191 |
| a2 | x = 0, y = 0.10 | (0.5646, 0.3272) | 4937 |
| b1 | x = 0.08, y = 0.01 | (0.2424, 0.2137) | 164,473 |
| b2 | x = 0.08, y = 0.02 | (0.2682, 0.2263) | 31,313 |
| b3 | x = 0.08, y = 0.03 | (0.2943, 0.2337) | 13,267 |
| b4 | x = 0.08, y = 0.04 | (0.3271, 0.2644) | 5827 |
| b5 | x = 0.08, y = 0.05 | (0.3821, 0.2857) | 2768 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wang, L.; Ran, W.; Deng, Z.; Shi, J.; Ren, C. Tunable Luminescence in Sr2MgSi2O7:Tb3+, Eu3+Phosphors Based on Energy Transfer. Materials 2017, 10, 227. https://doi.org/10.3390/ma10030227
Li M, Wang L, Ran W, Deng Z, Shi J, Ren C. Tunable Luminescence in Sr2MgSi2O7:Tb3+, Eu3+Phosphors Based on Energy Transfer. Materials. 2017; 10(3):227. https://doi.org/10.3390/ma10030227
Chicago/Turabian StyleLi, Minhong, Lili Wang, Weiguang Ran, Zhihan Deng, Jinsheng Shi, and Chunyan Ren. 2017. "Tunable Luminescence in Sr2MgSi2O7:Tb3+, Eu3+Phosphors Based on Energy Transfer" Materials 10, no. 3: 227. https://doi.org/10.3390/ma10030227