One-Pot Route towards Active TiO2 Doped Hierarchically Porous Cellulose: Highly Efficient Photocatalysts for Methylene Blue Degradation
Abstract
:1. Introduction
2. Results
2.1. Fabrication Parameters
2.2. Scanning Electron Microscope (SEM)
2.3. Nitrogen Adsorption-Desorption Isothems
2.4. Energy Dispersive Spectrometer (EDS) Analysis
2.5. Fourier Transform Infrared Spectroscopy (FT-IR) Analysis
2.6. X-ray Diffraction (XRD) Analysis
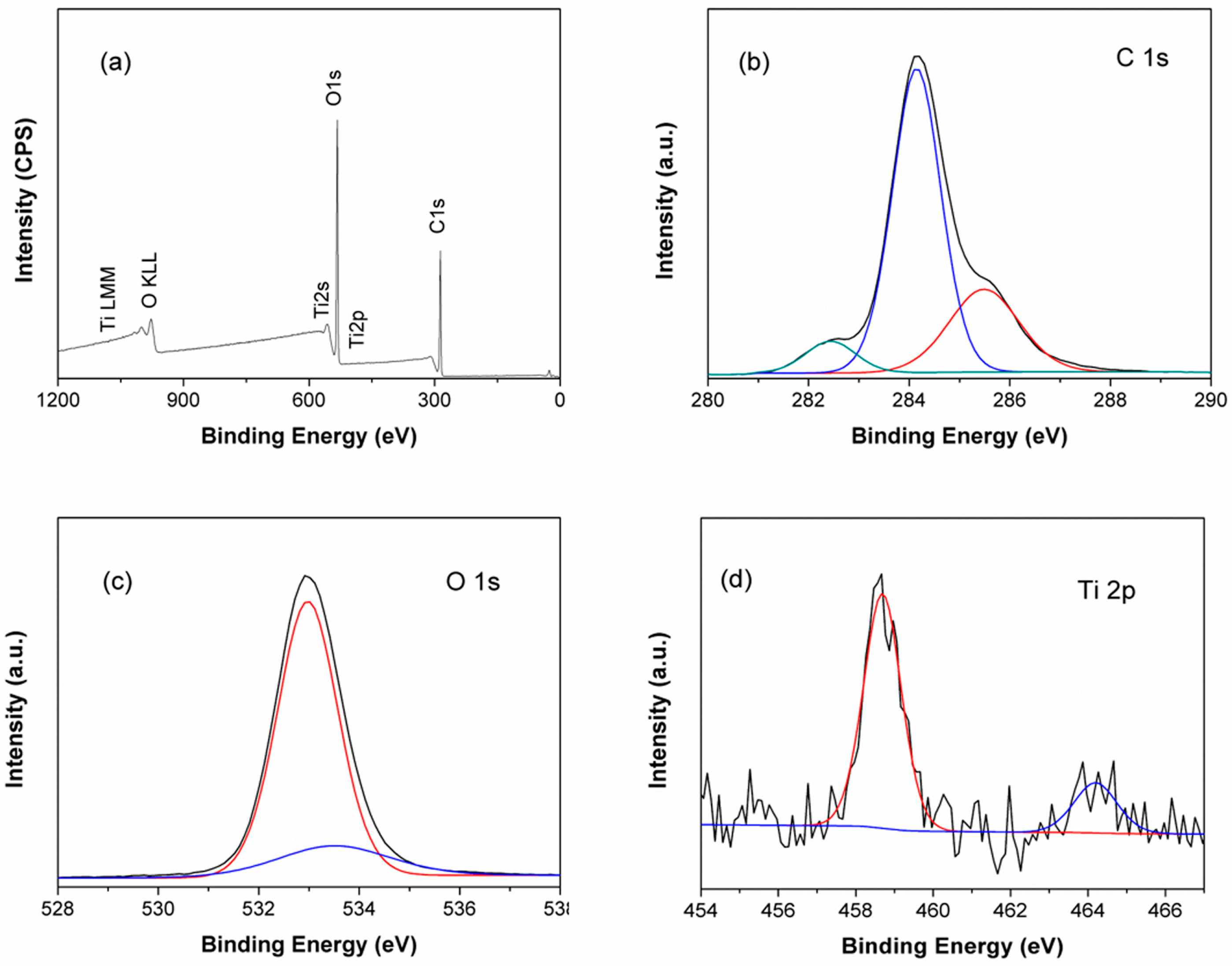
2.7. X-ray Photoelectron Spectroscopy (XPS) Analysis
2.8. Ultraviolet-Visible Spectroscopy (UV-Vis) Transmission Measurement
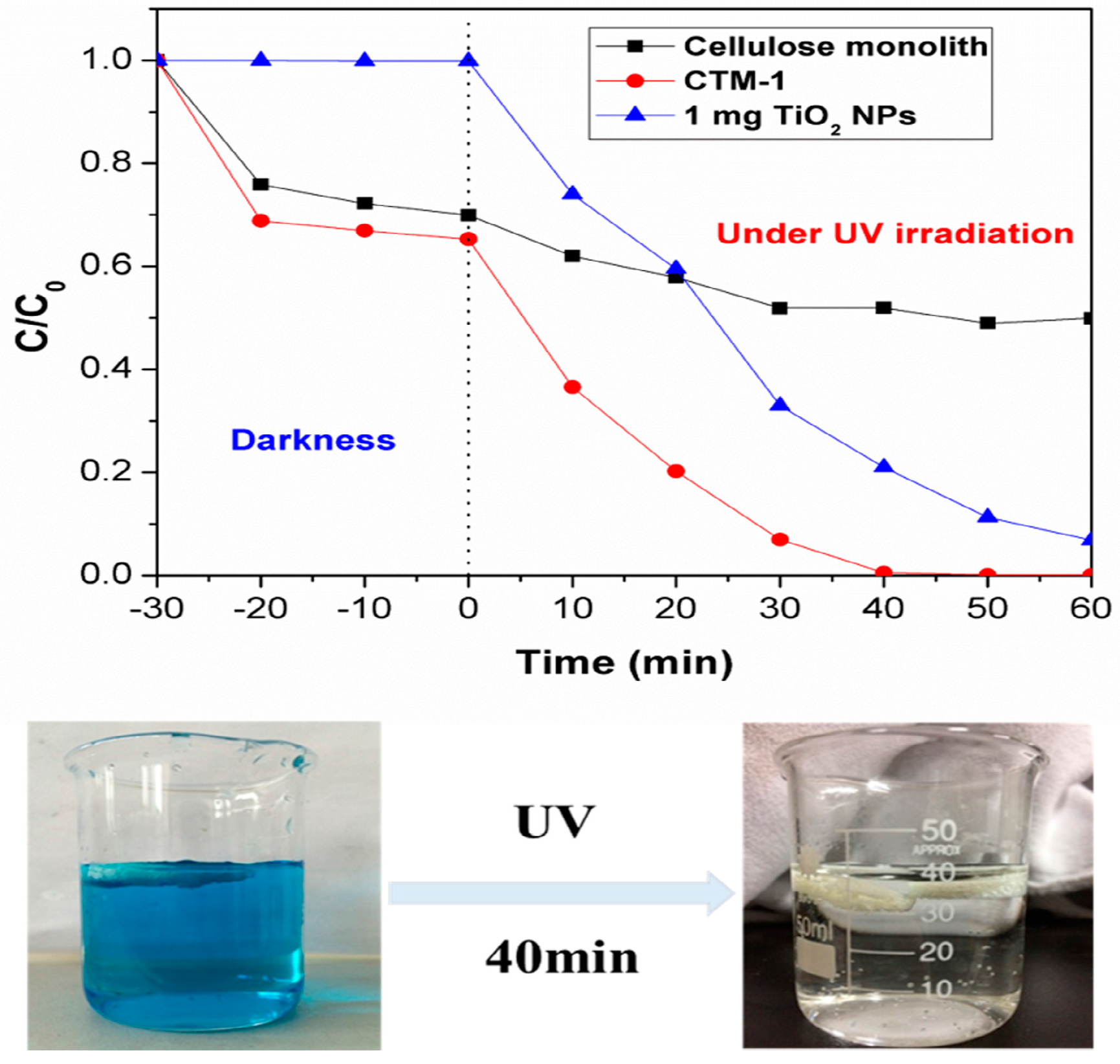
2.9. Photocatalytic Activity
2.10. Photostability Measurements
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Cellulose Solution
4.3. Preparation of Cellulose/TiO2 Monoliths
4.4. Characterizations
4.5. Photocatalytic Degradation of Methylene Blue (MB)
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: kinetic and mechanistic investigations—A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- Jin, X.; Xu, J.; Wang, X.; Xie, Z.; Liu, Z.; Liang, B.; Chen, D.; Shen, G. Flexible TiO2/cellulose acetate hybrid film as a recyclable photocatalyst. RSC Adv. 2014, 4, 12640. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolanb, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Postnova, I.; Kozlova, E.; Cherepanova, S.; Tsybulya, S.; Rempel, A.; Shchipunov, Y. Titania synthesized through regulated mineralization of cellulose and its photocatalytic activity. RSC Adv. 2015, 5, 8544–8551. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.; Zhang, J.; Schwank, J.W. A review on TiO2-based nanotubes synthesized via hydrothermal method: Formation mechanism, structure modification, and photocatalytic applications. Catal. Today 2014, 225, 34–51. [Google Scholar] [CrossRef]
- Gu, S.; Xie, J.; Li, C.M. Hierarchically porous graphitic carbon nitride: Large-scale facile synthesis and its application toward photocatalytic dye degradation. RSC Adv. 2014, 4, 59436–59439. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Jiang, G.; He, G. Hierarchically macro-mesoporous ZrO2-TiO2 composites with enhanced photocatalytic activity. Ceram. Int. 2015, 41, 5749–5757. [Google Scholar] [CrossRef]
- Han, C.; Wang, Y.; Lei, Y.; Wang, B. Modification of hierarchically porous SiC ultrafine fibers with tunable nitrogen-containing surface. Ceram. Int. 2016, 42, 5368–5374. [Google Scholar] [CrossRef]
- Ognibene, G.; Cristaldi, D.A.; Fiorenza, R.; Blanco, I.; Cicala, G.; Scire, S.; Fragala, M.E. Photoactivity of hierarchically nanostructured ZnO-PES fibre mats for water treatments. RSC Adv. 2016, 6, 42778–42785. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, S.; Zhang, H.; Liu, S.; Nawaz, F.; Xiao, F.S. Nanoporous polymer monoliths as adsorptive supports for robust photocatalyst of Degussa P25. J. Colloid Interface Sci. 2009, 339, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Abdal-hay, A.; Makhlouf, A.S.; Khalil, K.A. Novel, Facile, Single-Step Technique of Polymer/TiO(2) Nanofiber Composites Membrane for Photodegradation of Methylene Blue. ACS Appl. Mater. Interfaces 2015, 7, 13329–13341. [Google Scholar] [CrossRef] [PubMed]
- Morawski, A.W.; Kusiak-Nejman, E.; Przepiórski, J.; Kordala, R.; Pernak, J. Cellulose-TiO2 nanocomposite with enhanced UV–Vis light absorption. Cellulose 2013, 20, 1293–1300. [Google Scholar] [CrossRef]
- Wittmar, A.; Thierfeld, H.; Köcher, S.; Ulbricht, M. Routes towards catalytically active TiO2 doped porous cellulose. RSC Adv. 2015, 5, 35866–35873. [Google Scholar] [CrossRef]
- Wittmar, A.; Vorat, D.; Ulbricht, M. Two step and one step preparation of porous nanocomposite cellulose membranes doped with TiO2. RSC Adv. 2015, 5, 88070–88078. [Google Scholar] [CrossRef]
- Sun, X.; Fujimoto, T.; Uyama, H. Fabrication of a poly(vinyl alcohol) monolith via thermally impacted non-solvent-induced phase separation. Polym. J. 2013, 45, 1101–1106. [Google Scholar] [CrossRef]
- Sun, X.; Sun, G.; Wang, X. Morphology modeling for polymer monolith obtained by non-solvent-induced phase separation. Polymer 2017, 108, 432–441. [Google Scholar] [CrossRef]
- Xin, Y.; Xiong, Q.; Bai, Q.; Miyamoto, M.; Li, C.; Shen, Y.; Uyama, H. A hierarchically porous cellulose monolith: A template-free fabricated, morphology-tunable, and easily functionalizable platform. Carbohydr. Polym. 2017, 157, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, K.; ZHANG, B.-X.; Zou, F.; Sun, G.; Han, W.; Wang, X. Hierarchically porous cellulose monolith prepared by combination of an ice-template method and a non-solvent induced phase separation method. Chem. Lett. 2017, in press. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Mohamed, M.A.; Salleh, W.N.; Jaafar, J.; Ismail, A.F.; Mutalib, M.A.; Sani, N.A.A.; Asri, S.E.A.; Ong, C.S. Physicochemical characteristic of regenerated cellulose/N-doped TiO2nanocomposite membrane fabricated from recycled newspaper with photocatalytic activity under UV and visible light irradiation. Chem. Eng. J. 2016, 284, 202–215. [Google Scholar] [CrossRef]
- Zeng, J.L.; Cai, J.; Zhang, L. TiO2 immobilized in cellulose matrix for photocatalytic degradation of phenol under weak UV light irradiation. J. Phys. Chem. 2010, 114, 7806–7811. [Google Scholar]
- Wada, M.; Sugiyama, J.; Okano, T. Native celluloses on the basis of two crystalline phase (Iα/Iβ) system. J. Appl. Polym. Sci. 2003, 49, 1491–1496. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Voorwald, H.J.C.; Cioffi, M.O.H.; da Silva, M.L.C.P.; Rego, A.M.B.; Ferraria, A.M.; de Pinho, M.N. Sugarcane bagasse cellulose fibres and their hydrous niobium phosphate composites: Synthesis and characterization by XPS, XRD and SEM. Cellulose 2014, 21, 641–652. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Salleh, W.N.W.; Jaafar, J.; Ismail, A.F. Structural characterization of N-doped anatase-rutile mixed phase TiO2 nanorods assembled microspheres synthesized by simple sol-gel method. J. Sol-Gel Sci. Technol. 2015, 74, 513–520. [Google Scholar] [CrossRef]
- Sim, L.C.; Tan, W.H.; Leong, K.H.; Bashir, M.J.K.; Saravanan, P.; Surib, N.A. Mechanistic characteristics of surface modified organic semiconductor g-C3N4 nanotubes alloyed with titania. Materials 2017, 10, 28. [Google Scholar] [CrossRef]
- Snyder, A.; Bo, Z.; Moon, R.; Rochet, J.C.; Stanciu, L. Reusable photocatalytic titanium dioxide-cellulose nanofiber films. J. Colloid Interface Sci. 2013, 399, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, A.P.; Patrício, P.S.O.; Coura, I.R.; Pedroso, E.F.; Souza, P.P.; Mansur, H.S.; Mansur, A.; Oliveira, L.C.A. Nanostructured niobium oxyhydroxide dispersed Poly (3-hydroxybutyrate) (PHB) films: Highly efficient photocatalysts for degradation methylene blue dye. Appl. Catal. B Environ. 2016, 189, 141–150. [Google Scholar] [CrossRef]
- Zuo, H.-F.; Guo, Y.-R.; Li, S.-J.; Pan, Q.-J. Application of microcrystalline cellulose to fabricate ZnO with enhanced photocatalytic activity. J. Alloys Compd. 2014, 617, 823–827. [Google Scholar] [CrossRef]
- Zhang, B.-X.; Azuma, J.-I.; Uyama, H. Preparation and characterization of a transparent amorphous cellulose film. RSC Adv. 2015, 5, 2900–2907. [Google Scholar] [CrossRef]










| Type | Degradation Rate | Degradation Time (min) | Reusability | Ref. |
|---|---|---|---|---|
| TiO2/Polymer nanofibers | ~70% | 180 | 4 | [13] |
| TiO2-cellulose nanofiber films | ~75% | 60 | 5 | [28] |
| Niobium oxyhydroxide dispersed Poly(3-hydroxybutyrate) films | ~92% | 120 | 7 | [29] |
| Nanocrystalline ZnO/cellulose | 98.3% | 180 | 5 | [30] |
| TiO2/cellulose monolith | 99% | 40 | 9 | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Wang, K.; Shu, Y.; Zou, F.; Zhang, B.; Sun, G.; Uyama, H.; Wang, X. One-Pot Route towards Active TiO2 Doped Hierarchically Porous Cellulose: Highly Efficient Photocatalysts for Methylene Blue Degradation. Materials 2017, 10, 373. https://doi.org/10.3390/ma10040373
Sun X, Wang K, Shu Y, Zou F, Zhang B, Sun G, Uyama H, Wang X. One-Pot Route towards Active TiO2 Doped Hierarchically Porous Cellulose: Highly Efficient Photocatalysts for Methylene Blue Degradation. Materials. 2017; 10(4):373. https://doi.org/10.3390/ma10040373
Chicago/Turabian StyleSun, Xiaoxia, Kunpeng Wang, Yu Shu, Fangdong Zou, Boxing Zhang, Guangwu Sun, Hiroshi Uyama, and Xinhou Wang. 2017. "One-Pot Route towards Active TiO2 Doped Hierarchically Porous Cellulose: Highly Efficient Photocatalysts for Methylene Blue Degradation" Materials 10, no. 4: 373. https://doi.org/10.3390/ma10040373




