The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes
Abstract
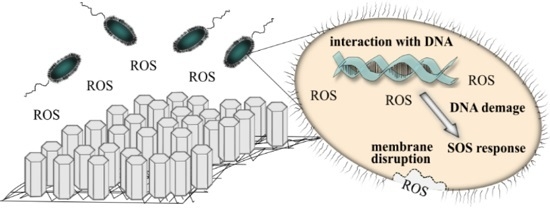
:1. Introduction
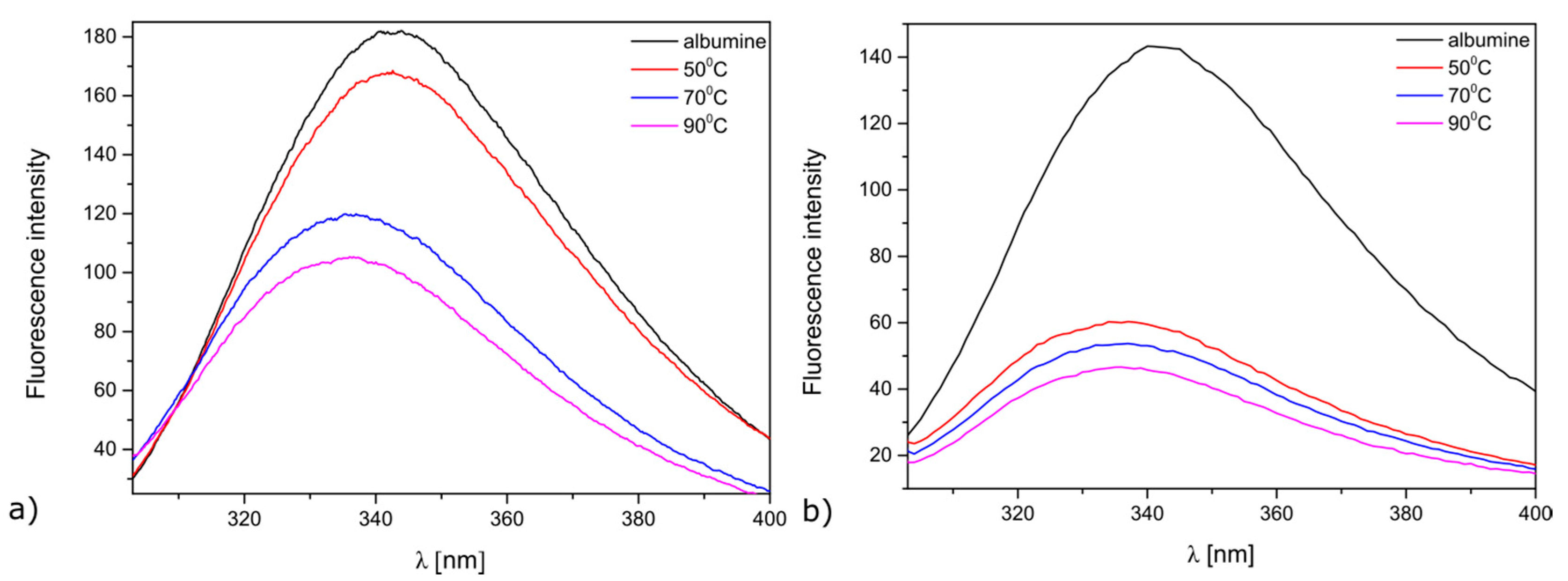
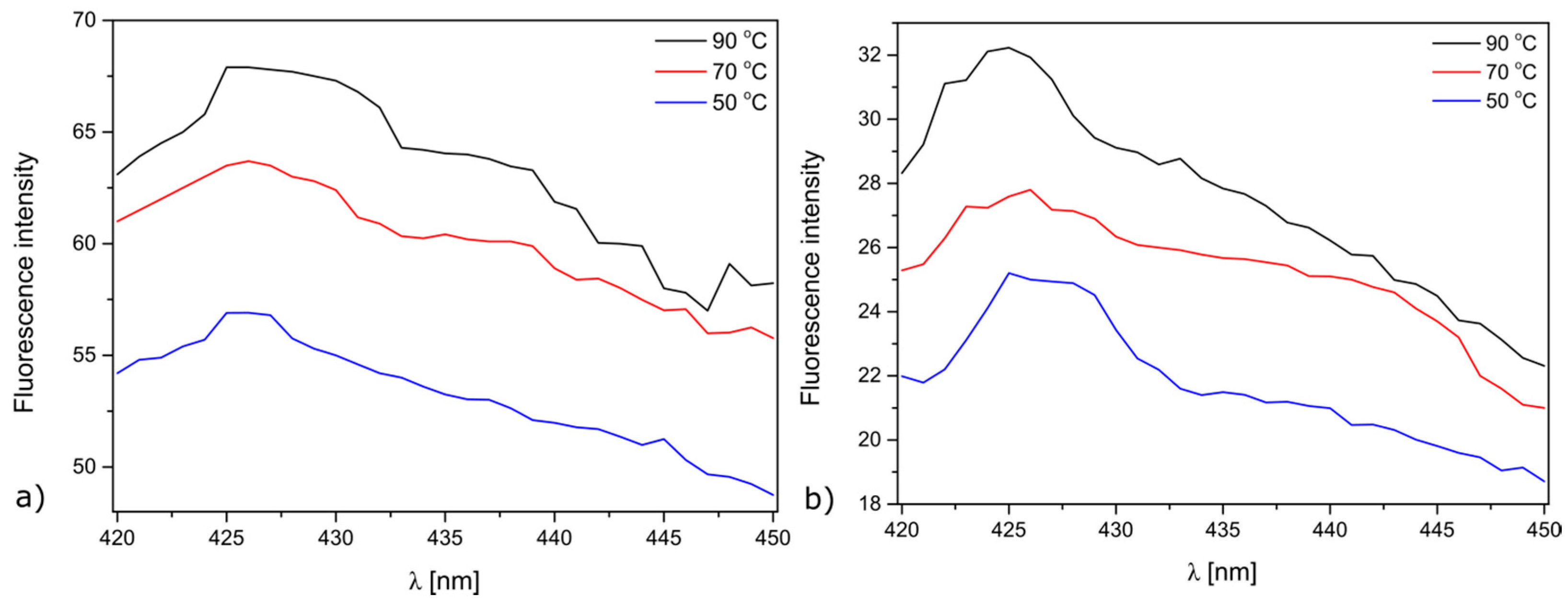
2. Results and Discussion
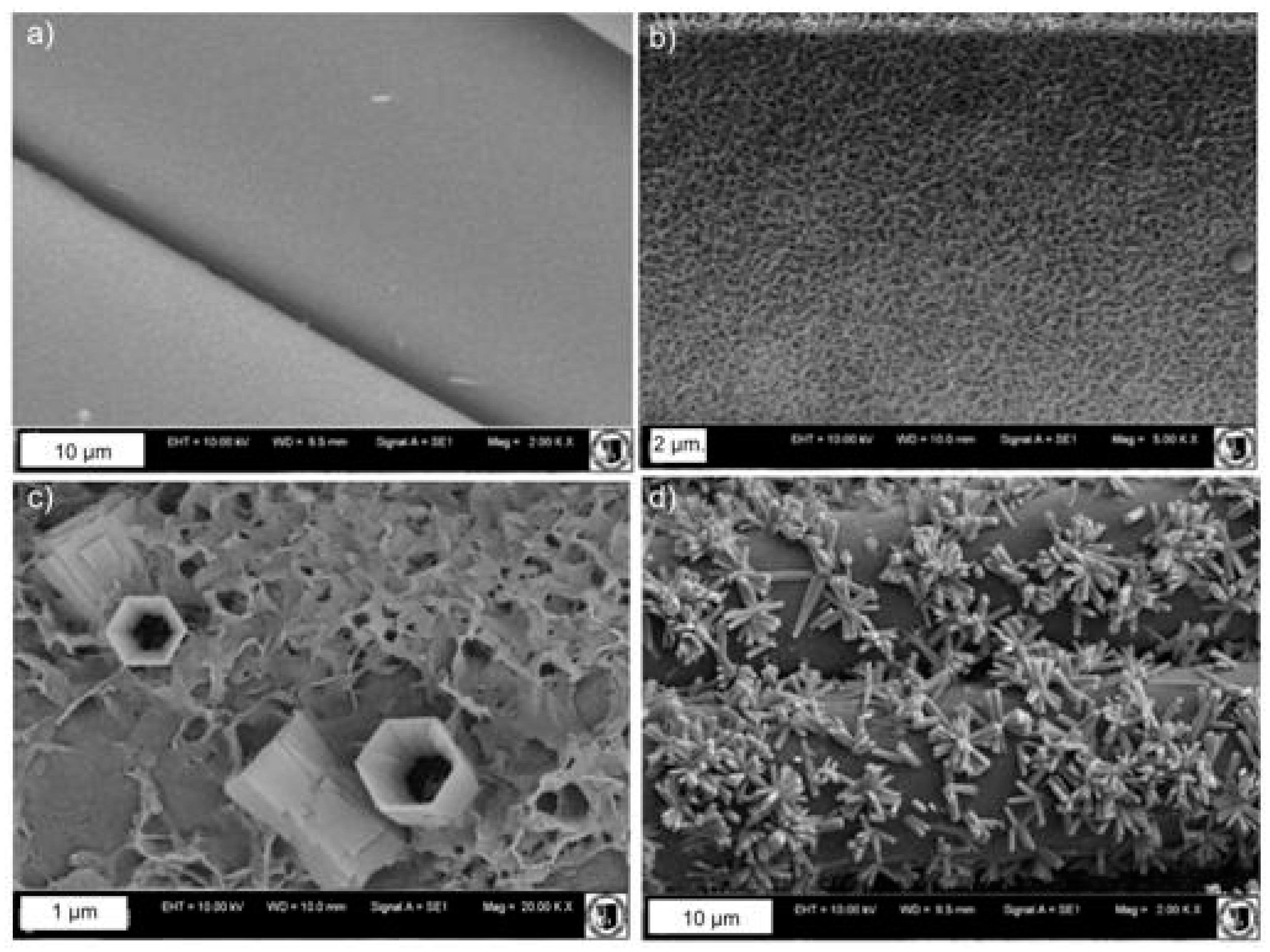
2.1. Surface Modification of Mersilene™ Meshes
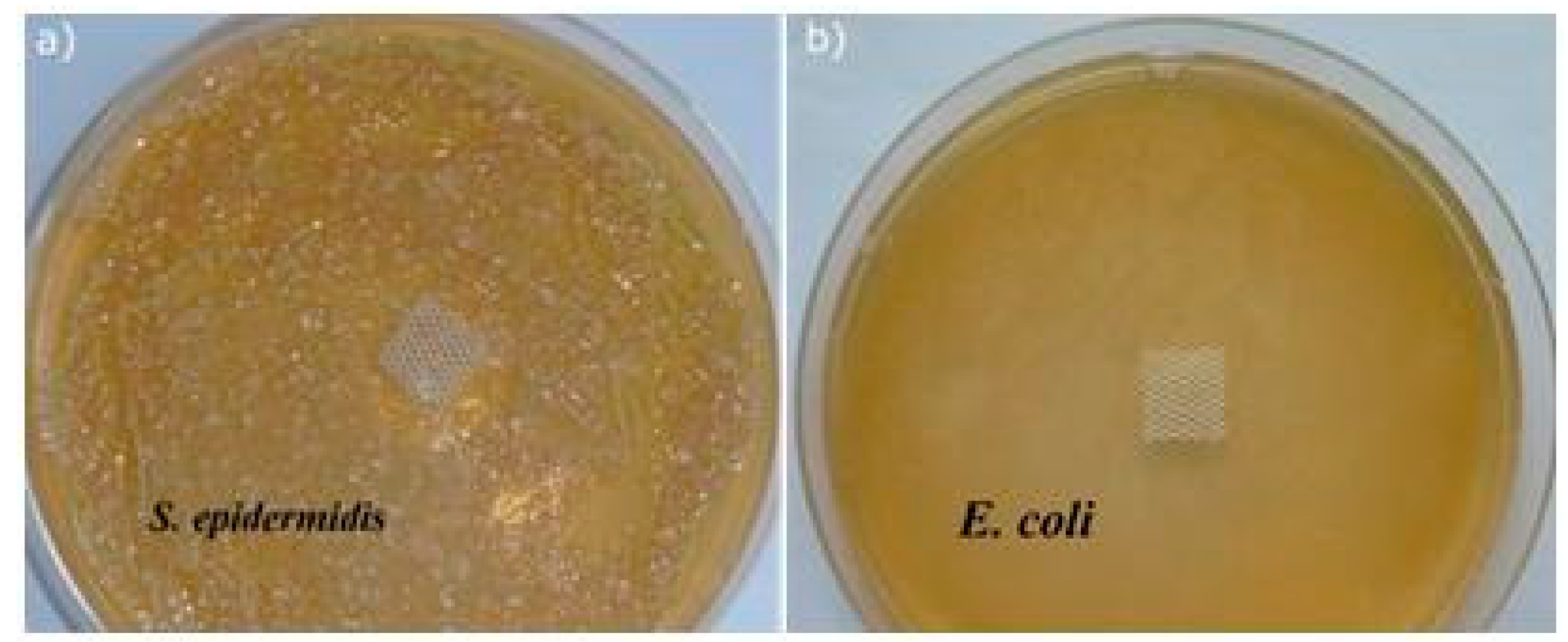
2.2. Antimicrobial Properties of the Modified MersileneTM Meshes
3. Experimental Section
3.1. Structural Characterization
3.2. Determination of Antimicrobial Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Insua, C.M.; Pereira, J.C.; de Oliveira, M.C. Obturator Hernia: The Plug Technique. Hernia 2001, 5, 161–163. [Google Scholar] [CrossRef]
- Horan, R.L.; Bramono, D.S.; Stanley, J.R.L.; Simmons, Q.; Chen, J.; Boepple, H.E.; Altman, G.H. Biological and Biomechanical Assessment of a Long-Term Bioresorbable Silk-Derived Surgical Mesh in an Abdominal Body Wall Defect Model. Hernia 2009, 13, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Junge, K.; Rosch, R.; Anurov, M.; Titkova, S.; Öttinger, A.; Klinge, U.; Schumpelick, V. Modification of collagen formation using supplemented mesh materials. Hernia 2006, 10, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial Properties of Chitosan and Mode of Action: A State of the Art Reviewing. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Prema, P.; Iniya, P.A.; Immanuel, G. Microbial Mediated Synthesis, Characterization, Antibacterial and Synergistic Effect of Gold Nanoparticles Using Klebsiella Pneumoniae (MTCC-4030). RSC Adv. 2016, 6, 4601–4607. [Google Scholar] [CrossRef]
- Varaprasad, K.; Raghavendra, G.M.; Jayaramudu, T.; Seo, J. Nano Zinc Oxide–Sodium Alginate Antibacterial Cellulose Fibres. Carbohydr. Polym. 2016, 135, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Taranath, T.C.; Patil, B.N. Limonia acidissima L. Leaf Mediated Synthesis of Zinc Oxide Nanoparticles: A Potent Tool against Mycobacterium Tuberculosis. Int. J. Mycobacterial. 2016, 5, 197–204. [Google Scholar]
- Yu, J.; Yu, X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Fiedot, M.; Rac, O.; Suchorska-Woźniak, P.; Karbownik, I.; Teterycz, H. Polymer-surfactant interactions and their influence on zinc oxide nanoparticles morphology. In Manufacturing Nanostructures; One Central Press (OCP): Manchester, UK, 2014; pp. 108–128. [Google Scholar]
- Hambali, N.A.; Yahaya, H.; Mahmood, M.R.; Terasako, T.; Hashim, A.M. Synthesis of Zinc Oxide Nanostructures on Graphene/Glass Substrate by Electrochemical Deposition: Effects of Current Density and Temperature. Nanoscale Res. Lett. 2014, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.H.; Park, I.; Pan, H.; Misra, N.; Rogers, M.S.; Grigoropoulos, C. P.; Pisano, A.P. ZnO nanowire network transistor fabrication on a polymer substrate by low-temperature, all-inorganic nanoparticle solution process. Appl. Phys. Lett. 2008, 92, 154102. [Google Scholar] [CrossRef]
- Fiedot, M.; Karbownik, I.; Maliszewska, I.; Rac, O.; Suchorska-Woźniak, P.; Teterycz, H. Deposition of One-Dimensional Zinc Oxide Structures on Polypropylene Fabrics and Their Antibacterial Properties. Text. Res. J. 2015, 85, 1340–1354. [Google Scholar] [CrossRef]
- Guo, M.Y.; Ng, A.M.C.; Liu, F.; Djurisic, A.B.; Chan, W.K.; Su, H.; Wong, K.S. Effect of native defects on photocatalytic properties of ZnO. J. Phys. Chem. C 2011, 115, 11095–11101. [Google Scholar] [CrossRef]
- Suchorska-Woźniak, P.; Rac, O.; Klimkiewicz, R.; Fiedot, M.; Teterycz, H. Dehydrogenation properties of ZnO and the impact of gold nanoparticles on the process. Appl. Catal. A 2016, 514, 135–145. [Google Scholar] [CrossRef]
- Richardson, J.J.; Lange, F.F. Controlling Low Temperature Aqueous Synthesis of ZnO. 1. Thermodynamic Analysis. Cryst. Growth Des. 2009, 9, 2570–2575. [Google Scholar] [CrossRef]
- Fiedot, M.; Teterycz, H.; Halek, P.; Malewicz, M. Effect of acidic environment on the shape and size of the 1D nano-and microstructures of zinc oxide. In Proceedings of the 2010 IEEE International Students and Young Scientists Workshop, Szklarska Poreba, Poland, 25–27 June 2010; pp. 52–55. [Google Scholar]
- Zhang, W.; Wang, P.; Fei, X.; Xiu, Y.; Jia, G. Growth mechanism and morphologies tuning of ZnO nanostructures. Int. J. Electrochem. Sci. 2015, 10, 4688–4695. [Google Scholar]
- Blumenstein, N.J.; Hofmeister, C.G.; Lindemann, P.; Huang, C.; Baier, J.; Leineweber, A.; Walheim, S.; Wöll, C.; Schimmel, T.; Bill, J. Chemical bath deposition of textured and compact zinc oxide thin films on vinyl-terminated polystyrene brushes. Beilstein J. Nanotechnol. 2016, 7, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, J.; Chevalier, Y.; Pelletier, J.; Couval, E.; Bouvier, D.; Bolzinger, M.A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A Physicochem. Eng. Asp. 2014, 457, 263–274. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Niu, J.; Chen, Y. Mechanism of photogenerated reactive oxygen species and correlation with the antibacterial properties of engineered metal-oxide nanoparticles. ACS Nano 2012, 6, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Talebian, N.; Amininezhad, S.M.; Doudi, M. Controllable synthesis of ZnO nanoparticles and their morphology-dependent antibacterial and optical properties. J. Photochem. Photobiol. B 2013, 120, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Xie, J.; Yan, K.; Duan, M. Growth Mechanism of Different Morphologies of ZnO Crystals Prepared by Hydrothermal Method. J. Mater. Sci. Technol. 2011, 27, 153–158. [Google Scholar] [CrossRef]
- Cullity, B.D.; Stock, S.R. Elements of X-ray Diffraction; Prentice Hall: Upper Saddle River, NJ, USA, 2001. [Google Scholar]
- Williamson, G.K.; Hall, W.H. X-ray Line Broadening from Filed Al and W. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial Activity of Metal Oxide Nanoparticles against Gram-positive and Gram-negative Bacteria: A Comparative Study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Similarities and differences in the responses of microorganisms to biocides. J. Antimicrob. Chemother. 2003, 52, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Sasidharan, A.; Divya Rani, V.V.; Menon, D.; Nair, S.; Manzoor, K.; Raina, S. Role of size scale of ZnO nanoparticles and microparticles on toxicity toward bacteria and osteoblast cancer cells. J. Mater. Sci. Mater. Med. 2009, 20, S235–S241. [Google Scholar] [PubMed]
- Sonohara, R.; Muramatsu, N.; Ohshima, H.; Kondo, T. Difference in surface properties between Escherichia coli and Staphylococcus aureus as revealed by electrophoretic mobility measurements. Biophys. Chem. 1995, 55, 273–277. [Google Scholar] [CrossRef]
- Gordon, T.; Perlstein, B.; Houbara, O.; Felner, I.; Banin, E.; Margel, S. Synthesis and characterization of zinc/iron oxide composite nanoparticles and their antibacterial properties. Coll. Surf. A Physicochem. Eng. Asp. 2011, 374, 1–8. [Google Scholar] [CrossRef]
- Applerot, G.; Perkas, N.; Amirian, G.; Girshevitz, O.; Gedanken, A. Coating of glass with ZnO via ultrasonic irradiation and a study of its antibacterial properties. Appl. Surf. Sci. 2009, 256, S3–S8. [Google Scholar] [CrossRef]
- Dmitriev, B.A.; Toukach, F.V.; Holst, O.; Rietschel, E.T.; Ehlers, S. Tertiary structure of Staphylococcus aureus cell wall murein. J. Bacteriol. 2004, 186, 7141–7148. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.S.; Adair, C.G.; Mawhinney, M.W.; Gorman, S.P. Standardization and comparison of methods employed for microbial cell surface hydrophobicity and charge determination. Int. J. Pharm. 1996, 131, 83–89. [Google Scholar] [CrossRef]
- Wang, J.; Huang, N.; Yang, P.; Leng, Y.X.; Sun, H.; Liu, Z.Y.; Chu, P.K. The effects of amorphous carbon films deposited on polyethylene terephthalate on bacterial adhesion. Biomaterials 2004, 25, 3163–3170. [Google Scholar] [CrossRef] [PubMed]
- Walencka, E.; Różalska, S.; Sadowska, B.; Różalska, B. The influence of Lactobacillus acidophilus-derived surfactants on Staphylococcal adhesion and biofilm formation. Folia Microbiol. (Praha) 2008, 53, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Rawlinson, L.A.B.; O’Gara, J.P.; Jones, D.S.; Brayden, D.J. Resistance of Staphylococcus aureus to the cationic antimicrobial agent poly(2-(dimethylaminoethyl)methacrylate) (pDMAEMA) is influenced by cell-surface charge and hydrophobicity. J. Med. Microbiol. 2011, 60, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Yoshikawa, T. Quantitative evaluation of antifungal activity of metallic oxide powders (MgO, CaO and ZnO) by an indirect conductometric assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Makhluf, S.; Dror, R.; Nitzan, Y.; Abramovich, Y.; Jelinek, R.; Gedanken, A. Microwave-assisted synthesis of nanocrystalline MgO and its use as a bactericide. Adv. Funct. Mater. 2005, 15, 1708–1715. [Google Scholar] [CrossRef]
- Adams, L.K.; Lyon, D.Y.; Alvarez, P.J.J. Comparative Ecotoxicity of Nanoscale TiO2, SiO2, and ZnO Water Suspensions. Water Res. 2006, 40, 3527–3532. [Google Scholar] [CrossRef] [PubMed]
- Brayner, R.; Ferrari-Iliou, R.; Brivois, N.; Djediat, S.; Benedetti, M.F.; Fievet, F. Toxicological Impact Studies Based on Escherichia coli Bacteria in Ultrafine ZnO Nanoparticles Colloidal Medium. Nano Lett. 2006, 6, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial Activity of ZnO Nanoparticle Suspensions on a Broad Spectrum of Microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Kasemets, K.; Ivask, A.; Dubourguier, H.C.; Kahru, A. Toxicity of Nanoparticles of ZnO, CuO and TiO2 to Yeast Saccharomyces Cerevisiae. Toxicol. In Vitro 2006, 23, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Xie, C. Zn2+ Release from Zinc and Zinc Oxide Particles in Simulated Uterine Solution. Colloids Surf. B 2006, 47, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, V.L.; Vijayaraghavan, R. Insight Into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
- Padmavathy, N.; Vijayaraghavan, R. Enhanced Bioactivity of ZnO Nanoparticles—An Antibacterial Study. Sci. Technol. Adv. Mater. 2008, 9, 035004. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, D.; Yi, Z.; Jiang, M.; Wang, L.; Zhou, Z.; Fan, X.; Wang, Y.; Hui, D. Antibacterial Mechanism Based on H2O2 Generation at Oxygen Vacancies in ZnO Crystals. Langmuir 2013, 29, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Lehman, S.E.; Morris, A.S.; Mueller, P.S.; Aliasger, S.K.; Grassian, V.H.; Larsen, S.C. Silica Nanoparticle-Generated ROS as a Predictor of Cellular Toxicity: Mechanistic Insights and Safety by Design. Environ. Sci. Nano 2016, 3, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Benzing, F.A.K.; Comba, P.; McRoberts, C.; Boyd, D.R.; Greiner, S.; Keppler, F. Abiotic Methanogenesis from Organosulphur Compounds under Ambient Conditions. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Polarz, S.; Strunk, J.; Ischenko, V.; van den Berg, M.W.E.; Hinrichsen, O.; Muhler, M.; Driess, M. On the Role of Oxygen Defects in the Catalytic Performance of Zinc Oxide. Angew. Chem. Int. Ed. 2006, 45, 2965–2969. [Google Scholar] [CrossRef] [PubMed]
- Ischenko, V.; Polarz, S.; Grote, D.; Stavarache, V.; Fink, K.; Driess, M. Zinc Oxide Nanoparticles with Defects. Adv. Funct. Mater. 2005, 15, 1945–1954. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation Into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO nanofluids). J. Nanopart. Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial Activity and Mechanism of Zinc Oxide Nanoparticles on Campylobacter Jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ding, Y.; Povey, M.; York, D. ZnO nanofluids—A Potential Antibacterial Agent. Prog. Nat. Sci. 2008, 18, 939–944. [Google Scholar] [CrossRef]
- Wahab, R.; Mishra, A.; Yun, S.I.; Hwang, I.H.; Mussarat, J.; Al-Khedhairy, A.A.; Kim, Y.S.; Shin, H.S. Fabrication, Growth Mechanism and Antibacterial Activity of ZnO Micro-Spheres Prepared via Solution Process. Biomass Bioenergy 2012, 39, 227–236. [Google Scholar] [CrossRef]
- Roca, A.I.; Cox, M.M. RecA Protein: Structure, Function, and Role in Recombinational DNA Repair. Prog. Nucleic Acid Res. Mol. Biol. 1997, 56, 129–223. [Google Scholar] [PubMed]
- Cox, M.M. Regulation of Bacterial RecA Protein Function. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Vandebriel, R.J.; de Jong, W.H. A review of mammalian toxicity of ZnO nanoparticles. J. Toxicol. Environ. Health A Nanotechnol. Sci. Appl. 2012, 5, 61–71. [Google Scholar]
- Sahu, D.; Kannan, G.M.; Vijayaraghavan, R. Size-dependent effect of zinc oxide on toxicity and inflammatory potential of human monocytes. J. Toxicol. Environ. Health 2014, 77, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Stecka, H.; Jedryczko, D.; Welna, M.; Pohl, P. Determination of traces of copper and zinc in honeys by the solid phase extraction pre-concentration followed by the flame atomic absorption spectrometry detection. Environ. Monit. Assess. 2014, 186, 6145–6155. [Google Scholar] [CrossRef] [PubMed]
- Kassab, K. Photophysical and Photosensitizing Properties of Selected Cyanines. J. Photochem. Photobiol. B 2002, 68, 15–22. [Google Scholar] [CrossRef]
- Bazylińska, U.; Pietkiewicz, J.; Saczko, J.; Nattich-Rak, M.; Rossowska, J.; Garbiec, A.; Wilk, K.A. Nanoemulsion-Templated Multilayer Nanocapsules for Cyanine-Type photosensitizer Delivery to Human Breast Carcinoma Cells. Eur. J. Pharm. Sci. 2012, 47, 406–420. [Google Scholar] [CrossRef] [PubMed]
- Bazylińska, U.; Lewińska, A.; Lamch, Ł.; Wilk, K.A. Polymeric Nanocapsules and Nanospheres for Encapsulation and Long Sustained Release of Hydrophobic Cyanine-Type Photosensitizer. Colloids Surf. A 2014, 442, 42–49. [Google Scholar] [CrossRef]
- Wolanov, Y.; Prikhodchenko, P.V.; Medvedev, A.G.; Pedahzur, R.; Lev, O. Zinc Dioxide Nanoparticulates: A Hydrogen Peroxide Source at Moderate pH. Environ. Sci. Technol. 2013, 47, 8769–8774. [Google Scholar] [CrossRef] [PubMed]
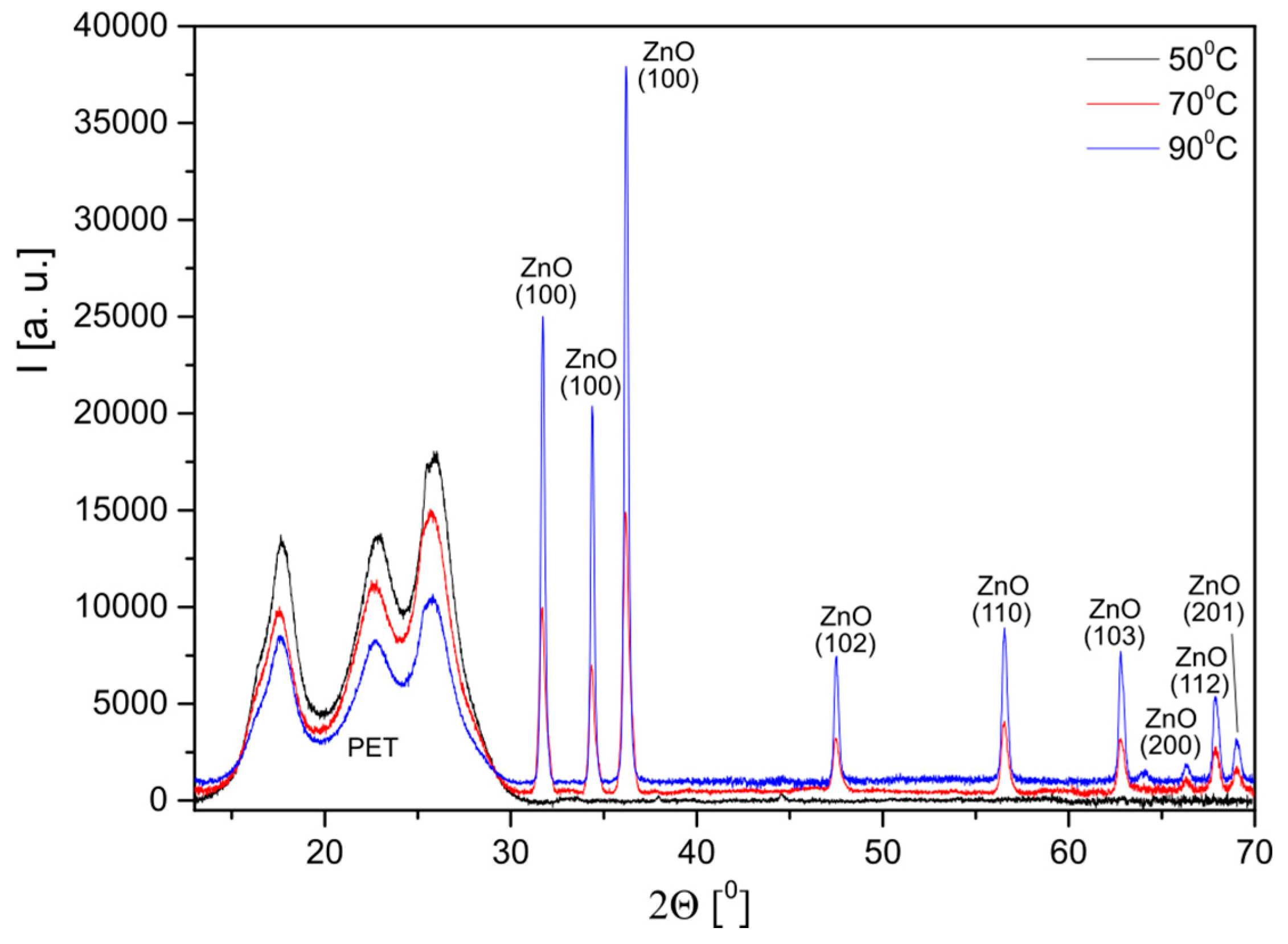
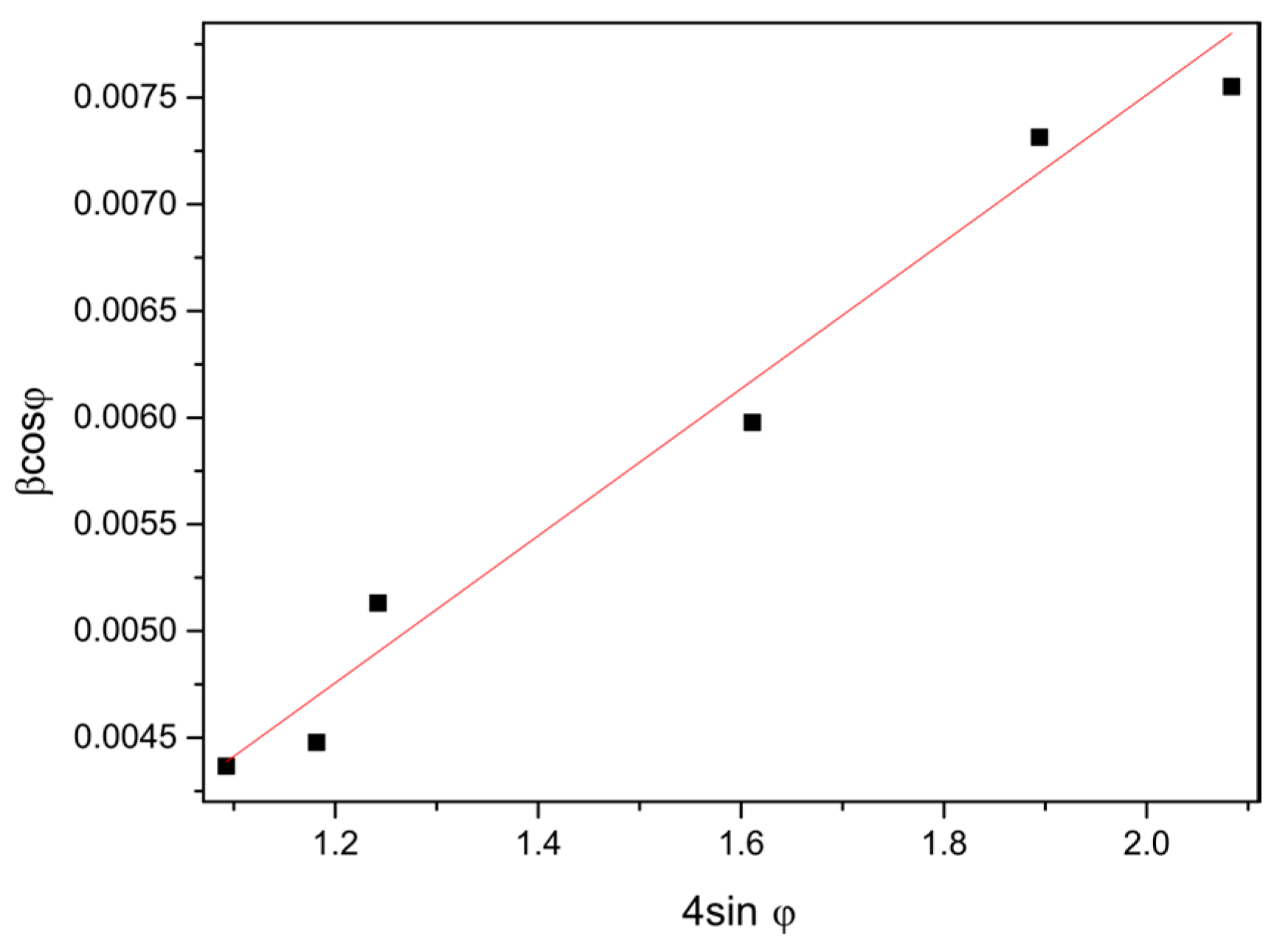
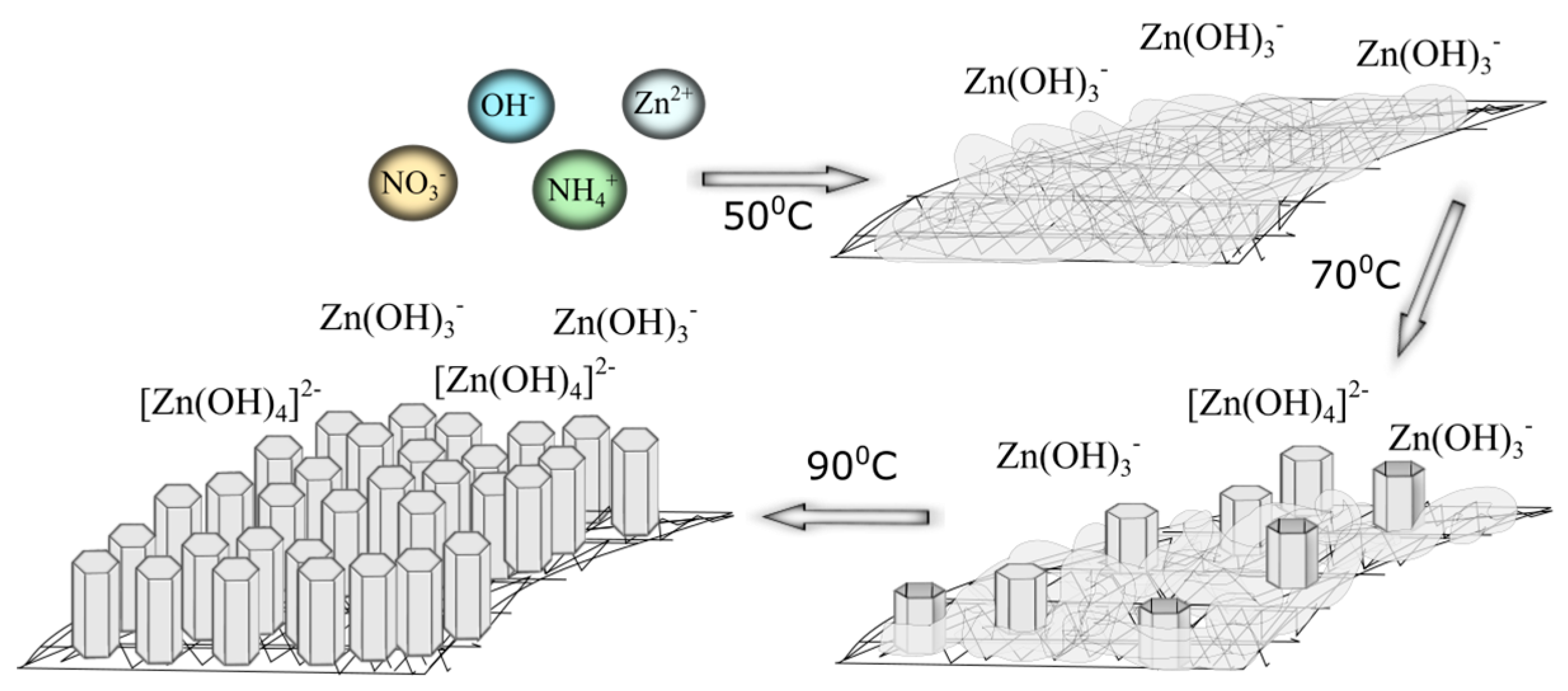
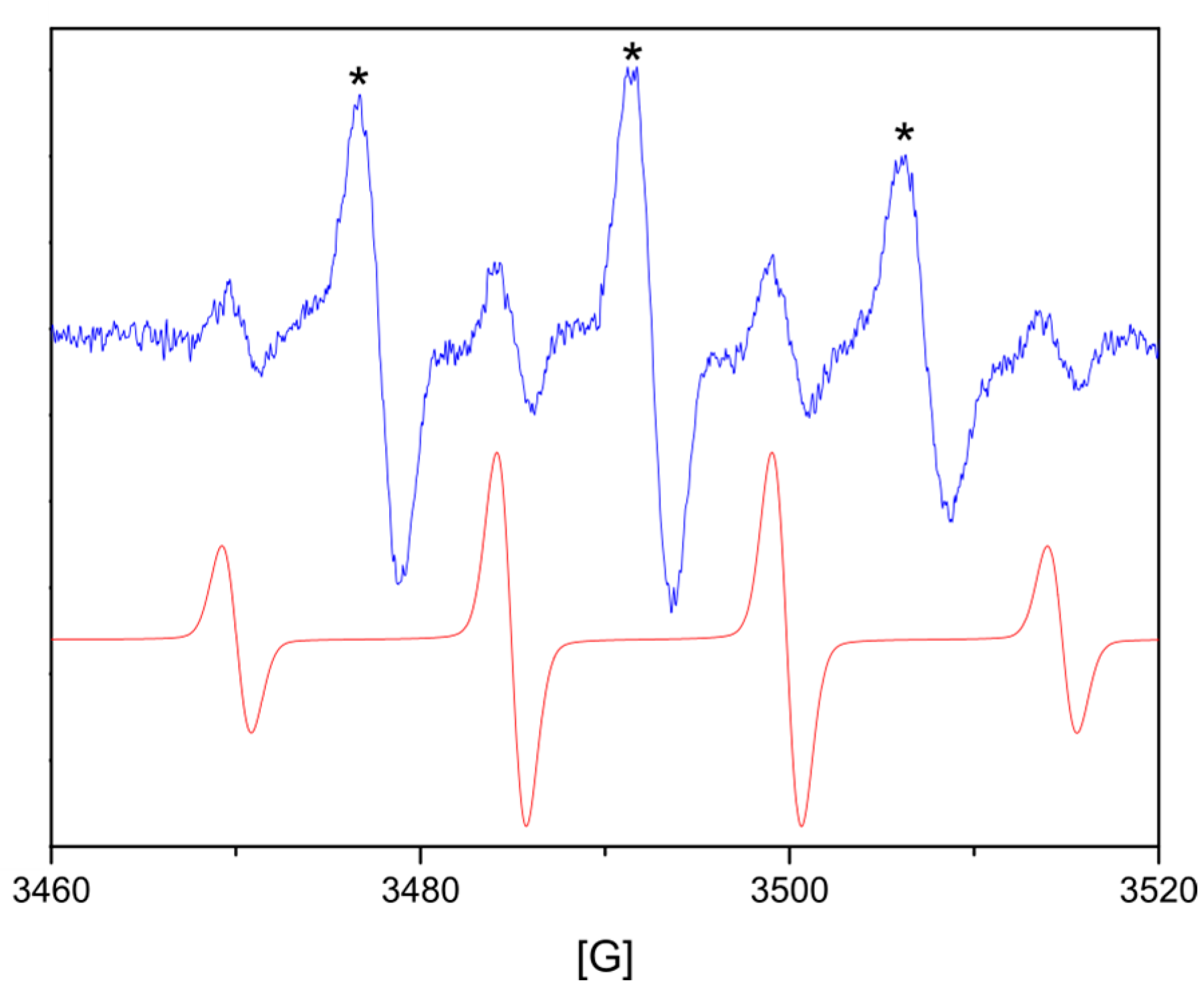
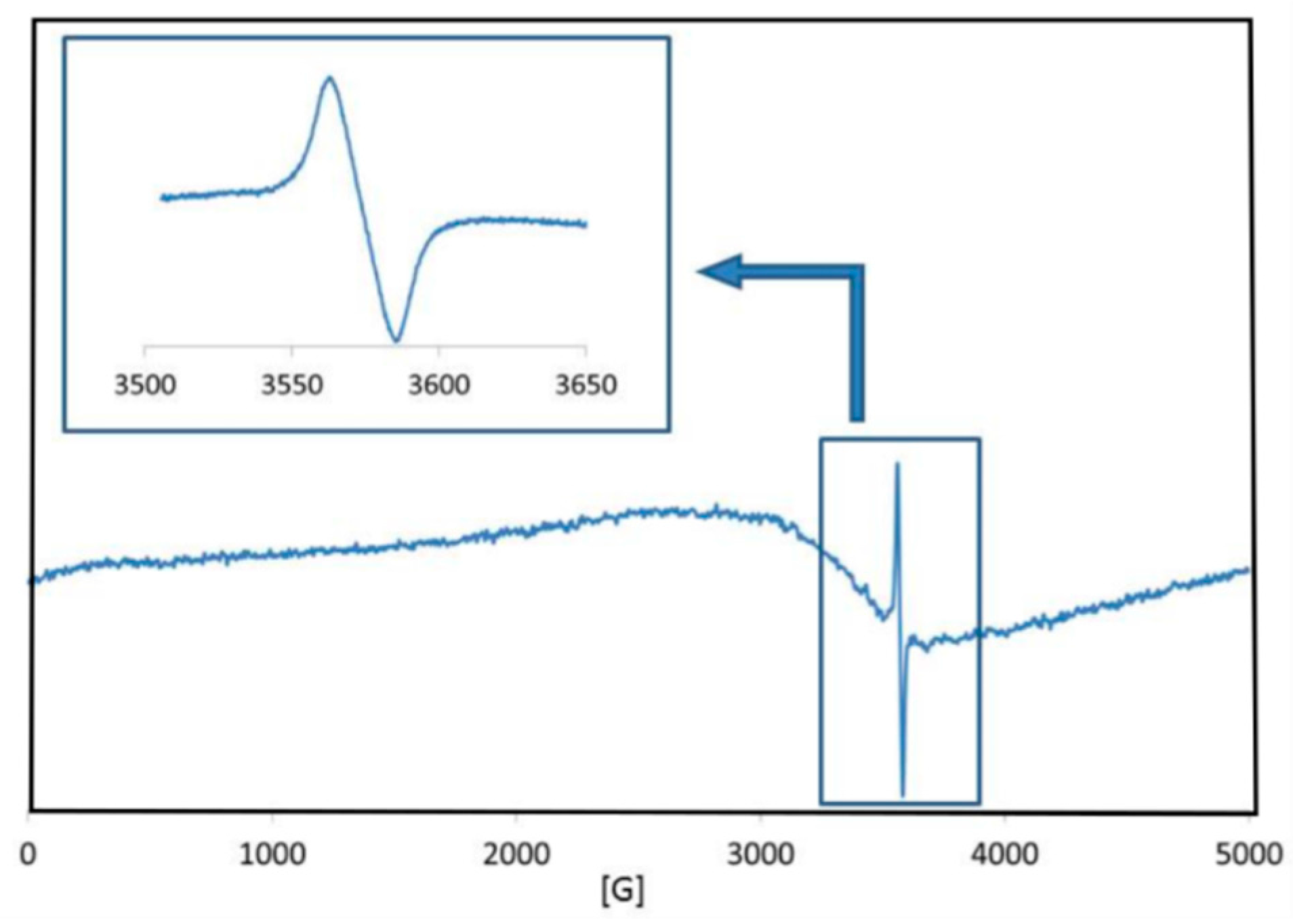

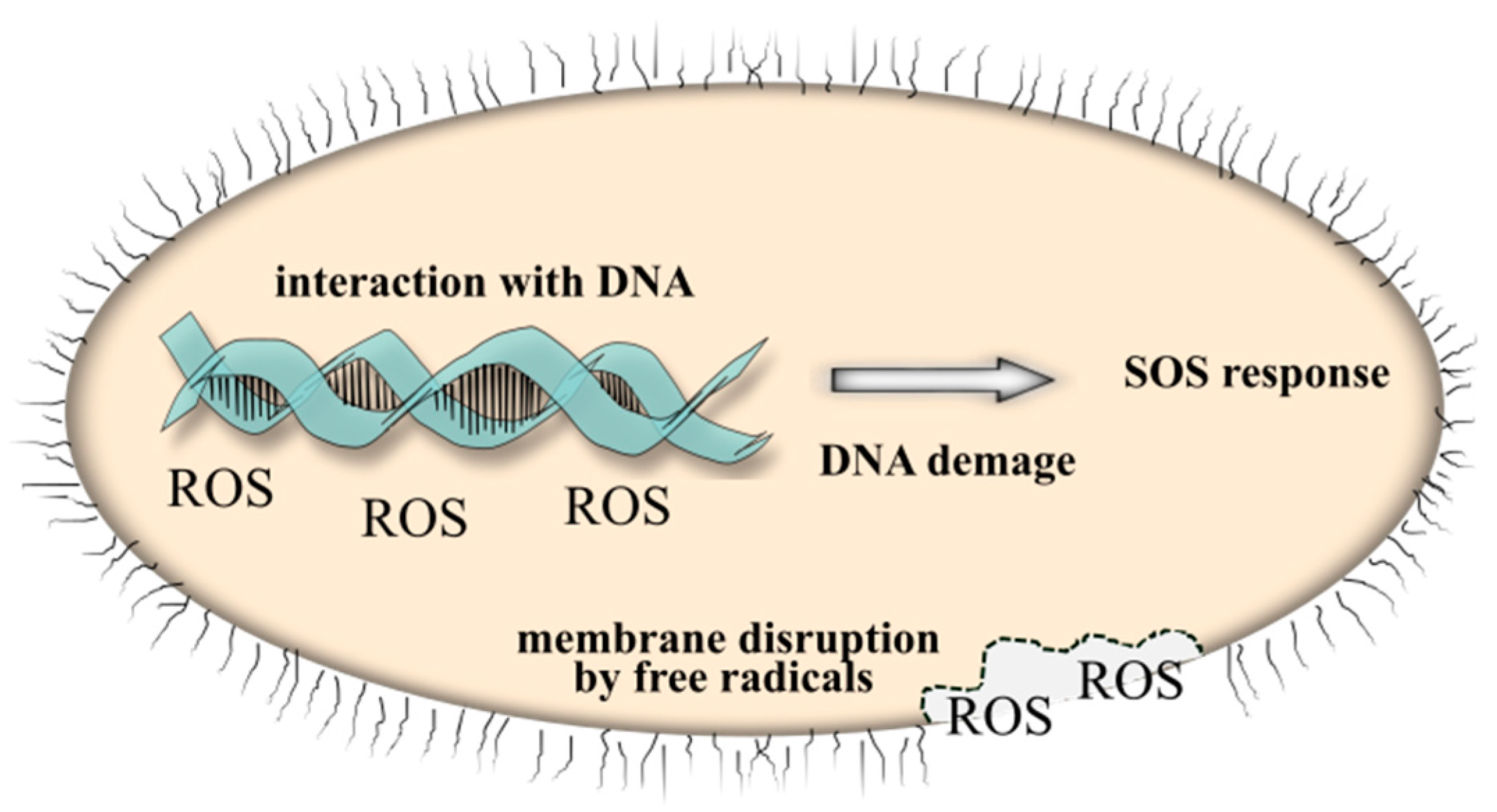
| T (°C) | d(100) (Å) | d(002) (Å) | a (Å) | c (Å) | V (Å3) | ε | D (nm) |
|---|---|---|---|---|---|---|---|
| 50 °C | - | - | - | - | - | - | - |
| 70 °C | 2.84 | 2.63 | 3.284 | 5.257 | 49.116 | 0.00726 | 182 |
| 90 °C | 2.82 | 2.61 | 3.259 | 5.220 | 48.023 | 0.00344 | 220 |
| standard | 2.81 | 2.60 | 3.250 | 5.204 | 47.603 | - | - |
| Sample | Duration of Treatment (h) | E. coli | S. aureus | S. epidermidis | C. albicans | ||||
|---|---|---|---|---|---|---|---|---|---|
| Reduction in Viability (%) | |||||||||
| s* | NB** | s | NB | s | NB | s | NB | ||
| 50 °C | 5 | 33 ± 2 | 22 ± 3 | 41 ± 1 | 28 ± 4 | 44 ± 2 | 32 ± 2 | 40 ± 3 | 37 ± 2 |
| 24 | 53 ± 2 | 43 ± 4 | 65 ± 2 | 56 ± 3 | 78 ± 3 | 67 ± 3 | 59 ± 2 | 42 ± 3 | |
| 70 °C | 5 | 43 ± 3 | 31 ± 3 | 47 ± 2 | 39 ± 2 | 85 ± 4 | 70 ± 4 | 52 ± 4 | 49 ± 4 |
| 24 | 59 ± 3 | 49 ± 2 | 59 ± 3 | 46 ± 3 | 90 ± 3 | 81 ± 1 | 75 ± 2 | 54 ± 4 | |
| 90 °C | 5 | 47 ± 2 | 36 ± 3 | 56 ± 3 | 53 ± 2 | 88 ± 3 | 71 ± 1 | 80 ± 3 | 70 ± 4 |
| 24 | 63 ± 3 | 57 ± 3 | 72 ± 3 | 67 ± 3 | 96 ± 3 | 89 ± 3 | 85 ± 2 | 78 ± 3 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiedot, M.; Maliszewska, I.; Rac-Rumijowska, O.; Suchorska-Woźniak, P.; Lewińska, A.; Teterycz, H. The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes. Materials 2017, 10, 353. https://doi.org/10.3390/ma10040353
Fiedot M, Maliszewska I, Rac-Rumijowska O, Suchorska-Woźniak P, Lewińska A, Teterycz H. The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes. Materials. 2017; 10(4):353. https://doi.org/10.3390/ma10040353
Chicago/Turabian StyleFiedot, Marta, Irena Maliszewska, Olga Rac-Rumijowska, Patrycja Suchorska-Woźniak, Agnieszka Lewińska, and Helena Teterycz. 2017. "The Relationship between the Mechanism of Zinc Oxide Crystallization and Its Antimicrobial Properties for the Surface Modification of Surgical Meshes" Materials 10, no. 4: 353. https://doi.org/10.3390/ma10040353