Electric Field-Driven Assembly of Sulfonated Polystyrene Microspheres
Abstract
:1. Introduction
2. Results
2.1. FTIR Studies
2.2. Optical Photography and Electron Microscopy Examination of Sulfonated PS Particles
2.3. Electrical Conductance and Dielectric Constant
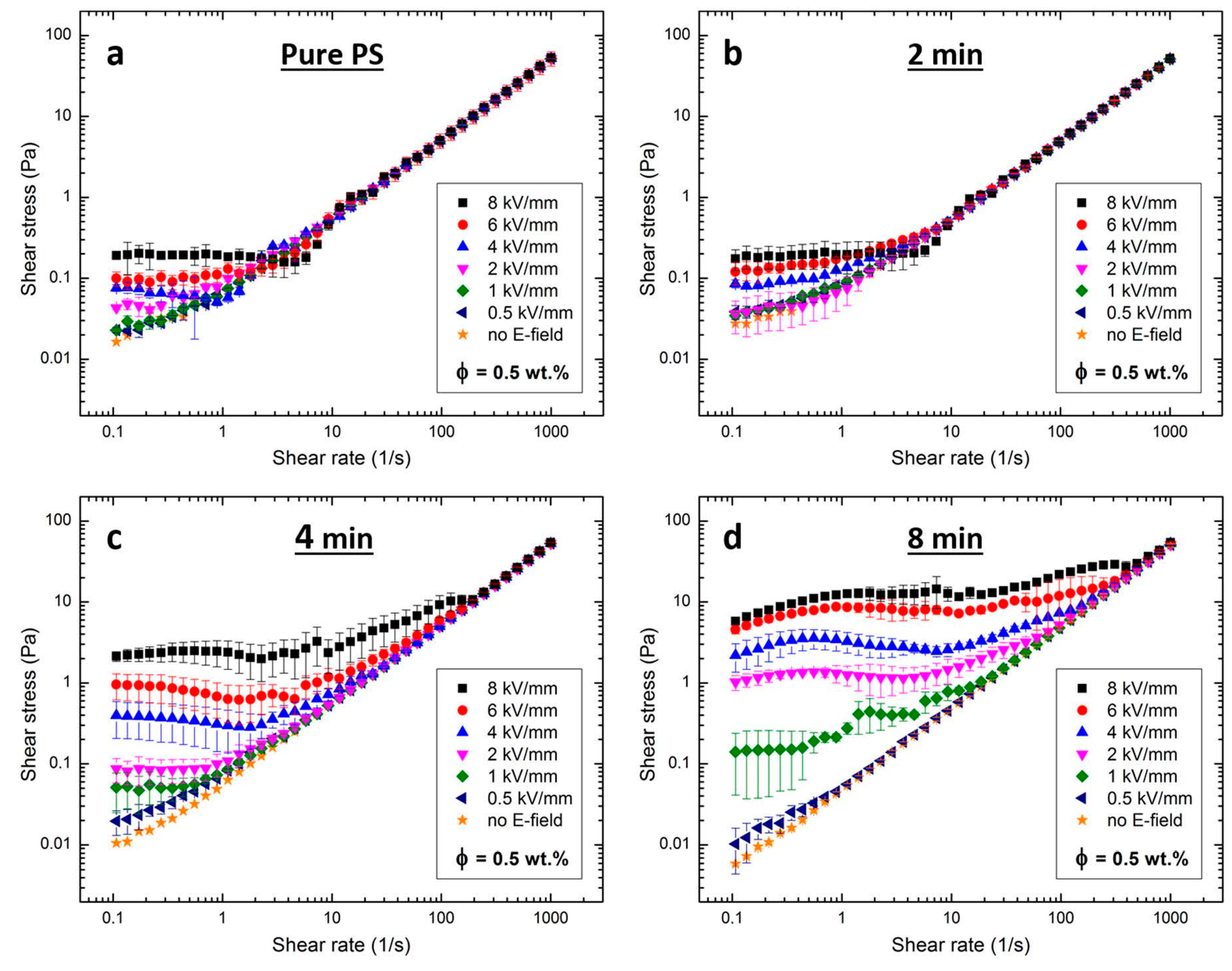
2.4. Electro-Rheology
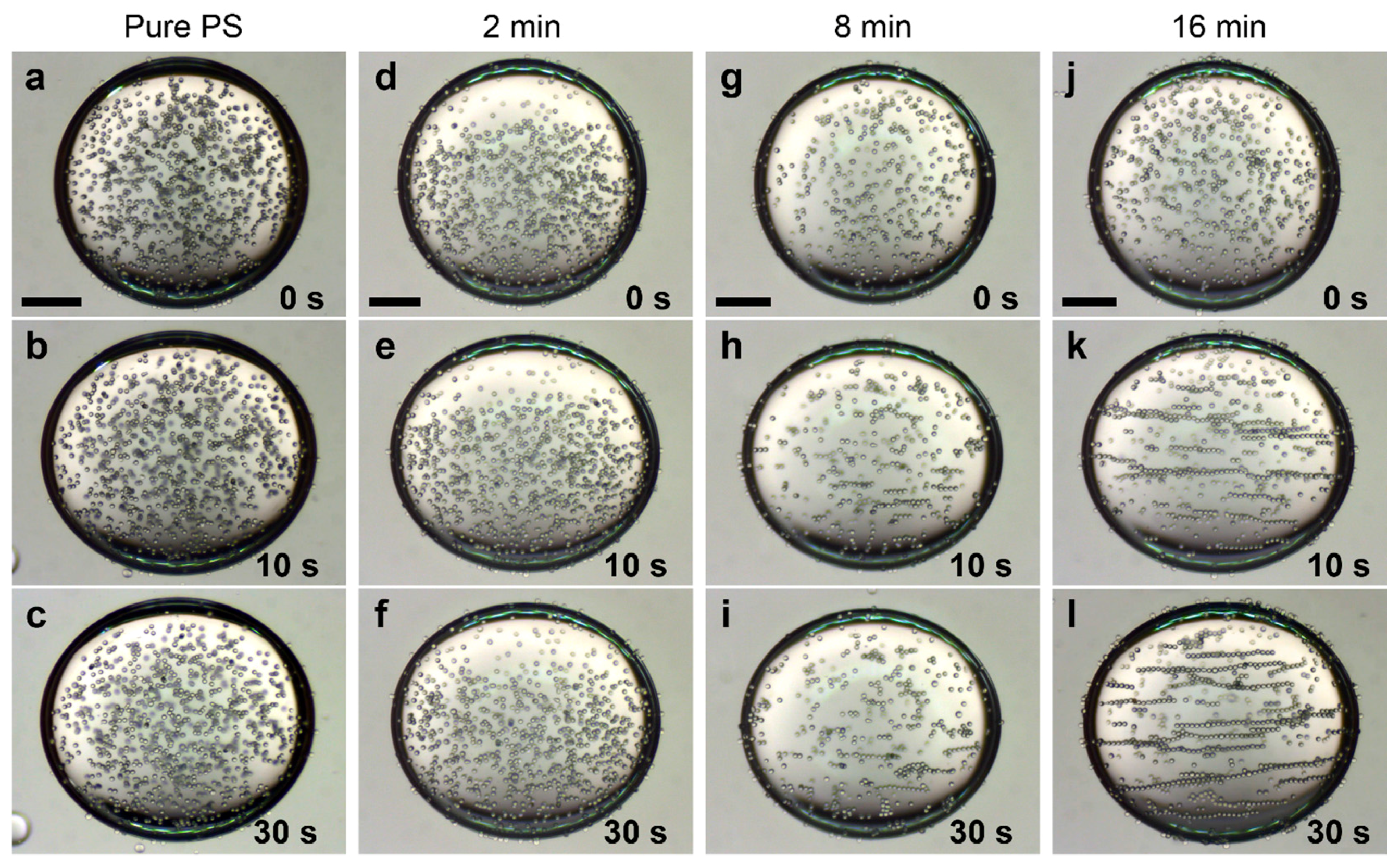
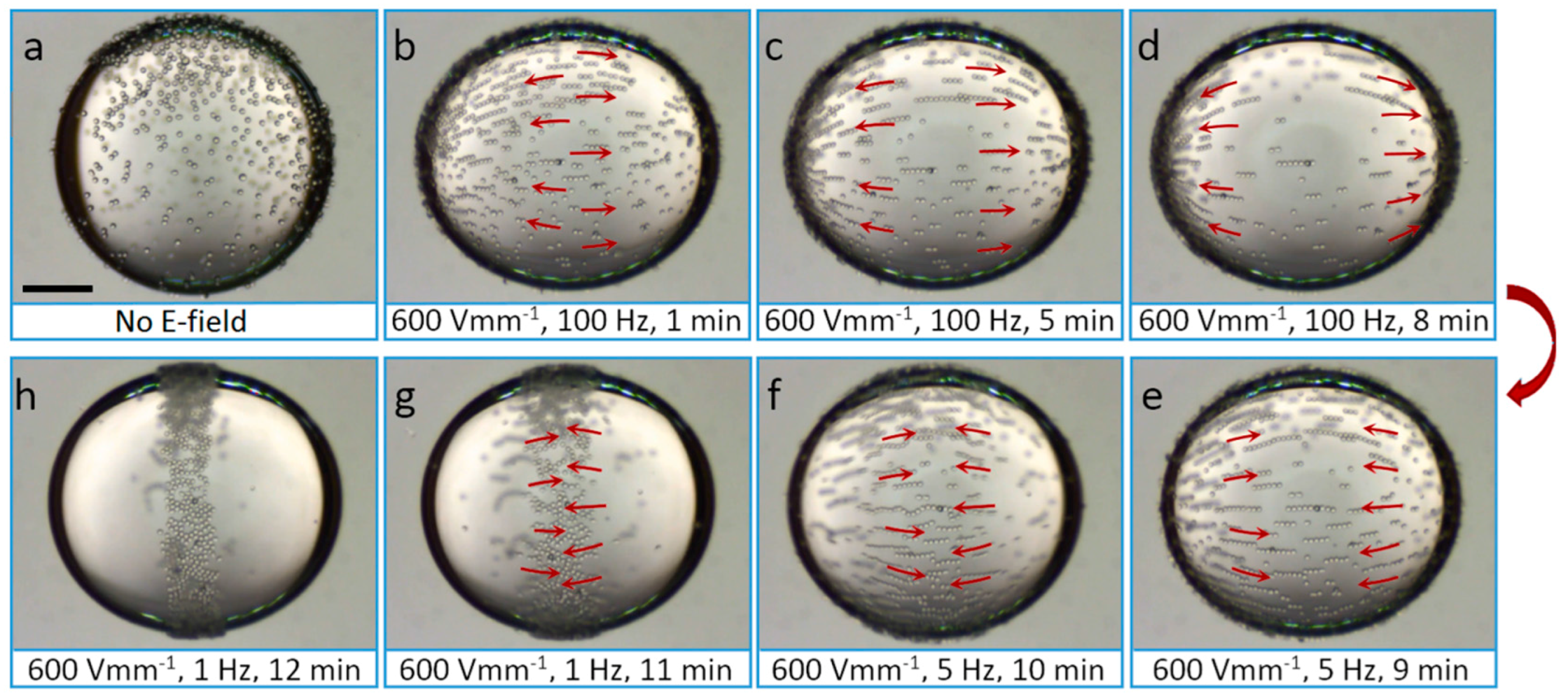
2.5. Electric Field-Driven Particle Assembly
3. Discussion and Conclusions
4. Materials and Methods
4.1. Sulfonation of PS Particles
4.2. FTIR Studies
4.3. Optical Photography and Scanning Electron Microscopy Imaging
4.4. Measurements of Dielectric Constant and Electric Conductivity
4.5. Rheometry
4.6. Experimental Set-Up for Electric Field-Driven Particle Assembly
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brochard-Wyart, F.; Quéré, D.; De Gennes, P.G. Capillarity And Wetting Phenomena: Drops, Bubbles, Pearls, Waves; Springer: New York, NY, USA, 2003. [Google Scholar]
- Dinsmore, A.D.; Hsu, M.F.; Nikolaides, M.G.; Marquez, M.; Bausch, A.R.; Weitz, D.A. Colloidosomes: Selectively Permeable Capsules Composed of Colloidal Particles. Science 2002, 298, 1006–1009. [Google Scholar] [CrossRef] [PubMed]
- Tabeling, P. Introduction to Microfluidics; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Pieranski, P. Two-Dimensional Interfacial Colloidal Crystals. Phys. Rev. Lett. 1980, 45, 569–572. [Google Scholar] [CrossRef]
- Lipowsky, P.; Bowick, M.J.; Meinke, J.H.; Nelson, D.R.; Bausch, A.R. Direct Visualization of Dislocation Dynamics in Grain-Boundary Scars. Nat. Mater. 2005, 4, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Bissig, H.; Dinsmore, A.D. Particles on Droplets: From Fundamental Physics to Novel Materials. Solid State Commun. 2006, 139, 547–556. [Google Scholar] [CrossRef]
- Rozynek, Z.; Mikkelsen, A.; Dommersnes, P.; Fossum, J.O. Electroformation of Janus and Patchy Capsules. Nat. Commun. 2014, 5, 3945. [Google Scholar] [CrossRef] [PubMed]
- Brugarolas, T.; Tu, F.; Lee, D. Directed Assembly of Particles Using Microfluidic Droplets and Bubbles. Soft Matter 2013, 9, 9046–9058. [Google Scholar] [CrossRef]
- Dommersnes, P.; Fossum, J.O. Surface Structuring of Particle Laden Drops Using Electric Fields. Eur. Phys. J. Spec. Top. 2016, 225, 715–728. [Google Scholar] [CrossRef]
- Ashby, N.P.; Binks, B.P. Pickering Emulsions Stabilised by Laponite Clay Particles. Phys. Chem. Chem. Phys. 2000, 2, 5640–5646. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions Stabilised Solely by Colloidal Particles. Adv. Colloid Interface Sci. 2003, 100, 503–546. [Google Scholar] [CrossRef]
- Tang, J.; Quinlan, P.J.; Tam, K.C. Stimuli-Responsive Pickering Emulsions: Recent Advances and Potential Applications. Soft Matter 2015, 11, 3512–3529. [Google Scholar] [CrossRef] [PubMed]
- Frijters, S.; Gunther, F.; Harting, J. Effects of Nanoparticles and Surfactant on Droplets in Shear Flow. Soft Matter 2012, 8, 6542–6556. [Google Scholar] [CrossRef]
- Mei, Y.; Li, G.; Moldenaers, P.; Cardinaels, R. Dynamics of Particle-Covered Droplets in Shear Flow: Unusual Breakup and Deformation Hysteresis. Soft Matter 2016, 12, 9407–9412. [Google Scholar] [CrossRef] [PubMed]
- Bécu, L.; Benyahia, L. Strain-Induced Droplet Retraction Memory in a Pickering Emulsion. Langmuir 2009, 25, 6678–6682. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Studies in Electrohydrodynamics. I. The Circulation Produced in a Drop by Electrical Field. Proc. R. Soc. Lond. A 1966, 291, 159–166. [Google Scholar] [CrossRef]
- Van Blaaderen, A.; Dijkstra, M.; van Roij, R.; Imhof, A.; Kamp, M.; Kwaadgras, B.; Vissers, T.; Liu, B. Manipulating the Self Assembly of Colloids in Electric Fields. Eur. Phys. J. Spec. Top. 2013, 222, 2895–2909. [Google Scholar] [CrossRef]
- Dorvee, J.R.; Derfus, A.M.; Bhatia, S.N.; Sailor, M.J. Manipulation of Liquid Droplets Using Amphiphilic, Magnetic One-Dimensional Photonic Crystal Chaperones. Nat. Mater. 2004, 3, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Singh, P.; Aubry, N. Destabilization of Pickering Emulsions Using External Electric Fields. Electrophoresis 2010, 31, 850–859. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.M.; Emrick, T.; Russell, T.P. Stabilizing Liquid Drops in Nonequilibrium Shapes by the Interfacial Jamming of Nanoparticles. Science 2013, 342, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Amah, E.; Shah, K.; Fischer, I.; Singh, P. Electrohydrodynamic Manipulation of Particles Adsorbed on the Surface of a Drop. Soft Matter 2016, 12, 1663–1673. [Google Scholar] [CrossRef] [PubMed]
- Nudurupati, S.; Janjua, M.; Singh, P.; Aubry, N. Effect of Parameters on Redistribution and Removal of Particles from Drop Surfaces. Soft Matter 2010, 6, 1157–1169. [Google Scholar] [CrossRef]
- Dommersnes, P.; Rozynek, Z.; Mikkelsen, A.; Castberg, R.; Kjerstad, K.; Hersvik, K.; Otto Fossum, J. Active Structuring of Colloidal Armour on Liquid Drops. Nat. Commun. 2013, 4, 2066. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Niu, L.; Xia, Z. Preparation of Raspberry-Like Silica Microcapsules via Sulfonated Polystyrene Template and Aniline Medium Assembly Method. Colloid Polym. Sci. 2014, 292, 3251–3259. [Google Scholar] [CrossRef]
- Fan, X.; Niu, L.; Wu, Y.H.; Cheng, J.; Yang, Z.R. Assembly Route toward Raspberry-Like Composite Particles and Their Controlled Surface Wettability through Varied Dual-Size Binary Roughness. Appl. Surf. Sci. 2015, 332, 393–402. [Google Scholar] [CrossRef]
- Ouriemi, M.; Vlahovska, P.M. Electrohydrodynamics of Particle-Covered Drops. J. Fluid Mech. 2014, 751, 106–120. [Google Scholar] [CrossRef]
- Rozynek, Z.; Dommersnes, P.; Mikkelsen, A.; Michels, L.; Fossum, J.O. Electrohydrodynamic Controlled Assembly and Fracturing of Thin Colloidal Particle Films Confined at Drop Interfaces. Eur. Phys. J. Spec. Top. 2014, 223, 1859–1867. [Google Scholar] [CrossRef]
- Li, M.; Li, D. Redistribution of Charged Aluminum Nanoparticles on Oil Droplets in Water in Response to Applied Electrical Field. J. Nanopart. Res. 2016, 18, 120. [Google Scholar] [CrossRef]
- Nudurupati, S.; Janjua, M.; Aubry, N.; Singh, P. Concentrating Particles on Drop Surfaces Using External Electric Fields. Electrophoresis 2008, 29, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Amah, E.C.; Fischer, I.S.; Singh, P. Transient Electrohydrodynamic Maniupation of Particles on the Surface of a Drop. Am. Soc. Mech. Eng. 2016. [Google Scholar] [CrossRef]
- Aubry, N.; Singh, P. Control of Electrostatic Particle-Particle Interactions in Dielectrophoresis. Europhys. Lett. 2006, 74, 623. [Google Scholar] [CrossRef]
- Nudurupati, S.; Janjua, M.; Singh, P.; Aubry, N. Electrohydrodynamic Removal of Particles from Drop Surfaces. Phys. Rev. E 2009, 80, 4. [Google Scholar] [CrossRef] [PubMed]
- Sherwood, J.D. Breakup of Fluid Droplets in Electric and Magnetic Fields. J. Fluid Mech. 1988, 188, 133–146. [Google Scholar] [CrossRef]
- Sheng, P.; Wen, W. Electrorheological Fluids: Mechanisms, Dynamics, and Microfluidics Applications. Annu. Rev. Fluid Mech. 2012, 44, 143–174. [Google Scholar] [CrossRef]
- Fossum, J.O.; Meheust, Y.; Parmar, K.P.S.; Knudsen, K.D.; Maloy, K.J.; Fonseca, D.M. Intercalation-Enhanced Electric Polarization and Chain Formation of Nano-Layered Particles. Europhys. Lett. 2006, 74, 438–444. [Google Scholar] [CrossRef]
- Wang, B.X.; Zhou, M.; Rozynek, Z.; Fossum, J.O. Electrorheological Properties of Organically Modified Nanolayered Laponite: Influence of Intercalation, Adsorption and Wettability. J. Mater. Chem. 2009, 19, 1816–1828. [Google Scholar] [CrossRef]
- Méheust, Y.; Parmar, K.; Schjelderupsen, B.; Fossum, J. The Electrorheology of Suspensions of Na-Fluorohectorite Clay in Silicone Oil. J. Rheol. 2011, 55, 809–833. [Google Scholar] [CrossRef]
- Wang, L.; Gong, X.; Wen, W. Electrorheological Fluid and Its Applications in Microfluidics. In Microfluidics; Springer: New York, NY, USA, 2011; pp. 91–115. [Google Scholar]
- Madeja, J.; Kesy, Z.; Kesy, A. Application of Electrorheological Fluid in a Hydrodynamic Clutch. Smart Mater. Struct. 2011, 20, 105005. [Google Scholar] [CrossRef]
- Furusho, J.; Sakaguchi, M.; Takesue, N.; Koyanagi, K.I. Development of Er Brake and Its Application to Passive Force Display. J. Intell. Mater. Syst. Struct. 2002, 13, 425–429. [Google Scholar] [CrossRef]
- Petek, N.K.; Romstadt, D.J.; Lizell, M.B.; Weyenberg, T.R. Demonstration of an Automotive Semi-Active Suspension Using Electrorheological Fluid; SAE Technical Paper 950586; SAE INTERNATIONAL: Warrendale, PA, USA, 1995. [Google Scholar] [CrossRef]
- Weiss, R.A.; Sen, A.; Willis, C.L.; Pottick, L.A. Block Copolymer Ionomers. 1. Synthesis and Physical-Properties of Sulfonated Poly(Styrene Ethylene/Butylene Styrene). Polymer 1991, 32, 1867–1874. [Google Scholar] [CrossRef]
- Barreira, S.V.P.; Garcia-Morales, V.; Pereira, C.M.; Manzanares, J.A.; Silva, F. Electrochemical Impedance Spectroscopy of Polyelectrolyte Multilayer Modified Electrodes. J. Phys. Chem. B 2004, 108, 17973–17982. [Google Scholar] [CrossRef]
- Martins, C.R.; Ruggeri, G.; de Paoli, M.-A. Synthesis in Pilot Plant Scale and Physical Properties of Sulfonated Polystyrene. J. Braz. Chem. Soc. 2003, 14, 797–802. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.L.; Nelson, N.C.; Sadow, A.D.; Slowing, I.I.; Overbury, S.H. Role of CO2 as a Soft Oxidant for Dehydrogenation of Ethylbenzene to Styrene over a High-Surface-Area Ceria Catalyst. ACS Catal. 2015, 5, 6426–6435. [Google Scholar] [CrossRef]
- Hazarika, M.; Malkappa, K.; Jana, T. Particle-Size-Dependent Properties of Sulfonated Polystyrene Nanoparticles. Polym. Int. 2012, 61, 1425–1432. [Google Scholar] [CrossRef]
- Siqueira-Petri, D.F.; Wenz, G.; Schunk, P.; Schimmel, T.; Bruns, M.; Dichtl, M.A. Surface Modification of Thin Polystyrene Films. Coll. Polym. Sci. 1999, 277, 673–679. [Google Scholar] [CrossRef]
- Coughlin, J.E.; Reisch, A.; Markarian, M.Z.; Schlenoff, J.B. Sulfonation Of Polystyrene: Toward The “Ideal” Polyelectrolyte. J. Polym. Sci. Pol. Chem. 2013, 51, 2416–2424. [Google Scholar] [CrossRef]
- Bekri-Abbes, I.; Bayoudh, S.; Baklouti, M.; Papon, E.; Leclercq, D. Converting Waste Polystyrene into Adsorbent: Optimisation of Reaction Parameters and Properties. Prog. Rubber Plast. Recycl. Technol. 2006, 22, 179–193. [Google Scholar]
- Kucera, F.; Jancar, J. Preliminary Study of Sulfonation of Polystyrene by Homogeneous and Heterogeneous Reaction. Chem. Pap. 1996, 50, 224–227. [Google Scholar]
- Benavides, R.; Oenning, L.W.; Paula, M.M.S.; Da Silva, L. Properties of Polystyrene/Acrylic Acid Membranes after Sulphonation Reactions. J. New Mater. Electrochem. Syst. 2014, 17, 85–90. [Google Scholar]
- Wallace, R.A. Electrical-Conduction in Sulfonated Polystyrene Films. J. Appl. Polym. Sci. 1973, 17, 231–238. [Google Scholar] [CrossRef]
- Wallace, R.A. Dielectric Behavior of Sulfonic Acid Polystyrene Films. J. Appl. Polym. Sci. 1973, 17, 223–230. [Google Scholar] [CrossRef]
- Barnes, H.A. The Yield Stress—A Review Or ’Pi Alpha Nu Tau Alpha Rho Epsilon Iota’—Everything Flows? J. Non-Newton. Fluid 1999, 81, 133–178. [Google Scholar] [CrossRef]
- Hao, T. Electrorheological Suspensions. Adv. Colloid Interface Sci. 2002, 97, 1–35. [Google Scholar] [PubMed]
- Rozynek, Z.; Wang, B.; Fossum, J.O.; Knudsen, K.D. Dipolar Structuring of Organically Modified Fluorohectorite Clay Particles. Eur. Phys. J. E 2012, 35, 9. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.C. Polarization Forces and Conductivity Effects in Electrorheological Fluids. J. Appl. Phys. 1992, 72, 1334–1340. [Google Scholar] [CrossRef]
- Pan, X.D.; Mckinley, G.H. Characteristics of Electrorheological Responses in an Emulsion System. J. Colloid Interface Sci. 1997, 195, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Saintillan, D. A Nonlinear Small-Deformation Theory for Transient Droplet Electrohydrodynamics. J. Fluid Mech. 2017, 810, 225–253. [Google Scholar] [CrossRef]
- Lanauze, J.A.; Walker, L.M.; Khair, A.S. Nonlinear Electrohydrodynamics of Slightly Deformed Oblate Drops. J. Fluid Mech. 2015, 774, 245–266. [Google Scholar] [CrossRef]
- Vlahovska, P.M. Electrohydrodynamic Instabilities of Viscous Drops. Phys. Rev. Fluids 2016, 1, 060504. [Google Scholar] [CrossRef]
- Rozynek, Z.; Kaczmarek-Klinowska, M.; Magdziarz, A. Assembly and Rearrangement of Particles Confined at a Surface of a Droplet, and Intruder Motion in Electro-Shaken Particle Films. Materials 2016, 9, 679. [Google Scholar] [CrossRef]
- Ouriemi, M.; Vlahovska, P.M. Electrohydrodynamic Deformation and Rotation of a Particle-Coated Drop. Langmuir 2015, 31, 6298–6305. [Google Scholar] [PubMed]
- Salipante, P.F.; Vlahovska, P.M. Electrohydrodynamics of Drops in Strong Uniform Dc Electric Fields. Phys. Fluids 2010, 22. [Google Scholar] [CrossRef]
- Lu, K.; Wen, W.; Li, C.; Xie, S. Frequency Dependence of Electrorheological Fluids in an Ac Electric Field. Phys. Rev. E 1995, 52, 6329–6332. [Google Scholar] [CrossRef]




| Reaction Time (min) | 0 | 2 | 4 | 8 | 16 | 32 | 64 |
|---|---|---|---|---|---|---|---|
| Transmittance Ratio | 1 | 1 | 1 | 1.001 | 1.002 | 1.007 | 1.009 |
| Reaction Time (min) | 0 | 2 | 4 | 8 | 16 | 32 |
|---|---|---|---|---|---|---|
| Dielectric constant, ε | 1.5 | 1.4 | 2.2 | 4.5 | 8.1 | 19.5 |
| Dielectric constant, ε (lyophilized) | 1.3 | 1.3 | 1.4 | 1.4 | 1.8 | 3.0 |
| Electrical conductivity, σ (nS/m) | 0.08 | 3.9 | 9.3 | 31.0 | 95.9 | 107.5 |
| Electrical conductivity, σ (nS/m) (lyophilized) | 0.08 | 0.8 | 1.6 | 1.6 | 8.5 | 30.1 |
| Silicone Oil Dispersions with: | E (kV/mm) | 0.5 | 1 | 2 | 4 | 6 | 8 |
|---|---|---|---|---|---|---|---|
| pure PS particles | τ (Pa) | ~0.02 | ~0.02 | ~0.04 | ~0.07 | ~0.10 | ~0.19 |
| PS particles sulfonated for 2 min | τ (Pa) | ~0.03 | ~0.03 | ~0.03 | ~0.08 | ~0.11 | ~0.18 |
| PS particles sulfonated for 4 min | τ (Pa) | ~0.02 | ~0.05 | ~0.08 | ~0.40 | ~1.00 | ~2.00 |
| PS particles sulfonated for 8 min | τ (Pa) | ~0.01 | ~0.13 | ~1.00 | ~2.00 | ~4.00 | ~5.50 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikkelsen, A.; Wojciechowski, J.; Rajňák, M.; Kurimský, J.; Khobaib, K.; Kertmen, A.; Rozynek, Z. Electric Field-Driven Assembly of Sulfonated Polystyrene Microspheres. Materials 2017, 10, 329. https://doi.org/10.3390/ma10040329
Mikkelsen A, Wojciechowski J, Rajňák M, Kurimský J, Khobaib K, Kertmen A, Rozynek Z. Electric Field-Driven Assembly of Sulfonated Polystyrene Microspheres. Materials. 2017; 10(4):329. https://doi.org/10.3390/ma10040329
Chicago/Turabian StyleMikkelsen, Alexander, Jarosław Wojciechowski, Michal Rajňák, Juraj Kurimský, Khobaib Khobaib, Ahmet Kertmen, and Zbigniew Rozynek. 2017. "Electric Field-Driven Assembly of Sulfonated Polystyrene Microspheres" Materials 10, no. 4: 329. https://doi.org/10.3390/ma10040329
APA StyleMikkelsen, A., Wojciechowski, J., Rajňák, M., Kurimský, J., Khobaib, K., Kertmen, A., & Rozynek, Z. (2017). Electric Field-Driven Assembly of Sulfonated Polystyrene Microspheres. Materials, 10(4), 329. https://doi.org/10.3390/ma10040329







