Effect of Immobilized Antithrombin III on the Thromboresistance of Polycarbonate Urethane
Abstract
:1. Introduction
2. Methods
2.1. Production of PCU Foils and Spiral PCU Grafts
2.2. Surface Modifications
2.3. Elasticity and Wettability of Surface Modifications
2.4. Bacterial Adhesion
2.5. Platelet Adhesion—Hemocompatibility
2.6. Chandler-Loop Experiments
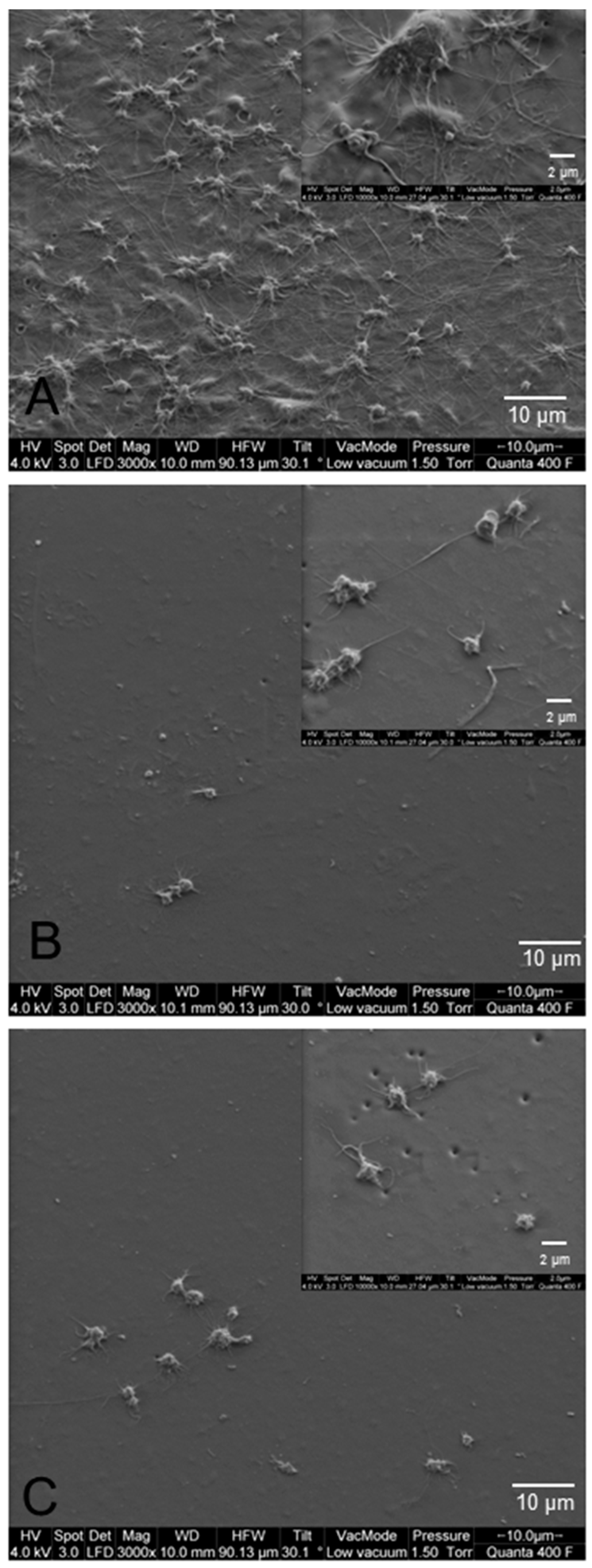
2.7. Scanning Electron Microscopy (SEM)
2.8. Statistics
3. Results
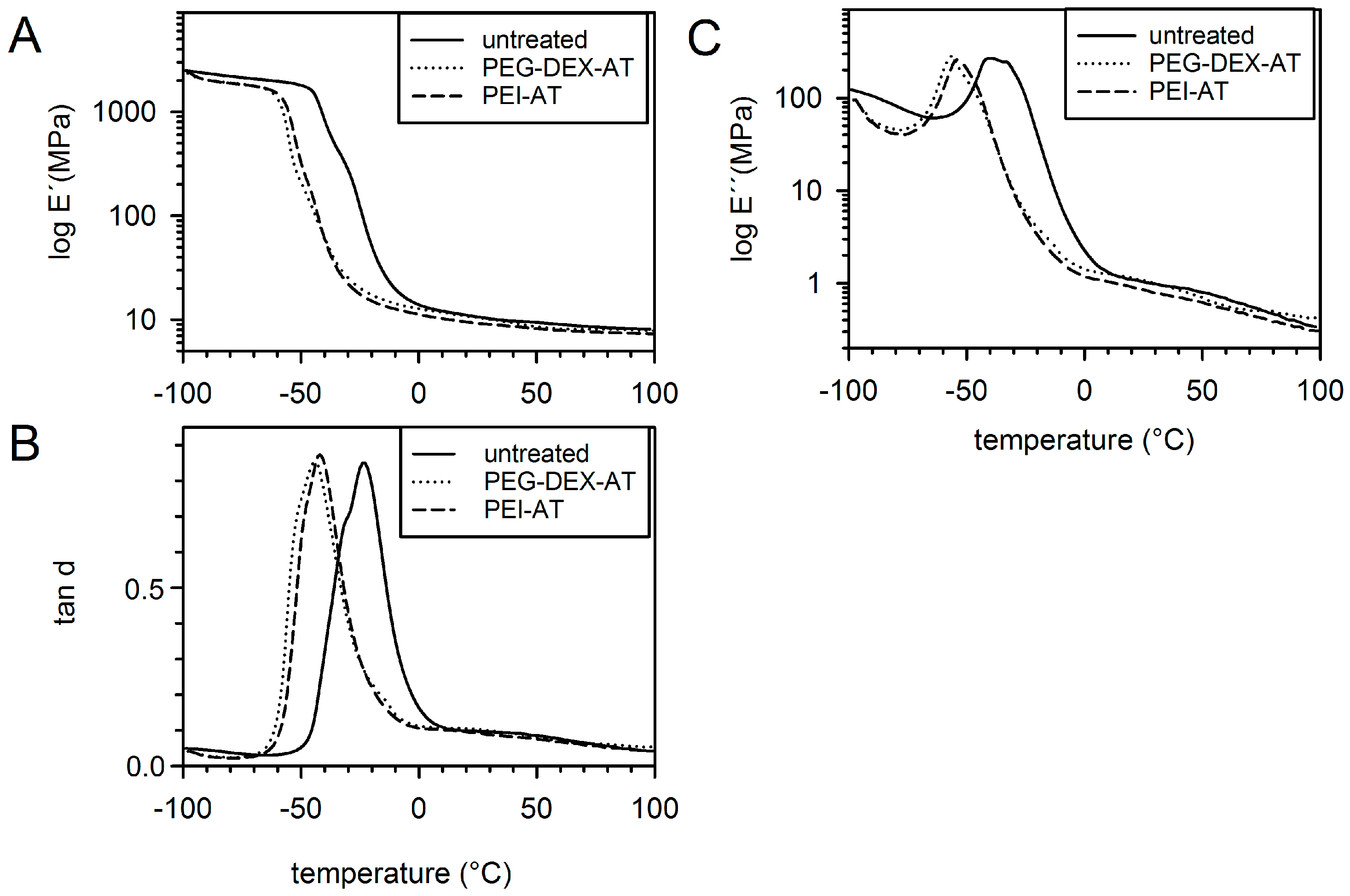
3.1. Mechanical Properties
3.2. Wettability
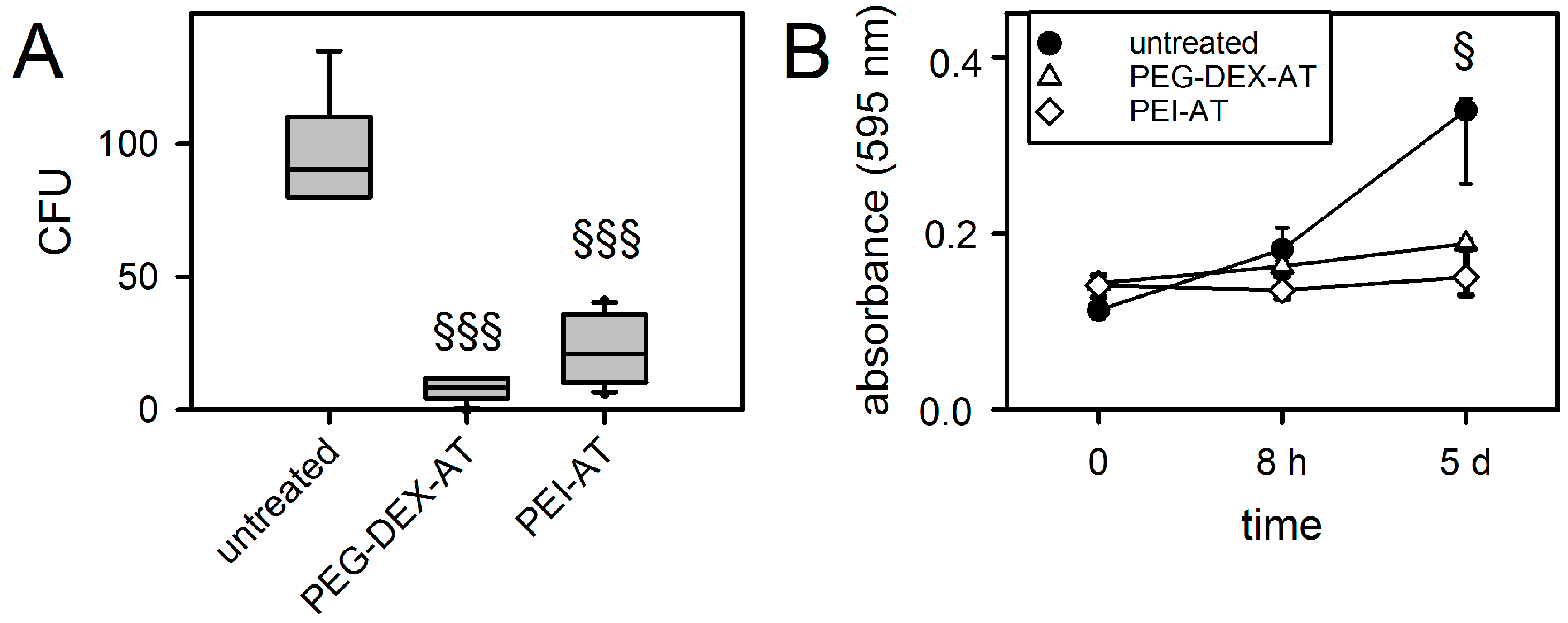
3.3. Biofilm Formation on Modified PCU
3.4. Hemocompatibility under Static Culture Conditions
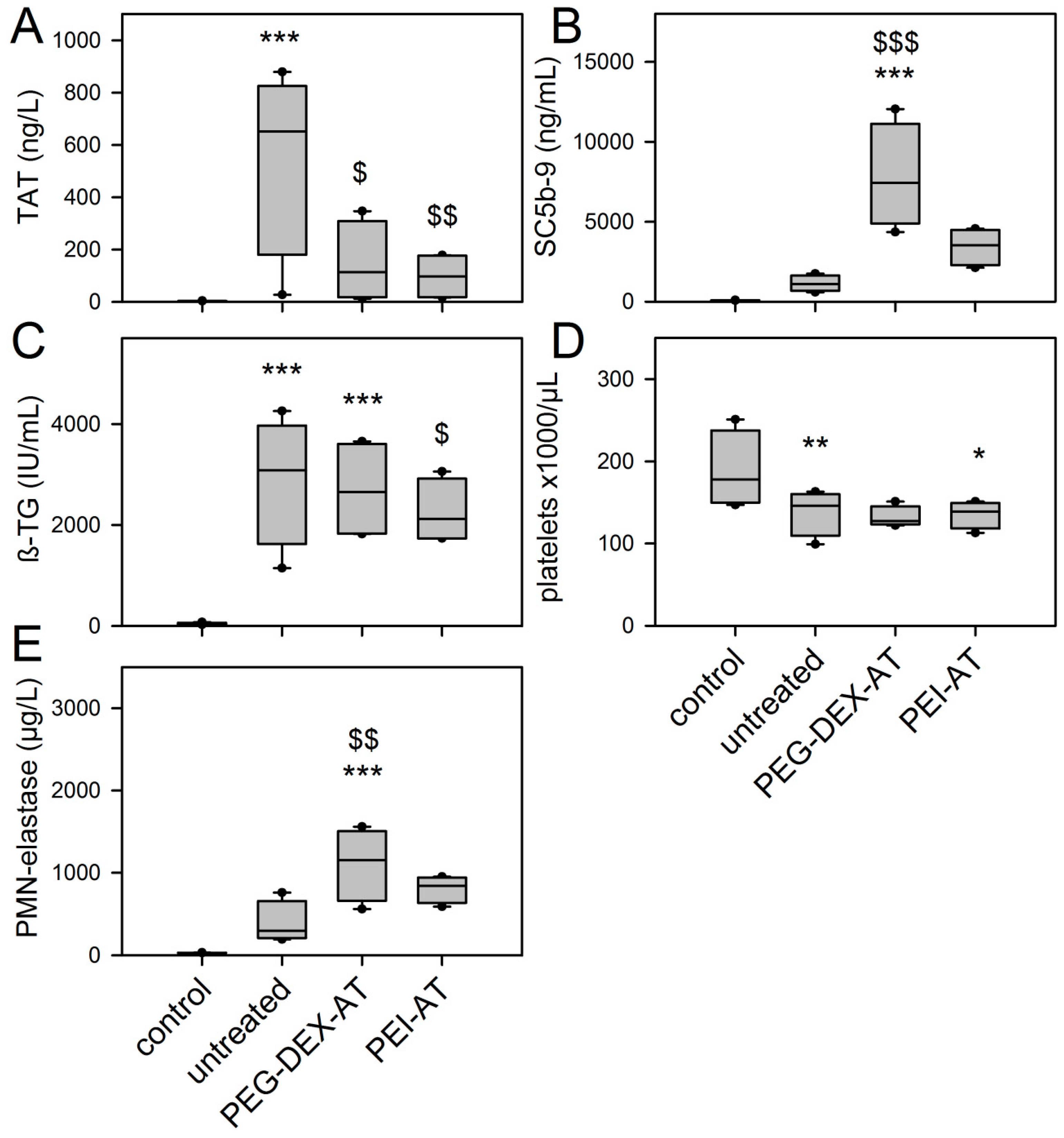
3.5. Chandler-Loop Experiments
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Funding
References
- Reed, A.M.; Potter, J.; Szycher, M. A solution grade biostable polyurethane elastomer: ChronoFlex® AR. J. Biomater. Appl. 1994, 8, 210–236. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, M.C.; Marois, Y.; Roy, R.; Mehri, Y.; Wagner, E.; Zhang, Z.; King, M.W.; Yang, M.; Hahn, C.; Guidoin, R. Selection of a polyurethane membrane for the manufacture of ventricles for a totally implantable artificial heart: Blood compatibility and biocompatibility studies. Artif. Organs 2000, 24, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Chandy, T.; Van Hee, J.; Nettekoven, W.; Johnson, J. Long-term in vitro stability assessment of polycarbonate urethane micro catheters: Resistance to oxidation and stress cracking. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 89, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Arjun, G.N.; Ramesh, P. Structural characterization, mechanical properties, and in vitro cytocompatibility evaluation of fibrous polycarbonate urethane membranes for biomedical applications. J. Biomed. Mater. Res. A 2012, 100, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Wang, Y.; Zhang, Z.; Ma, D.; Wang, X. Synthesis of polycarbonate urethane elastomers and effects of the chemical structures on their thermal, mechanical and biocompatibility properties. Heliyon 2016, 2, e00125. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Y.; Kuo, J.F.; Chen, C.Y. Synthesis, characterization and platelet adhesion studies of novel ion-containing aliphatic polyurethanes. Biomaterials 2000, 21, 161–171. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Giordano, C.; Macchi, P.; Malpezzi, L.; Pedrocchi-Fantoni, G.; Servi, S. Synthesis, platelet adhesion and cytotoxicity studies of new glycerophosphoryl-containing polyurethanes. Int. J. Artif. Organs 2007, 30, 133–143. [Google Scholar] [PubMed]
- Eckman, P.M.; John, R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012, 125, 3038–3047. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.; Mozetic, M.; Stana-Kleinschek, K.; Fröhlich, M.; Turk, B.; Vesel, A. Covalent binding of heparin to functionalized PET materials for improved haemocompatibility. Materials 2015, 8, 1526–1544. [Google Scholar] [CrossRef]
- Michanetzis, G.P.; Katsala, N.; Missirlis, Y.F. Comparison of haemocompatibility improvement of four polymeric biomaterials by two heparinization techniques. Biomaterials 2003, 24, 677–688. [Google Scholar] [CrossRef]
- Salacinski, H.J.; Hamilton, G.; Seifalian, A.M. Surface functionalization and grafting of heparin and/or RGD by an aqueous-based process to a poly(carbonate-urea)urethane cardiovascular graft for cellular engineering applications. J. Biomed. Mater. Res. A 2003, 66, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Gerlitz, B.; Grinnell, B.W.; Meyerhoff, M.E. Polymeric coatings that mimic the endothelium: Combining nitric oxide release with surface-bound active thrombomodulin and heparin. Biomaterials 2007, 28, 4047–4055. [Google Scholar] [CrossRef] [PubMed]
- Butruk-Raszeja, B.; Trzaskowski, M.; Ciach, T. Cell membrane-mimicking coating for blood-contacting polyurethanes. J. Biomater. Appl. 2015, 29, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Major, T.C.; Brisbois, E.J.; Jones, A.M.; Zanetti, M.E.; Annich, G.M.; Bartlett, R.H.; Handa, H. The effect of a polyurethane coating incorporating both a thrombin inhibitor and nitric oxide on hemocompatibility in extracorporeal circulation. Biomaterials 2014, 35, 7271–7285. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Brisbois, E.; Handa, H.; Annich, G.; Meyerhoff, M.; Bartlett, R.; Major, T. The immobilization of a direct thrombin inhibitor to a polyurethane as a nonthrombogenic surface coating for extracorporeal circulation. J. Mater. Chem. B Mater. Biol. Med. 2016, 4, 2264–2272. [Google Scholar] [CrossRef] [PubMed]
- Ranucci, M.; Frigiola, A.; Menicanti, L.; Ditta, A.; Boncilli, A.; Brozzi, S. Postoperative antithrombin levels and outcome in cardiac operations. Crit. Care Med. 2005, 33, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Stiller, B.; Lemmer, J.; Merkle, F.; Alexi-Meskishvili, V.; Wenig, Y.; Hübler, M.; Koster, A.; Drews, T.; Lange, P.E.; Hetzer, R. Consumption of blood products during mechanical circulatory support in children: Comparison between ECMO and a pulsatile ventricular assist device. Intensive Care Med. 2004, 30, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Chryssos, A.; Stroup, S.J.; Pifer, M.M.; Tawil, M.; Conrad, C.G. Management of Heparin-Resistant Patients with Benefits? Maximizing Biocompatibility in Cardiopulmonary Bypass: Combining ATryn® Recombinant Antithrombin III and Carmeda® Heparin-Bonded Perfusion Circuits: A Case Series. J. Extra Corpor. Technol. 2015, 47, 44–47. [Google Scholar] [PubMed]
- Sask, K.N.; Berry, L.R.; Chan, A.K.; Brash, J.L. Polyurethane modified with an antithrombin-heparin complex via polyethylene oxide linker/spacers: Influence of PEO molecular weight and PEO-ATH bond on catalytic and direct anticoagulant functions. J. Biomed. Mater. Res. A 2012, 100, 2821–2828. [Google Scholar] [CrossRef] [PubMed]
- Lukas, K.; Thomas, U.; Gessner, A.; Wehner, D.; Schmid, T.; Schmid, C.; Lehle, K. Plasma functionalization of polycarbonaturethane to improve endothelialization—Effect of shear stress as a critical factor for biocompatibility control. J. Biomater. Appl. 2016, 30, 1417–1428. [Google Scholar] [CrossRef] [PubMed]
- Sinn, S.; Scheuermann, T.; Deichelbohrer, S.; Ziemer, G.; Wendel, H.P. A novel in vitro model for preclinical testing of the hemocompatibility of intravascular stents according to ISO 10993-4. J. Mater. Sci. Mater. Med. 2011, 22, 1521–1528. [Google Scholar] [CrossRef] [PubMed]
- Moffat, K.L.; Marra, K.G. Biodegradable poly(ethylene glycol) hydrogels crosslinked with genipin for tissue engineering applications. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 181–187. [Google Scholar] [CrossRef] [PubMed]
- McArthur, S.L.; McLean, K.M.; Kingshott, P.; St John, H.A.W.; Catelier, R.C.; Griesser, H.J. Effect of polysaccharide structure on protein adsorption. Colloids Surf. B Biointerfaces 2000, 17, 37–48. [Google Scholar] [CrossRef]
- Lehle, K.; Buttstaedt, J.; Birnbaum, D.E. Expression of adhesion molecules and cytokines in vitro by endothelial cells seeded on various polymer surfaces coated with titaniumcarboxonitride. J. Biomed. Mater. Res. A 2003, 65, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus epidermidis—The ‘accidental’ pathogen. Nat. Rev. Microbiol. 2009, 7, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Wendel, H.P.; Hauser, N.; Briquet, F.; Ziemer, G. Hemocompatibility of medical connectors with biopassive or bioactive surface coatings. J. Biomater. Appl. 2002, 17, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Lamba, N.; Woodhouse, K.; Cooper, S. Polyurethanes in Biomedical Applications; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Christenson, E.M.; Dadsetan, M.; Wiggins, M.; Anderson, J.M.; Hiltner, A. Poly(carbonate urethane) and poly(ether urethane) biodegradation: In vivo studies. J. Biomed. Mater. Res. A 2004, 69, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Park, K.D.; Kim, Y.S.; Han, D.K.; Kim, Y.H.; Lee, E.H.; Suh, H.; Choi, K.S. Bacterial adhesion on PEG modified polyurethane surfaces. Biomaterials 1998, 19, 851–859. [Google Scholar] [CrossRef]
- Schaer, T.P.; Stewart, S.; Hsu, B.B.; Klibanov, A.M. Hydrophobic polycationic coatings that inhibit biofilms and support bone healing during infection. Biomaterials 2012, 33, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 2013, 34, 8533–8554. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Wang, J.; Klibanov, A.M. One-step, painting-like coating procedures to make surfaces highly and permanently bactericidal. Biotechnol. Prog. 2006, 22, 584–589. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, F.L.; Kwa, L.J.; Porapakkham, P.; Rajadurai, S.; Jones, K.; van de Merwe, J.; Billah, B.; Porapakkham, P.; Esmore, D.S.; Halvorsen, D.S.; et al. Bacteraemia in ventricular assist devices: A common complication that need not affect clinical outcomes. Heart. Lung. Circ. 2014, 23, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Lin, S.; Shen, J. Enhanced blood compatibility of polyurethane functionalized with sulfobetaine. Colloids Surf. B Biointerfaces 2008, 66, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Coll Ferrer, M.C.; Eckmann, U.N.; Composto, R.J.; Eckmann, D.M. Hemocompatibility and biocompatibility of antibacterial biomimetic hybrid films. Toxicol. Appl. Pharmacol. 2013, 272, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Liu, L.S.; Matsuo, R.; Imanishi, Y. Synthesis and nonthrombogenicity of polymer membrane with surface-graft polymers carrying thrombin inhibitor. J. Biomed. Mater. Res. 1992, 26, 1065–1080. [Google Scholar] [CrossRef] [PubMed]
- Christensen, K.; Larsson, R.; Emanuelsson, H.; Elgue, G.; Larsson, A. Coagulation and complement activation. Biomaterials 2001, 22, 349–355. [Google Scholar] [CrossRef]
- Tepe, G.; Wendel, H.P.; Khorchidi, S.; Schmehl, J.; Wiskirchen, J.; Pusich, B.; Claussen, C.D.; Duda, S.H. Thrombogenicity of various endovascular stent types: An in vitro evaluation. J. Vasc. Interv. Radiol. 2002, 13, 1029–1035. [Google Scholar] [CrossRef]
- Touma, H.; Sahin, I.; Gaamangwe, T.; Gorbet, M.B.; Peterson, S.D. Numerical investigation of fluid flow in a chandler loop. J. Biomech. Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hathcock, J.J. Flow effects on coagulation and thrombosis. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1729–1737. [Google Scholar] [CrossRef] [PubMed]
- Stang, K.; Krajewski, S.; Neumann, B.; Kurz, J.; Post, M.; Stoppelkamp, S.; Fennrich, S.; Avci-Adali, M.; Armbruster, D.; Schlensak, C.; et al. Hemocompatibility testing according to ISO 10993-4: Discrimination between pyrogen- and device-induced hemostatic activation. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 422–428. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukas, K.; Stadtherr, K.; Gessner, A.; Wehner, D.; Schmid, T.; Wendel, H.P.; Schmid, C.; Lehle, K. Effect of Immobilized Antithrombin III on the Thromboresistance of Polycarbonate Urethane. Materials 2017, 10, 335. https://doi.org/10.3390/ma10040335
Lukas K, Stadtherr K, Gessner A, Wehner D, Schmid T, Wendel HP, Schmid C, Lehle K. Effect of Immobilized Antithrombin III on the Thromboresistance of Polycarbonate Urethane. Materials. 2017; 10(4):335. https://doi.org/10.3390/ma10040335
Chicago/Turabian StyleLukas, Karin, Karin Stadtherr, Andre Gessner, Daniel Wehner, Thomas Schmid, Hans Peter Wendel, Christof Schmid, and Karla Lehle. 2017. "Effect of Immobilized Antithrombin III on the Thromboresistance of Polycarbonate Urethane" Materials 10, no. 4: 335. https://doi.org/10.3390/ma10040335




