Research Advances of Microencapsulation and Its Prospects in the Petroleum Industry
Abstract
:1. Introduction
2. Research Advances in Microencapsulation
2.1. Development of Microcapsule Structure Polymers
- To protect core materials from adverse environmental effects (pH, temperature, humidity, and other substances)
- To control the active components for delayed (timed) release or long-acting (sustained) release
- To combine two incompatible components for a multifunctional structure
2.2. Types of Microcapsules
2.3. Formation Principle of Microcapsule-Based Structures
2.3.1. Interfacial Polymerization
2.3.2. Coacervation/Phase Separation
2.3.3. Solvent Evaporation
2.4. The Mechanism of a Microcapsule and Its Development in Oilfield Chemicals
3. Microencapsulation in Oilfield Additives
3.1. Current Situation
3.2. Materials
3.2.1. Shell Materials
3.2.2. Core Materials
3.3. Preparation and Evaluation Methods
3.4. Main Product and Mechanism
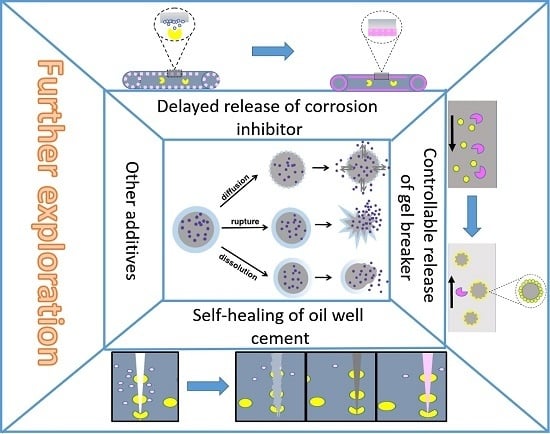
3.4.1. Microencapsulated Delayed Release Breaker
3.4.2. Microencapsulated Oilfield Corrosion Inhibitor
3.4.3. Microencapsulated Self-Healing Agent
3.4.4. Microencapsulation of Other Additives
4. Prospects for Oilfield Additives with Microcapsule Structures
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Obi, C.I. The petroleum industry: A paradox or (sp) oiler of development. J. Contemp. Afr. Stud. 2010, 28, 443–457. [Google Scholar] [CrossRef]
- Alagusundaram, M.; Chetty, M.S.; Umashankari, C. Microspheres as a novel drug delivery system—A review. Int. J. Chem. Tech. Res. 2009, 1, 526–534. [Google Scholar]
- Misal, R.; Waghmare, A.; Aqueel, S. Recent advances in microencapsulation: A review. Int. J. Pharm. Tech. 2013, 5, 2520–2535. [Google Scholar]
- Sliwka, W. Microencapsulation. Angew. Chem. Int. Ed. 1975, 14, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.I.; Kim, D.M.; Yu, H.C.; Chung, C.M. Microcapsule-type organogel-based self-healing system having secondary damage preventing capability. ACS Appl. Mater. Interfaces 2016, 8, 11070–11075. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.Z.; Sun, W.; Zuo, X.B. Study of feasibility of heat melt adhesive being used in crack self-healing of cement-based materials. Appl. Mech. Mater. 2011, 99–100, 1087–1091. [Google Scholar] [CrossRef]
- Cui, H.; Tucker-Burden, C.; Cauffiel, S.M.D.; Barry, A.K.; Iwakoshi, N.N.; Weber, C.J.; Safley, S.A. Long-term metabolic control of autoimmune diabetes in spontaneously diabetic nonobese diabetic mice by nonvascularized microencapsulated adult porcine islets. Transplantation 2009, 88, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Grandoso, L.; Ponce, S.; Manuel, I.; Arrúec, A.; Ruiz-Ortegaa, J.A.; Ulibarria, I.; Oriveb, G.; Hernándezb, R.M.; Rodríguezb, A.; Rodríguez-Puertasa, R.; et al. Long-term survival of encapsulated GDNF secreting cells implanted within the striatum of parkinsonized rats. Int. J. Pharm. 2007, 343, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Jin, Z.; Nishino, N.; Kato, H.; Shimizu, Y.; Niiya, T.; Murai, N.; Enami, Y.; Mitamura, K.; Koizumi, T.; et al. Intrasplenic transplantation of encapsulated hepatocytes decreases mortality and improves liver functions in fulminant hepatic failure from 90% partial hepatectomy in rats. Transplantation 2005, 79, 783–790. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ding, D.; Ren, G.; Xu, X.; Yin, X.; Hu, Y. Sustained delivery of endostatin improves the efficacy of therapy in Lewis lung cancer model. J. Control. Release 2009, 134, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Shen, B.; Wang, Z.; Shi, M.; Li, H.; Peng, C.; Zhao, Q.; Gao, C. Layered microcapsules for daunorubicin loading and release as well as in vitro and in vivo studies. Polym. Adv. Technol. 2008, 19, 36–46. [Google Scholar] [CrossRef]
- Kim, S.R.; Getachew, B.A.; Park, S.J.; Kwon, O.S.; Ryu, W.H.; Taylor, A.D.; Bae, J.; Kim, J.H. Toward microcapsule-embedded self-healing membranes. Environ. Sci. Technol. Lett. 2016, 3, 216–221. [Google Scholar] [CrossRef]
- Anal, A.K.; Singh, H. Recent advances in microencapsulation of probiotics for industrial applications and targeted delivery. Trends Food Sci. Technol. 2007, 18, 240–251. [Google Scholar] [CrossRef]
- Saez, V.; Hernández, J.R.; Peniche, C. Microspheres as delivery systems for the controlled release of peptides and proteins. Biotecnol. Apl. 2007, 24, 108–116. [Google Scholar]
- Patravale, V.B.; Mandawgade, S.D. Novel cosmetic delivery systems: An application update. Int. J. Cosmet. Sci. 2008, 30, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Leelarasamee, N.; Howard, S.A.; Malanga, C.J.; Ma, J.K.H.; Castranova, V.; Ma, J.Y.C. A method for the preparation of polylactic acid microcapsules of controlled particle size and drug loading. J. Microencapsul. 1988, 5, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Langdon, C.J.; De Bevoise, A.E. Effect of microcapsule type on delivery of dietary protein to a marine suspension-feeder, the oyster Crassostrea gigas. Mar. Biol. 1990, 105, 437–443. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.; Bao, Y.; Liu, J.; Zhang, J. Research advances in polymer emulsion based on “core-shell” structure particle design. Adv. Colloid Interface Sci. 2013, 197, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Han, N.X.; Xing, F.A. Comprehensive Review of the Study and Development of Microcapsule Based Self-Resilience Systems for Concrete Structures at Shenzhen University. Materials 2016, 10, 2. [Google Scholar] [CrossRef]
- Lee, D.; Park, Y.; Cho, S.H.; Yoo, M.; Jung, N.; Yun, M.; Ko, W.; Jeon, S. Microthermogravimetry of a single microcapsule using silicon microresonators. Anal. Sci. 2010, 82, 5815–5818. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, H.; Fukumori, Y.A. Novel positively thermosensitive controlled- release microcapsule with membrane of nano-sized poly(N-isopropylacrylamide) gel dispersed in ethylcellulose matrix. J. Control. Release 2000, 63, 107–119. [Google Scholar] [CrossRef]
- Sawada, K.; Urakawa, H. Preparation of photosensitive color-producing microcapsules utilizing in situ polymerization method. Dyes Pigments 2005, 65, 45–49. [Google Scholar] [CrossRef]
- Jonsson, M.; Nordin, O.; Malmström, E.; Hammer, C. Suspension polymerization of thermally expandable core/shell particles. Polymer 2006, 47, 3315–3324. [Google Scholar] [CrossRef]
- Kawaguchi, Y.; Oishi, T. Synthesis and properties of thermoplastic expandable microspheres: The relation between crosslinking density and expandable property. J. Appl. Polym. Sci. 2004, 93, 505–512. [Google Scholar] [CrossRef]
- Jeoung, S.K.; Han, I.S.; Jung, Y.J.; Hong, S.; Shim, S.E.; Hwang, Y.J.; Lee, P.C.; Ha, J.U. Fabrication of thermally expandable core–shell microcapsules using organic and inorganic stabilizers and their application. J. Appl. Polym. Sci. 2016, 133, 44247–44252. [Google Scholar] [CrossRef]
- Posillico, E.G. Microencapsulation Technology for large-scale antibody production. Nat. Biotechnol. 1986, 4, 114–117. [Google Scholar] [CrossRef]
- Dubey, R.; Shami, T.C.; Rao, K.U.B. Microencapsulation technology and applications. Defence Sci. J. 2009, 59, 82–95. [Google Scholar]
- Dewettinck, K.; Huyghebaert, A. Fluidized bed coating in food technology. Trends Food Sci. Technol. 1999, 10, 163–168. [Google Scholar] [CrossRef]
- Gouin, S. Microencapsulation: Industrial appraisal of existing technologies and trends. Trends Food Sci. Technol. 2004, 15, 330–347. [Google Scholar] [CrossRef]
- Knezevic, Z.; Gosak, D.; Hraste, M.; Jalsenjako, I. Fluid-bed microencapsulation of ascorbic acid. J. Microencapsul. 1998, 15, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Fukumori, Y.; Fukuda, T.; Hanyu, Y.; Takeuchi, Y.; Osako, Y. Coating of pharmaceutical powders by fluidized bed process. I. Aqueous enteric coating with methacrylic acid-ethylacrylate copolymer and the dissolution behavior of products. Chem. Pharm. Bull. 1987, 35, 2949–2957. [Google Scholar] [CrossRef] [PubMed]
- Morgan, P.W.; Kwolek, S.L. Interfacial polycondensation. II. fundamentals of polymer formation at liquid interfaces. J. Polym. Sci. 1959, 40, 299–327. [Google Scholar] [CrossRef]
- Schaefgen, J.R.; Koontz, F.H.; Tietz, R.F. Interfacial polycondensation. VIII. application to A-B type monomers. J. Polym. Sci. 1959, 40, 377–387. [Google Scholar] [CrossRef]
- Chang, T.M.S.; MacIntosh, F.C.; Mason, S.G. Semipermeable aqueous microcapsules: I. preparation and properties. Can. J. Physiol. Pharm. 1966, 44, 115–128. [Google Scholar] [CrossRef]
- Koishi, M.; Fukuhara, N.; Kondo, T. Studies on microcapsules. II. preparation of polyphthalamide microcapsules. Chem. Pharm. Bull. 1969, 17, 804–809. [Google Scholar] [CrossRef]
- Yang, J.; Keller, M.W.; Moore, J.S.; White, S.R.; Sottos, N.R. Microencapsulation of isocyanates for self-healing polymers. Macromolecules 2008, 41, 9650–9655. [Google Scholar] [CrossRef]
- Lv, L.; Schlangen, E.; Yang, Z.; Xing, F. Micromechanical Properties of a New Polymeric Microcapsule for Self-Healing Cementitious Materials. Materials 2016, 9, 1025. [Google Scholar] [CrossRef]
- Hirech, K.; Payan, S.; Carnelle, G.; Legrand, J. Microencapsulation of an insecticide by interfacial polymerisation. Powder Technol. 2003, 130, 324–330. [Google Scholar] [CrossRef]
- Magdassi, S. Delivery systems in cosmetics. Colloids Surf. A 1997, 123, 671–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Rochefort, D. Characterisation and applications of microcapsules obtained by interfacial polycondensation. J. Microencapsul. 2012, 29, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Mcilroy, D.A.; Blaiszik, B.J.; Caruso, M.M.; White, S.R.; Moore, J.S.; Sottos, N.R. Microencapsulation of a reactive liquid-phase amine for self-healing epoxy composites. Macromolecules 2010, 43, 1855–1859. [Google Scholar] [CrossRef]
- He, Z.; Jiang, S.; Li, Q.; Wang, J.; Zhao, Y.; Kang, M. Facile and cost-effective synthesis of isocyanate microcapsules via polyvinyl alcohol-mediated interfacial polymerization and their application in self-healing materials. Compos. Sci. Technol. 2017, 138, 15–23. [Google Scholar] [CrossRef]
- Okada, J.; Kusai, A.; Ueda, S. Factors affecting microencapsulability in simple gelatin coacervation method. J. Microencapsul. 1985, 2, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.Z.; Gu, X.; Zhu, X.; Zhang, Z. Spreadable dispersion of insect sex pheromone capsules, preparation via complex coacervation and release control of the encapsulated pheromone component molecule. Biomed. Microdevices 2009, 11, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Weiβ, G.; Knoch, A.; Laicher, A.; Stanislaus, F.; Daniels, R. Simple coacervation of hydroxypropyl methylcellulose phthalate (HPMCP) II. microencapsulation of ibuprofen. Int. J. Pharm. 1995, 124, 97–105. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Mokhtarian, N.; Ghasemi, M. Production of protein nanoparticles for food and drug delivery system. Afr. J. Biotechnol. 2009, 8, 4738–4743. [Google Scholar]
- Niu, F.; Dong, Y.; Shen, F.; Wang, J.; Liu, Y.; Su, Y.; Xu, R.; Wang, J.; Yang, Y. Phase separation behavior and structural analysis of ovalbumin–gum arabic complex coacervation. Food Hydrocolloid 2015, 43, 1–7. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S.; Yamauchi, R. Microencapsulation: A review of applications in the food and pharmaceutical industries. Rev. Agric. Sci. 2016, 4, 56–65. [Google Scholar] [CrossRef]
- Deshmukh, R.; Wagh, P.; Naik, J. Solvent evaporation and spray drying technique for micro-and nanospheres/particles preparation: A review. Dry. Technol. 2016, 34, 1758–1772. [Google Scholar] [CrossRef]
- Yang, X.; Gao, N.; Hu, L.; Li, J.; Sun, Y. Development and evaluation of novel microcapsules containing poppy-seed oil using complex coacervation. J. Food Eng. 2015, 161, 87–93. [Google Scholar] [CrossRef]
- Saravanan, M.; Rao, K.P. Pectin–gelatin and alginate–gelatin complex coacervation for controlled drug delivery: Influence of anionic polysaccharides and drugs being encapsulated on physicochemical properties of microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- Wang, J.C.; Chen, S.H.; Xu, Z.C. Synthesis and properties research on the nanocapsulated capsaicin by simple coacervation method. J. Disper. Sci. Technol. 2008, 29, 687–695. [Google Scholar] [CrossRef]
- Butstraen, C.; Salaün, F. Preparation of microcapsules by complex coacervation of gum Arabic and chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.G.; Bozza, F.T.; Thomazini, M.; Favaro-Trindade, C.S. Microencapsulation of xylitol by double emulsion followed by complex coacervation. Food Chem. 2015, 171, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Rocha-Selmi, G.A.; Bozza, F.T.; Thomazini, M.; Bolinia, H.M.A.; Fávaro-Trindadeb, C.S. Microencapsulation of aspartame by double emulsion followed by complex coacervation to provide protection and prolong sweetness. Food Chem. 2013, 139, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Adhikari, B.; Barrow, C.J. Optimisation of the microencapsulation of tuna oil in gelatin–sodium hexametaphosphate using complex coacervation. Food Chem. 2014, 158, 358–365. [Google Scholar]
- Li, M.; Rouaud, O.; Poncelet, D. Microencapsulation by solvent evaporation: State of the art for process engineering approaches. Int. J. Pharm. 2008, 363, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Noviendri, D.; Jaswir, I.; Taher, M.; Mohamed, F.; Salleh, H.M.; Noorbatcha, I.A.; Octavianti, F.; Lestari, W.; Hendri, R.; Ahmad, H.; et al. Fabrication of fucoxanthin-loaded microsphere(F-LM) by two steps double-emulsion solvent evaporation method and characterization of fucoxanthin before and after Microencapsulation. J. Oleo Sci. 2016, 65, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Manekar, N.C.; Puranik, P.K.; Joshi, S.B. Microencapsulation of propranolol hydrochloride by the solvent evaporation technique. J. Microencapsul. 1992, 9, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Youan, B.B.C. Microencapsulation of superoxide dismutase into poly(ε- caprolactone) microparticles by reverse micelle solvent evaporation. Drug Deliv. 2003, 10, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lu, Z.; Wu, H.; Lv, F. Study on the antibiotic activity of microcapsule curcumin against foodborne pathogens. Int. J. Food Microbiol. 2009, 136, 71–74. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Johannesson, B.; Geiker, M. A review: Self-healing in cementitious materials and engineered cementitious composite as a self-healing material. Constr. Build. Mater. 2012, 28, 571–583. [Google Scholar] [CrossRef]
- Li, W.; Zhu, X.; Zhao, N.; Jiang, Z. Preparation and properties of melamine urea-formaldehyde microcapsules for self-healing of cementitious materials. Materials 2016, 9, 152. [Google Scholar] [CrossRef]
- Kim, D.M.; Yu, H.C.; Yang, H.I.; Cho, Y.J.; Lee, K.M.; Chung, C.M. Microcapsule-Type Self-Healing Protective Coating for Cementitious Composites with Secondary Crack Preventing Ability. Materials 2017, 10, 114. [Google Scholar] [CrossRef]
- Dong, B.; Wang, Y.; Fang, G.; Han, N.; Xing, F.; Lu, Y. Smart releasing behavior of a chemical self-healing microcapsule in the stimulated concrete pore solution. Cem. Concr. Compos. 2015, 56, 46–50. [Google Scholar] [CrossRef]
- Qureshi, T.S.; Kanellopoulos, A.; Al-Tabbaa, A. Encapsulation of expansive powder minerals within a concentric glass capsule system for self-healing concrete. Constr. Build. Mater. 2016, 121, 629–643. [Google Scholar] [CrossRef]
- Yang, C.Y.; Tsay, S.Y.; Tsiang, R.C. Encapsulating aspirin into a surfactant-free ethyl cellulose microsphere using non-toxic solvents by emulsion solvent-evaporation technique. J. Microencapsul. 2001, 18, 223–236. [Google Scholar] [PubMed]
- Pathak, Y.V.; Shingatgiri, M.; Dorle, A.K. In vivo performance of pentaestergum-coated aspirin microcapsules. J. Microencapsul. 2008, 4, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Horsup, D.I.; Clark, J.C.; Binks, B.P.; Fletcher, P.D.I.; Hicks, J.T. The fate of oilfield corrosion inhibitors in multiphase systems. Corrosion 2010, 66, 410–418. [Google Scholar] [CrossRef]
- Cantu, L.A.; Boyd, P.A. Laboratory and field evaluation of a combined fluid-loss-control additive and gel breaker for Fracturing Fluids. SPE Prod. Eng. 2013, 5, 253–260. [Google Scholar] [CrossRef]
- Nelson, G. Application of microencapsulation in textiles. Int. J. Pharm. 2002, 242, 55–62. [Google Scholar] [CrossRef]
- Cuddington, J.T.; Moss, D.L. Technological change, depletion, and the U.S. petroleum industry. Am. Econ. Rev. 2001, 91, 1135–1148. [Google Scholar] [CrossRef]
- Heurlin, M.; Stankevič, T.; Mickevičius, S.; Yngman, S.; Lindgren, D.; Mikkelsen, A.; Feidenhans’I, R.; Borgström, M.T.; Samuelson, L. Structural properties of wurtzite InP-InGaAs nanowire core–shell hetero structures. Nano Lett. 2015, 15, 2462–2467. [Google Scholar] [CrossRef] [PubMed]
- Tasker, A.L.; Hitchcock, J.P.; He, L.; Baxter, E.A.; Biggs, S.; Cayre, O.J. The effect of surfactant chain length on the morphology of poly (methyl methacrylate) microcapsules for fragrance oil encapsulation. J. Colloid Interface Sci. 2016, 484, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachari, P.; Hashaikeh, R.; Tiner, M. Modified cellulose morphologies and its composites: SEM and TEM analysis. Micron 2011, 42, 751–761. [Google Scholar] [CrossRef] [PubMed]
- O'Connell, J.H.; Lee, M.E.; Yagoub, M.Y.A.; Swart, H.C.; Coetsee, E. Characterization of crystallite morphology for doped strontium fluoride nanophosphors by TEM and XRD. Physica B 2016, 480, 169–173. [Google Scholar] [CrossRef]
- Xu, P.; Xu, J.; He, M.; Song, L.; Chen, D.; Guo, G.; Dai, H. Morphology and chemical characteristics of micro-and Nano-particles in the haze in Beijing studied by XPS and TEM/EDX. Sci. Total Environ. 2016, 565, 827–832. [Google Scholar] [CrossRef] [PubMed]
- Abel, K.A.; Boyer, J.C.; Andrei, C.M.; Van Veggel, F.C. Analysis of the shell thickness distribution on NaYF4/NaGdF4 core/shell nanocrystals by EELS and EDS. J. Phys. Chem. Lett. 2011, 2, 185–189. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Z.; Wu, Z.; Zhou, Y. Synthesis and detection the oxidization of Co cores of Co@ SiO2 core-shell nanoparticles by in situ XRD and EXAFS. Nanoscale Res. Lett. 2015, 10, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.B.; Brittman, S.; Yu, Y.; Dasgupta, N.P.; Yang, P. Core-shell CdS-Cu2S nanorod array solar cells. Nano Lett. 2015, 15, 4096–4101. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ba, M.; Du, Y.; He, Z.M.; Chen, J.B. Effects of chloride ions on carbonation rate of hardened cement paste by X-ray CT techniques. Constr. Build. Mater. 2016, 122, 619–627. [Google Scholar] [CrossRef]
- Lv, L.; Yang, Z.; Chen, G.; Zhu, G.; Han, N.; Schlangen, E.; Xing, F. Synthesis and characterization of a new polymeric microcapsule and feasibility investigation in self-healing cementitious materials. Constr. Build. Mater. 2016, 105, 487–495. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Retarding effectiveness of hexitols on the hydration of the silicate phases of cement: Interaction with the aluminate and sulfate phases. Cem. Concr. Res. 2016, 90, 137–143. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Effects of functionality and stereochemistry of small organic molecules on the hydration of tricalcium silicate. Cem. Concr. Res. 2016, 87, 97–104. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Impacts of hexitols on the hydration of a tricalcium aluminate-calcium sulfate mixture. Cem. Concr. Res. 2016, 89, 177–186. [Google Scholar] [CrossRef]
- Nalet, C.; Nonat, A. Ionic complexation and adsorption of small organic molecules on calcium silicate hydrate: Relation with their retarding effect on the hydration of C3S. Cem. Concr. Res. 2016, 89, 97–108. [Google Scholar] [CrossRef]
- Dong, B.; Fang, G.; Ding, W. Self-healing features in cementitious material with urea–formaldehyde/epoxy microcapsules. Constr. Build. Mater. 2016, 106, 608–617. [Google Scholar] [CrossRef]
- Wang, J.Y.; Soens, H.; Verstraete, W.; De Belie, N. Self-healing concrete by use of microencapsulated bacterial spores. Cem. Concr. Res. 2014, 56, 139–152. [Google Scholar] [CrossRef]
- Fancher, C.M.; Han, Z.; Levin, I.; Page, K.; Reich, B.J.; Smith, R.C.; Wilson, A.G.; Jonesa, J.L. Use of bayesian inference in crystallographic structure refinement via full diffraction profile analysis. Sci. Rep. 2016, 6, 31625–31637. [Google Scholar] [CrossRef] [PubMed]
- Yio, M.H.N.; Wong, H.S.; Buenfeld, N.R. 3D Monte Carlo simulation of backscattered electron signal variation across pore-solid boundaries in cement-based materials. Cem. Concr. Res. 2016, 89, 320–331. [Google Scholar] [CrossRef]
- Chitez, A.S.; Jefferson, A.D. A coupled thermo-hygro-chemical model for characterising autogenous healing in ordinary cementitious materials. Cem. Concr. Res. 2016, 88, 184–197. [Google Scholar] [CrossRef]
- Dai, C.; Wang, K.; Liu, Y.; Li, H.; Wei, Z.; Zhao, M. Reutilization of Fracturing Flowback Fluids in Surfactant Flooding for Enhanced Oil Recovery. Energy Fuels 2015, 29, 2304–2311. [Google Scholar] [CrossRef]
- Nolte, K.G. Fracturing Fluid Breaker System which Is Activated by Fracture Closure. U.S. Patent 4,506,734, 26 March 1985. [Google Scholar]
- Walles, W.E.; Williamson, T.D.; Tomkinson, D.L. Method for Treating Subterranean Formations. U.S. Patent 4,741,401, 3 May 1988. [Google Scholar]
- Gulbis, J.; King, M.T.; Hawkins, G.W.; Brannon, H.D. Encapsulated breaker for aqueous polymeric fluids. SPE Prod. Eng. 1992, 7, 9–14. [Google Scholar] [CrossRef]
- Wu, J.Q.; Wu, X.M.; Liu, X.J.; Zhang, N.; Liu, J. New hydraulic fracturing fluid with microencapsulated heat-generating system. Oil Drill. Prod. Technol. 2005, 27, 67–70. [Google Scholar]
- Barati, R.; Johnson, S.J.; Mccool, S.; Green, D.W.; Willhite, G.P.; Liang, J.T. Fracturing fluid cleanup by controlled release of enzymes from polyelectrolyte complex nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1292–1298. [Google Scholar] [CrossRef]
- Rahimi, A.; Amiri, S. Anticorrosion hybrid nanocomposite coatings with encapsulated organic corrosion inhibitors. J. Coat. Technol. Res. 2015, 12, 587–593. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Kadhum, A.A.H.; Alobaidy, A.H.M.; Mohamad, A.B.; Hoon, P.S. Novel corrosion inhibitor for mild steel in HCl. Materials 2014, 7, 662. [Google Scholar] [CrossRef]
- Shi, S.C.; Su, C.C. Corrosion inhibition of high speed steel by biopolymer HPMC derivatives. Materials 2016, 9, 612. [Google Scholar] [CrossRef]
- Zaid, G.H.; Sanders, D.W. Binary corrosion inhibitors offer improved corrosion control. SPE Prod. Facil. 2005, 20, 133–137. [Google Scholar] [CrossRef]
- Thanawala, K.; Mutneja, N.; Khanna, A.S.; Raman, R.K. Development of self-healing coatings based on linseed oil as autonomous repairing agent for corrosion resistance. Materials 2014, 7, 7324–7338. [Google Scholar] [CrossRef]
- Zheludkevich, M.L.; Shchukin, D.G.; Yasakau, K.A.; Möhwald, H.; Ferreira, M.G. Anticorrosion coatings with self-healing effect based on nanocontainers impregnated with corrosion inhibitor. Chem. Mater. 2007, 19, 402–411. [Google Scholar] [CrossRef]
- Choi, H.; Song, Y.K.; Kim, K.Y.; Park, J.M. Encapsulation of triethanolamine as organic corrosion inhibitor into nanoparticles and its active corrosion protection for steel sheets. Surf. Coat. Technol. 2012, 206, 2354–2362. [Google Scholar] [CrossRef]
- Amar, H.; Braisaz, T.; Villemin, D.; Moreau, B. Thiomorpholin-4-ylmethyl-phosphonic acid and morpholin-4-methyl-phosphonic acid as corrosion inhibitors for carbon steel in natural seawater. Mater. Chem. Phys. 2008, 110, 1–6. [Google Scholar] [CrossRef]
- Gite, V.V.; Tatiya, P.D.; Marathe, R.J.; Mahulikar, P.P.; Hundiwale, D.G. Microencapsulation of quinoline as a corrosion inhibitor in polyurea microcapsules for application in anticorrosive PU coatings. Prog. Org. Coat. 2015, 83, 11–18. [Google Scholar] [CrossRef]
- Huang, M.; Yang, J. Facile microencapsulation of HDI for self-healing anticorrosion coatings. J. Mater. Chem. 2011, 21, 11123–11130. [Google Scholar] [CrossRef]
- Kuang, F.; Shi, T.; Wang, J.; Jia, F. Microencapsulation technology for thiourea corrosion inhibitor. J. Solid State Electrochem. 2009, 13, 1729–1735. [Google Scholar] [CrossRef]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Carey, J.W.; Wigand, M.; Chipera, S.J.; WoldeGabriel, G.; Pawar, R.; Lichtnera, P.C.; Wehner, S.C.; Raines, M.A.; Guthrie, J.G.D. Analysis and performance of oil well cement with 30 years of CO2 exposure from the SACROC Unit, West Texas, USA. Int. J. Greenh. Gas Control 2007, 1, 75–85. [Google Scholar] [CrossRef]
- Peled, A.; Jones, J.; Shah, S.P. Effect of matrix modification on durability of glass fiber reinforced cement composites. Mater. Struct. 2005, 38, 163–171. [Google Scholar] [CrossRef]
- Zhou, S.; Zhu, H.; Yan, Z.; Ju, J.W.; Zhang, L. A micromechanical study of the breakage mechanism of microcapsules in concrete using PFC2D. Constr. Build. Mater. 2016, 115, 452–463. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Giannaros, P.; Al-Tabbaa, A. The effect of varying volume fraction of microcapsules on fresh, mechanical and self-healing properties of mortars. Constr. Build. Mater. 2016, 122, 577–593. [Google Scholar] [CrossRef]
- Huang, H.; Ye, G. Simulation of self-healing by further hydration in cementitious materials. Cem. Concr. Compos. 2012, 34, 460–467. [Google Scholar] [CrossRef]
- Tittelboom, K.V.; Belie, N.D. Self-Healing in cementitious materials-a review. Materials 2013, 6, 2182–2217. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Su, J.F.; Schlangen, E.; Liu, Y.; Zhang, J.; Han, N.; Xing, F. Fabrication and characterization of self-healing microcapsules containing bituminous rejuvenator by a nano-inorganic/organic hybrid method. Constr. Build. Mater. 2016, 121, 471–482. [Google Scholar] [CrossRef]
- Yang, Z.; Hollar, J.; He, X.; Shi, X. A self-healing cementitious composite using oil core/silica gel shell microcapsules. Cem. Concr. Compos. 2011, 33, 506–512. [Google Scholar] [CrossRef]
- Xu, J.; Yao, W. Multiscale mechanical quantification of self-healing concrete incorporating non-ureolytic bacteria-based healing agent. Cem. Concr. Res. 2014, 64, 1–10. [Google Scholar] [CrossRef]
- Kanellopoulos, A.; Qureshi, T.S.; Al-Tabbaa, A. Glass encapsulated minerals for self-healing in cement based composites. Constr. Build. Mater. 2015, 98, 780–791. [Google Scholar] [CrossRef]
- Wang, J.; Van Tittelboom, K.; De Belie, N.; Verstraete, W. Use of silica gel or polyurethane immobilized bacteria for self-healing concrete. Constr. Build. Mater. 2012, 26, 532–540. [Google Scholar] [CrossRef]
- Van Tittelboom, K.; De Belie, N.; Van Loo, D.; Jacobs, P. Self-healing efficiency of cementitious materials containing tubular capsules filled with healing agent. Cem. Concr. Compos. 2011, 33, 497–505. [Google Scholar] [CrossRef]
- García, Á.; Schlangen, E.; Van de Ven, M.; Sierra-Beltrán, G. Preparation of capsules containing rejuvenators for their use in asphalt concrete. J. Hazard. Mater. 2010, 184, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Wiktor, V.; Jonkers, H.M. Quantification of crack-healing in novel bacteria-based self-healing concrete. Cem. Concr. Compos. 2011, 33, 763–770. [Google Scholar] [CrossRef]
- Sisomphon, K.; Copuroglu, O.; Fraaij, A. Application of encapsulated lightweight aggregate impregnated with sodium monofluorophosphate as a self-healing agent in blast furnace slag mortar. Heron 2011, 56, 13–32. [Google Scholar]
- Huang, H.; Ye, G. Self-healing of cracks in cement paste affected by additional Ca2+ ions in the healing agent. J. Intell. Mater. Syst. Struct. 2014, 26, 1–12. [Google Scholar] [CrossRef]
- Liu, B.; Ke, J.L.; Deng, X.; Zhu, G.M.; Luo, Y.C.; Han, N.X.; Xing, F. A waterproof epoxy resin microcapsule for the encapsulation of self-healing bacterium. In Proceedings of the Fifth International Conference on Self-Healing Materials, Durham, NC, USA, 22–24 June 2015. [Google Scholar]
- Ge, S.; Wan, F.; Lu, J.; Yu, J. Molecular self-Assembled microcapsules prepared by in situ polymerization technology for self-Healing cement materials. J. Inorg. Organomet. Polym. Mater. 2011, 21, 841–845. [Google Scholar] [CrossRef]
- Chen, X.; Dai, J.; Zhou, M.; Hu, C. Preparation of crosslinker microcapsules for in-depth profile control and study on the release model. Spéc. Petrochem. 2014, 31, 13–17. [Google Scholar]
- Li, B.; Dong, G.; Zhang, C. Storage stability and solubility of poly (urea-formaldehyde) microcapsules containing A-olefin drag reducing polymer. J. Appl. Polym. Sci. 2011, 122, 1450–1456. [Google Scholar] [CrossRef]
- Schock, J.; Liebl, S.; Achterhold, K.; Pfeiffer, F. Obtaining the spacing factor of microporous concrete using high-resolution dual energy X-ray micro CT. Cem. Concr. Res. 2016, 89, 200–205. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Liu, Z.; Zhao, P.; Liu, C. In-situ tracking of water transport in cement paste using X-ray computed tomography combined with CsCl enhancing. Mater. Lett. 2015, 160, 381–383. [Google Scholar] [CrossRef]








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Guo, J.; Yu, Y.; Cao, L.; Xu, Y. Research Advances of Microencapsulation and Its Prospects in the Petroleum Industry. Materials 2017, 10, 369. https://doi.org/10.3390/ma10040369
Hu M, Guo J, Yu Y, Cao L, Xu Y. Research Advances of Microencapsulation and Its Prospects in the Petroleum Industry. Materials. 2017; 10(4):369. https://doi.org/10.3390/ma10040369
Chicago/Turabian StyleHu, Miaomiao, Jintang Guo, Yongjin Yu, Lei Cao, and Yang Xu. 2017. "Research Advances of Microencapsulation and Its Prospects in the Petroleum Industry" Materials 10, no. 4: 369. https://doi.org/10.3390/ma10040369