1. Introduction
Silicon plays an extremely important role in semiconductor materials [
1,
2,
3,
4,
5,
6,
7,
8,
9]. In fact, silicon cannot be substituted in the modern semiconductor industry. Almost ninety percent of semiconductor components are based on silicon. In addition, silicon is the second most abundant element on earth, and its processing is most mature at the semiconductor industry level. Moreover, silicon is an optional material in many electronics applications [
10]. In particular, silicon is one of the most important photovoltaic (PV) materials in the solar cell industry [
2,
10]. Monocrystalline, polycrystalline, and amorphous silicon are types of silicon materials used in solar cells. Due to the existing processing conditions, diamond silicon is the primary material used in the solar cell market. However, diamond silicon (space group:
Fd-3
m) is not a direct band-gap semiconductor, and there is a large energy difference (2.3 eV) between the indirect band gap and the direct band gap, which reduces its solar energy absorption efficiency [
10,
11]. Under extreme conditions, a crystal may undergo a phase transition [
10], which may reduce the solar energy absorption efficiency. Besides, the band gap of silicone monolayers can be tuned from semimetallic to semiconducting by oxygen adatoms [
12]. Thus, researchers continue to search for silicon allotropes with direct band gap or better physical properties.
To meet the needs of industry for materials with efficient solar energy absorption, many silicon allotropes have been reported. Many semiconductor silicon allotropes have been proposed [
2,
4,
8,
9,
10,
11,
13]. Wang et al. [
4] found six other metastable silicon allotropes with direct or quasi-direct band gaps of 0.39–1.25 eV utilizing Vienna An-initio Simulation Package (VASP) code. They found that these six metastable silicon allotropes not only have a direct band gap or quasi-direct band gap but also have better optical properties than diamond silicon. Recently, Fan et al. [
10] investigated four silicon allotropes, including one quasi-direct band-gap phase (
Amm2) with a band gap of 0.74 eV and three indirect band-gap phases (
C2/
m-16,
C2/
m-20, and
I-4) with band gaps of 0.56, 0.53, and 1.28 eV, respectively. The
B/
G values indicate that all four phases of silicon are brittle. Moreover, in Ref. [
13], Feng et al. not only report the structural properties of two new allotropes, but also elucidate the formation of silicone on an Ag (111) surface. In addition, they found that the two phases have quite similar formation energies and stabilities. Feng et al. [
13] obtained silicene by epitaxial growth on conductive substrates. However, the strong silicene-substrate interaction may depress its superior electronic properties [
14]. Thus, Du et al. [
14] improved the growth method of silicone on Ag (111) by oxygen intercalation and also successfully obtained silicone through the oxidization of bilayer silicene on an Ag (111) surface. In addition, the interactions between the top layer of silicone and the underlying silicene oxide or substrate were weakened by oxidization in this method. Oh et al. [
15] presented super-stable pure-silicon superlattice structures that can be applied in solar cell industry and can lead to the realization of pure Si-based optoelectronic devices. Moreover, the minimum band gap of the structure is smaller than that of diamond silicon (the experimental value of diamond silicon 1.12 eV [
11]) [
15]. While the enthalpy of many of the superlattcies is lower than
P2/
m Si. The enthalpy is very close to diamond silicon. In addition, Lee et al. [
16] reported two inverse design approaches for the discovery of new photovoltaic materials and successfully predicted favourable structures using the Conformational Space Annealing algorithm. One of the advantages of this approach is that the crystal structures do not need to be known. Kim et al. [
17] reported a structure in
Cmcm space group, and this structure is an indirect band-gap semiconductor with a band gap of 1.41 eV. Besides, they also examined the dynamical stability. And the structure is dynamically stable to 10 GPa. Guo et al. [
18] found a missing structure, the h-Si
6 silicon phase in the
P6
3/
mmc space group, by using silicon triangles as building blocks. Using first-principles calculations, they confirmed that this structure has thermal, dynamical, and mechanical stability. In addition, h-Si
6 is a direct band-gap semiconductor with a band gap of 0.61 eV and has remarkably better optical properties than diamond silicon.
In this work, a new silicon allotrope is proposed. The original structure of
P2/
m-Si is composed of a lattice similar to that of carbon, in which Si is substituted for C [
19]. The calculated formation enthalpy (0.08 eV/atom larger than that of diamond silicon [
11]) indicates that
P2/
m-Si has a higher thermodynamic stability than that of
tP16-Si (0.28 eV/atom larger than that of diamond silicon [
11]). Moreover, this paper also examines, in detail, physical properties such as structural properties, elastic properties and elastic anisotropic properties. In Ref. [
20], the authors present a very similar silicon allotrope with strong absorption in the visible region for photovoltaic applications. In addition, the structure in Ref. [
20] has a total energy that is higher than that of diamond silicon by less than 0.15 eV/atom, while the energy of
P2/
m-Si is higher than that of diamond silicon by 0.08 eV/atom. This restriction assures that the experimental synthesis of these structures is energetically feasible [
20].
3. Results and Discussion
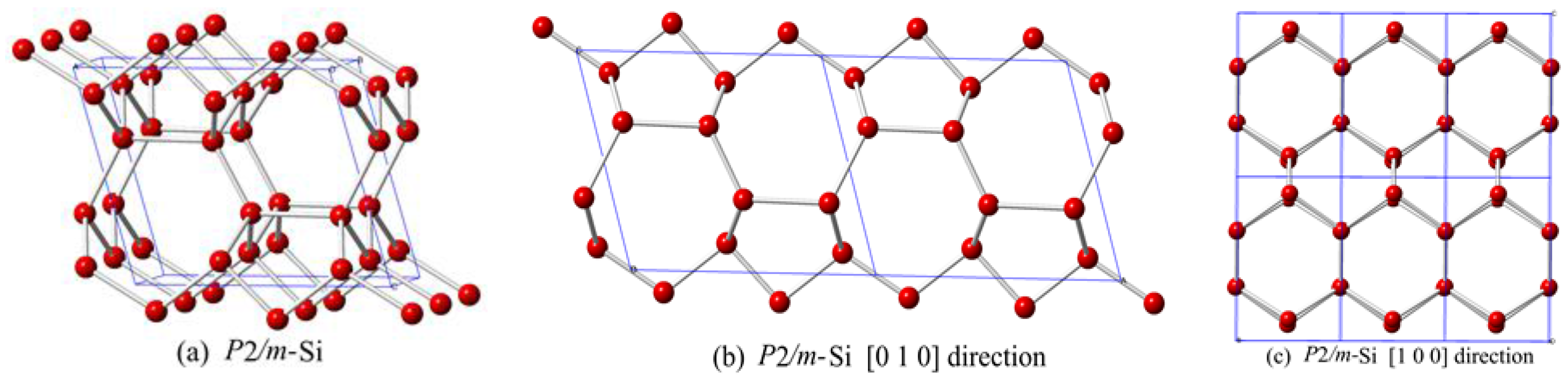
This paper reports a new silicon allotrope in the
P2/
m space group. The crystal structure of
P2/
m-Si is shown in
Figure 1. The structure of
P2/
m-Si is composed of six-membered silicon rings that are similar to graphite. The zigzag six-membered silicon ring affords
P2/
m-Si a lower density than other silicon allotrope materials. The positions of silicon atoms in
P2/
m-Si are Si1 (0.1114, 0.5000, 0.8949), Si2 (0.1217, 0.0000, 0.6741), Si3 (0.5411, 0.0000, 0.3330), and Si4 (0.4194, 0.5000, 0.1340).
P2/
m-Si can be described as corrugated graphite sheets interconnected by an alternating sequence of odd 5- or 7-membered rings of silicon, when viewed along the [010] direction, as shown in
Figure 1b. However, the structure consists of 6-membered rings of silicon when viewed in the [100] direction. The lattice parameters of
P2/
m-Si at zero pressure are shown in
Table 1. From
Table 1, the calculated lattice parameters of diamond silicon (
a = 5.426Å) are in excellent agreement with the reported experimental results (
a = 5.431 Å) [
29]. The values obtained by PBE are closer to the experimental values for diamond silicon than the other methods. Thus, the following discussion mainly gives results calculated by the PBE method. In addition, the calculated results in this work for space group
P222
1 are consistent with the results of Fan et al. [
5].
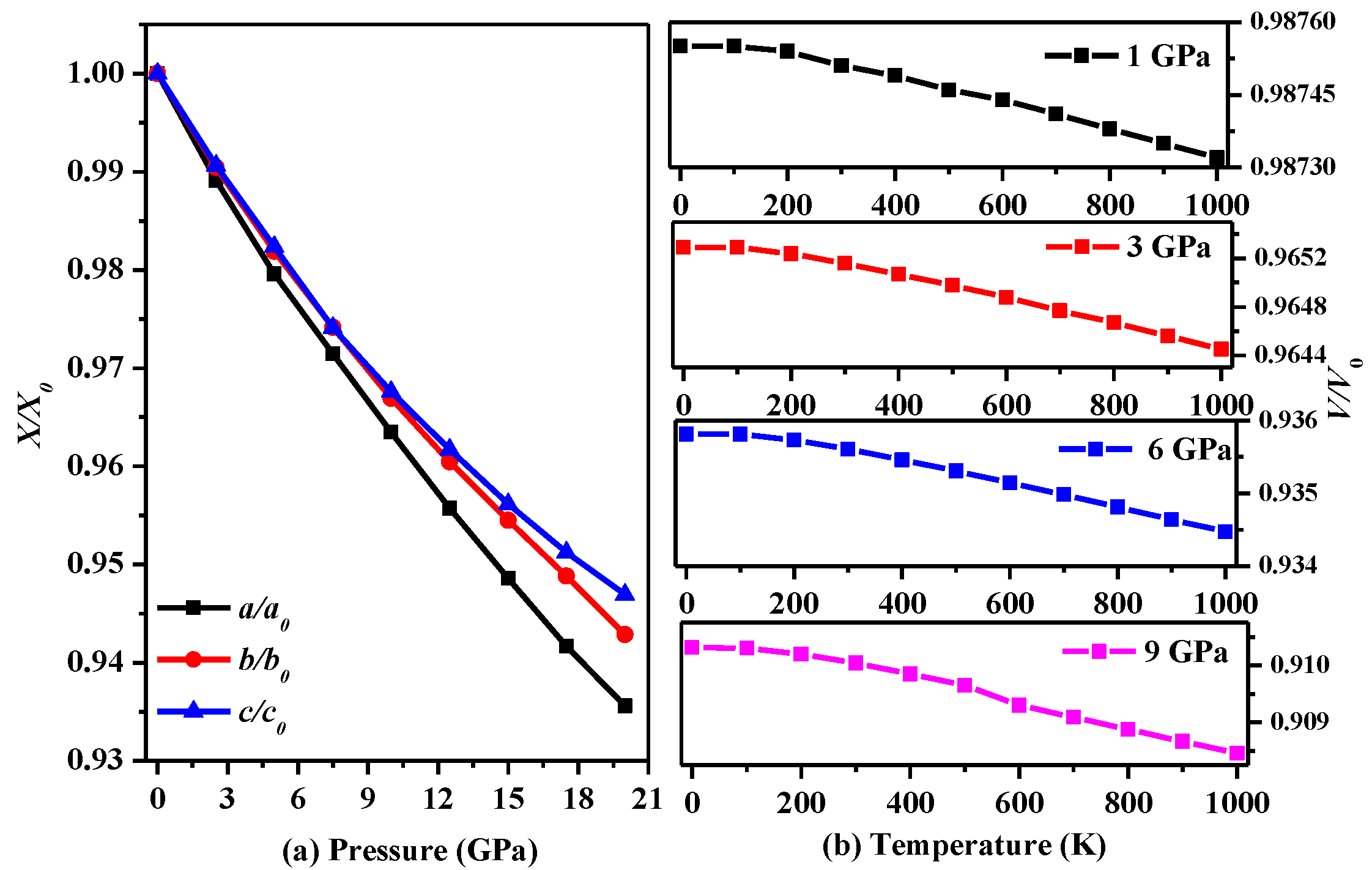
In addition, the influences of temperature and pressure on the lattice parameters are further studied, and the results are shown in
Figure 2.
Figure 2a presents trends in the lattice constants of
P2/
m-Si with increasing pressure. As seen in
Figure 2a, it is very difficult to compress
P2/
m-Si in the
c-axis direction, while compression in the
a-axis direction is the easiest. In addition, the difference in the lattice parameters of
P2/
m-Si and diamond silicon along the
a-axis is larger than that along the other axes. In Ref. [
5],
P222
1-Si is shown to be compressed easily in the
b-axis direction. In addition, the decline in the slope of the lattice parameters lessens when the pressure is larger than 12 GPa.
Figure 2b describes the trend in the volume with increasing pressure at different temperatures. The trend in the volume at different temperatures remains roughly the same as the pressure increases, indicating that this material may be used in environments that undergo serious temperature changes.
The elastic constants C
ij, bulk modulus
B, shear modulus
G and Young’s modulus
E of
P2/
m-Si calculated by the PBE method are given in
Table 2.
Table 2 also lists the elastic constants and the elastic modulus of diamond silicon and other silicon allotropes. Monoclinic symmetry gives 13 independent elastic constants [
32], which are listed in
Table 2. If the allotrope is a stable monoclinic structure, the elastic constants of
P2/
m-Si should satisfy the mechanical stability criteria of monoclinic symmetry [
32].
From
Table 2, as the mechanical stability criteria of monoclinic symmetry are satisfied, it can be concluded that
P2/
m-Si is mechanically stable [
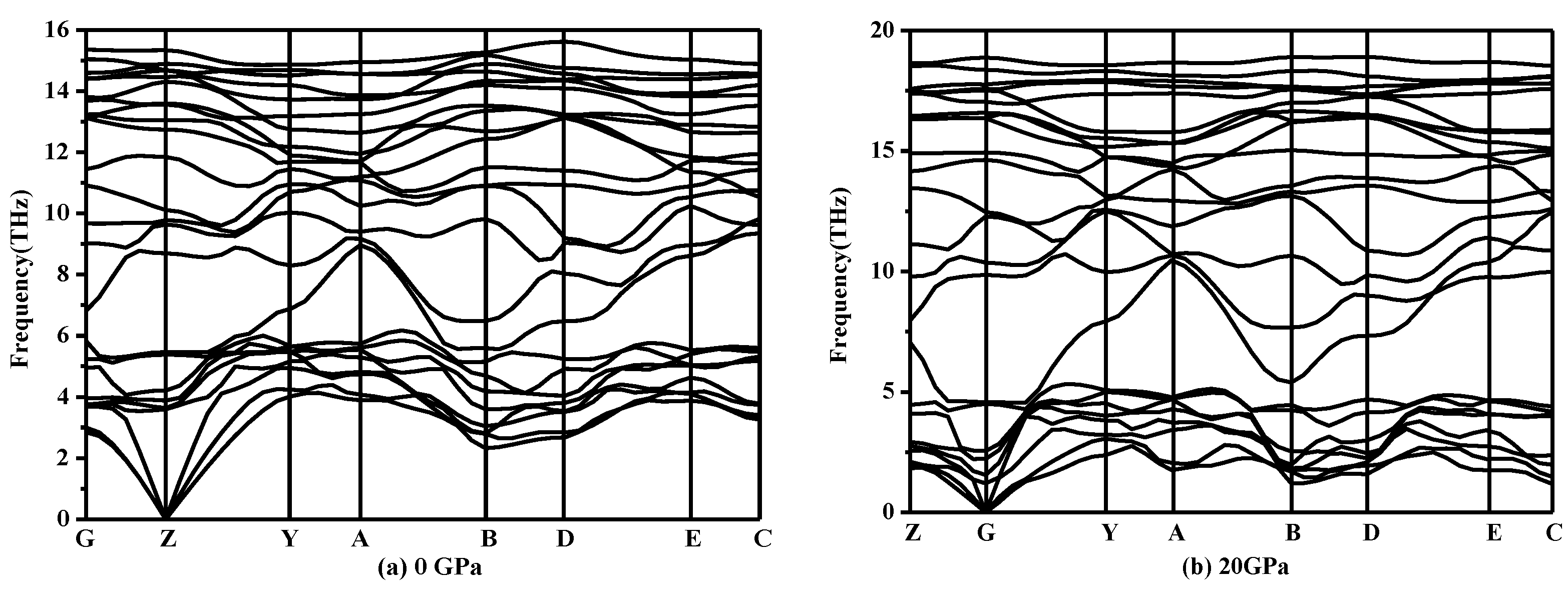
34]. Meanwhile, the phonon spectra of
P2/
m-Si was also investigated under different pressures. The phonon spectra should show no imaginary frequencies over the entire Brillouin zone, which indicates that the phase is dynamically stable [
35]. The phonon spectra of
P2/
m-Si at different pressures are shown in
Figure 3. The phonon spectra of
P2/
m-Si shows no imaginary frequencies over the entire Brillouin zone between 0 GPa and 20 GPa, which indicates that
P2/
m-Si is dynamically stable up to 20 GPa. Above this pressure,
P2/
m-Si is destabilized, indicating that a structural transformation occurs.
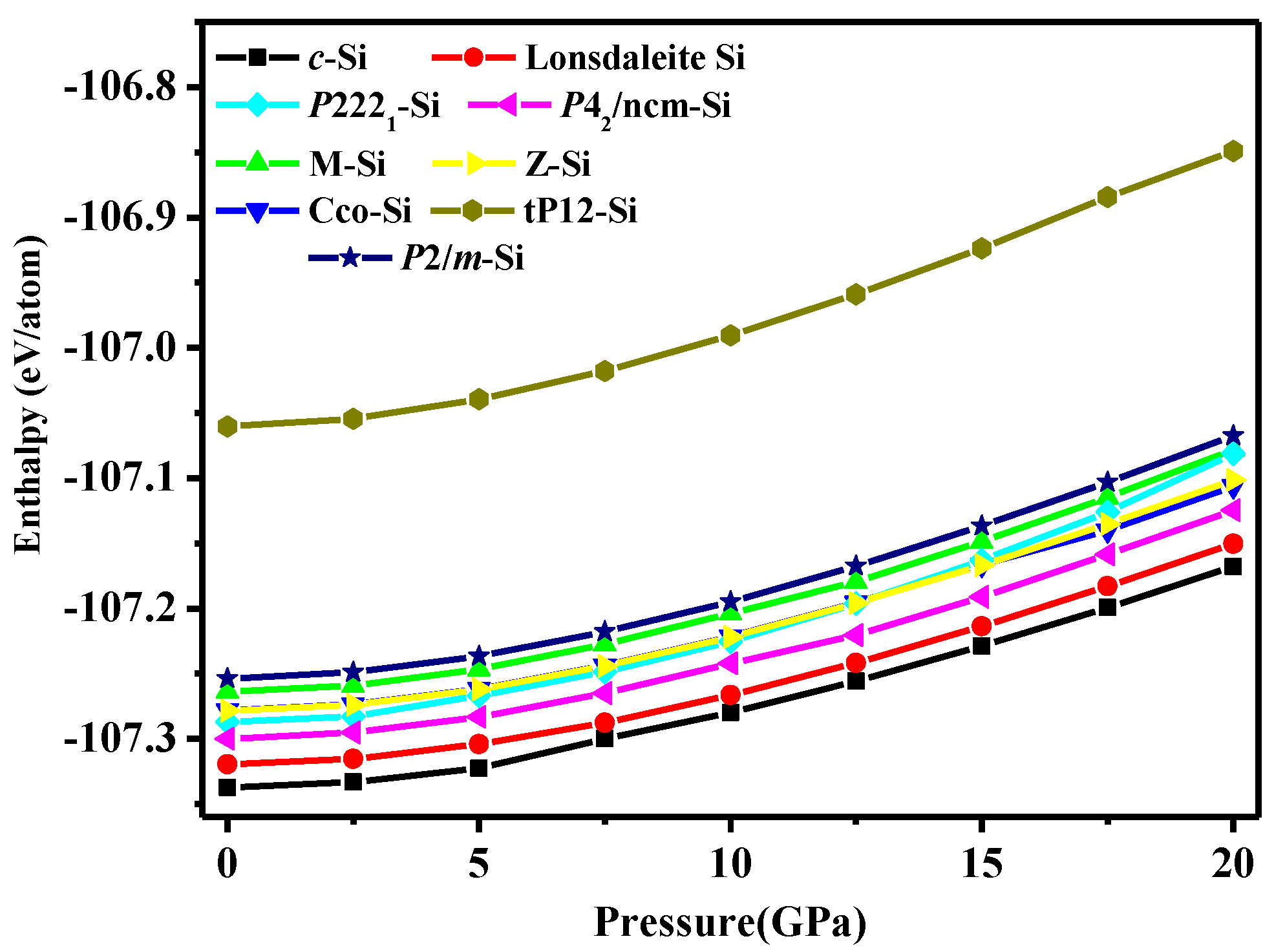
The enthalpy of
P2/
m-Si is an important factor in the stability, which measures the thermodynamic stability. The enthalpies of different silicon allotropes as the pressure increases are shown in
Figure 4. It is clear that diamond silicon is the most stable phase over the entire pressure range in
Figure 4. In Ref. [
11] and this work, the most unfavourable structure is the
tP12 phase (which is higher in energy than diamond silicon by 0.276 and 0.269 eV/atom at zero pressure in this work and in Ref. [
4], respectively), but
tP12-Si is still dynamically and mechanically stable [
4]. The curve of
tP12-Si is also shown in
Figure 4, while the energy of
P2/
m-Si is lower than that of
tP12-Si by 0.19 eV/atom at zero pressure and 0.22 eV/atom at a pressure of 20 GPa. Simultaneously,
P2/
m-Si is higher in energy than diamond silicon by 0.08 eV/atom at zero pressure. In addition, the enthalpy of
P2/
m-Si is smaller than that of
Amm2 (−170.23 eV/atom),
C2/
m-16 (−170.21 eV/atom),
C2/
m-20 (−170.24 eV/atom), and
I-4 Si (−170.24 eV/atom) [
10], respectively. The calculated formation enthalpy indicates that
P2/
m-Si has a higher thermodynamic stability than that of
Amm2 Si,
C2/
m-16 Si,
C2/
m-20 Si,
I-4 Si, and superlattices. There are many intermediate phases between
P2/
m-Si and diamond silicon, and the excess enthalpy of each structure relative to diamond silicon is shown in
Table 3. Most of the structures have enthalpies higher than diamond silicon (−170.34 eV/atom). Although the energies of
M-Si,
Cco-Si,
P222
1-Si,
Z-Si,
tP12-Si, and
P2/
m-Si are higher than that of diamond silicon, while their enthalpies are very close to that of diamond silicon. The order of enthalpy from high to low is
tP12-Si >
P2/
m Si >
M Si >
Pmmn Si >
Cco Si >
Pbam Si >
P222
1 Si >
P4
2/
ncm Si >
Cmcm Si >
P2
1/
m Si > Lonsdaleite Si. The enthalpy of many of the superlattcies is lower than
P2/
m Si. However,
tP12-Si is still thermodynamically dynamically and mechanically stable. So the
P2/
m Si is thermodynamically stable.
Table 2 lists the bulk moduli, shear moduli, Young’s moduli, and Poisson’s ratios of
P2/
m-Si, diamond silicon and other silicon allotropes. The bulk modulus and shear modulus are both important characteristics of a material. The bulk modulus and shear modulus represent the resistance to fracture and plastic deformation, respectively [
34]. The bulk modulus of
P2/
m-Si is slightly smaller than that of diamond silicon. The shear modulus of
P2/
m-Si is also slightly smaller than that of diamond silicon but is the greatest among those of
P2/
m-Si,
C2/
m-16 Si,
C2/
m-20 Si, and
I-4 Si [
10]. The hardness of a material can determine the plastic and elastic properties to some extent and can be predicted by following formula [
34]:
In the above formula,
k is equal to
G/
B. The calculated hardness of
P2/
m-Si is 10.0 GPa, while the hardness of diamond silicon is 12.4 GPa. However, the hardness of
P2/
m-Si is slightly larger than that of the
C2/
m-16,
C2/
m-20,
I-4 and
Amm2 phases (
C2/
m-16: 8.4 GPa,
C2/
m-20: 9.8 GPa,
I-4: 7.6 GPa,
Amm2: 9.1 GPa) [
10]. In
P2/
m-Si, there are eight different bond lengths: 2.3945, 2.4582, 2.3365, 2.3420, 2.3274, 2.3522, 2.3008, and 2.4099 Å, and the average bond length is 2.3652 Å, which is slightly smaller than that of diamond silicon (2.3729 Å) [
10]. The bond lengths of
P2/
m-Si are very close to that of diamond silicon, indicating that the bond energy of the Si-Si bonds should be as high as that of diamond silicon.
P2/
m-Si has the smallest average bond length among the
C2/
m-16,
C2/
m-20,
I-4, and
Amm2 phases, and thus the hardness of
P2/
m-Si is the greatest. On the other hand, there is only one bond length in diamond silicon, but in the other silicon allotropes, including
P2/
m-Si, there are seven, eight, and even nine or more bond lengths in which the extra bond lengths are all larger than that of diamond silicon. Thus, the bond energy of these extra Si-Si bonds is weaker than that of diamond silicon, making the hardness of the other silicon allotropes, including
P2/
m-Si, lower than that of diamond silicon.
The
B/
G ratio of
P2/
m-Si is 1.48, as calculated by the PBE method, meaning
P2/
m-Si is a brittle material, according to the theory proposed by Puge in which the
B/
G value of a brittle material is less than 1.75 [
36,
37]. The
B/
G ratio of
P2/
m-Si is slightly greater than that of diamond silicon (1.38); that is to say, diamond silicon is more brittle. The
B/
G ratio of
C2/
m-16 Si is 1.60,
C2/
m-20 Si is 1.50,
I-4 Si is 1.68,
Amm2 Si is 1.54 [
10], and
P222
1 Si is 1.54 [
5]. Therefore,
P2/
m-Si is the most brittle among
C2/
m-16 Si,
C2/
m-20 Si,
I-4 Si,
Amm2 Si, and
P222
1 Si. In addition, Poisson’s ratio
v, which gives the ratio of the fraction of expansion and the fraction of compression, can be obtained by the following equation [
26]:
According to Ref. [
38], the Poisson’s ratio
v of a ductile material is usually larger than 0.26, while brittle materials usually have a small
v (
v < 0.26) [
38]. Moreover, the Poisson’s ratio
v of
P2/
m-Si is consistent with its
B/
G, and
P2/
m-Si is characterized as a brittle material via its Poisson’s ratio
v. At the same time, the Poisson’s ratio of
Amm2 (0.23) is slightly larger than that of
P2/
m-Si. The Poisson’s ratio of
I-4 Si is the largest (0.25), and that of diamond silicon is the smallest (0.21) by only a difference of 0.04 [
10].
Young’s modulus, which is calculated from the bulk modulus and shear modulus in Formula (11) [
39], is the physical attribute describing the resistance of the material to deformation.
According to the listed values of
E in
Table 2, the Young’s modulus of
P2/
m-Si is smaller than that of diamond silicon and larger than that of
C2/
m-20,
I-4,
Amm2 and
C2/
m-16 Si. That is, the mechanical properties of
P2/
m-Si are the greatest among
C2/
m-20,
I-4,
Amm2 and
C2/
m-16 Si.
It is well known that the elastic anisotropy is an important parameter in engineering science and crystal physics. Crystals show different physical and chemical properties in different directions. In addition, the anisotropy represents the extent of this property. The universal anisotropic index
AU and percentage of elastic anisotropy for the bulk modulus and shear modulus can be calculated by the following equations [
39,
40]:
where
BV represents the bulk modulus found using the Voigt method and
BR represents the bulk modulus found using the Reuss method. In addition,
GV and
GR represent the shear modulus found using the Voigt and Reuss method, respectively.
AU takes both bulk and shear effects into consideration, and the extent of anisotropy increases with larger values of
AU [
4,
26,
41]. The interrelated factors of elastic anisotropy for
P2/
m-Si are shown in
Table 4. From
Table 4, the
AU of
P2/
m-Si is smaller than that of diamond silicon. The order from large to small according to
AU in
Table 4 is diamond silicon >
P2/
m Si >
C2/
m-20 Si >
C2/
m-16 Si >
Amm2 Si >
I-4 Si [
10].
AB and
AG of the
P2/
m phase are the smallest among the listed allotropes, which indicates that it has least anisotropy in its shear modulus among these silicon allotropes [
34]. The
AB values of
P2/
m-Si and
I-4 Si are almost the same as that of diamond silicon, which indicates that they have the least anisotropy in the bulk modulus, similar to diamond silicon.
The shear anisotropy factors are calculated in this work to verify the
P2/
m-Si elastic anisotropy along different orientations (shown in
Table 4). In addition,
Table 4 also lists the compression anisotropy factors and the shear anisotropy factors of
P2/
m-Si and the
C2/
m-20,
C2/
m-16,
Amm2,
I-4, and
Fd-3
m phases. The value of the shear anisotropy factors represents the level of anisotropy in the bonding between atoms in different planes. The three formulas defining the shear anisotropy factors are as follows [
34,
42]:
where
A1 is the factor for the (100) shear plane between the [011] and [010] directions,
A2 is for the (010) shear plane between the [101] and [001] directions, and
A3 is for the (001) shear plane between the [110] and [010] directions. A value with a larger offset than 1 implies that the crystal behaves more like an anisotropic material, and a value in the vicinity of 1 indicates an isotropic crystal. The results in
Table 4 indicate that
P2/
m-Si shows more distinct performance. The shear anisotropy factors of
P2/
m-Si along different directions are very different. In particular, there is a larger offset between
A3 and 1, while the other two directions are close to 1. The value for the three directions of diamond silicon are the all smaller than 1 by 0.227 (diamond silicon:
A1: 0.773). It is clear that
P2/
m-Si exhibits a larger anisotropy in
A3 than that of diamond silicon, while
P2/
m-Si exhibits a smaller anisotropy in
A1 and
A2 than diamond silicon. Meanwhile, the
Amm2 and
C2/
m-20 phases exhibit a larger anisotropy in
A3 than diamond silicon, while the
I-4 phase exhibits the smallest anisotropy in
A1 and
A2.
The fundamental physical and chemical properties of materials depend on the electronic structure. However, the DFT method typically underestimates the electronic band structure [
36]. The true band gap is usually larger than the simulation result. The Heyd-Scuseria-Ernzerhof (HSE06) functional was examined in consideration of this problem. The results of HSE06 are relatively accurate. The hybrid functional HSE06 was used in the following form [
36,
43,
44]:
where the HF mixing parameter
μ is 0.25 and the screening parameter
ω is 0.207 Å
−1 [
44,
45]. The electronic band structure of
P2/
m-Si was calculated using the GGA-PBE and HSE06 functionals, and the electronic band structures of
P2/
m-Si are shown in
Figure 5a.
Figure 5a clearly shows that
P2/
m-Si is an indirect-band-gap semiconductor with a band gap of 1.51 eV, as determined by the HSE06 hybrid functional. The result calculated by the HSE06 hybrid functional is much larger than the result from the GGA-PBE functional (0.87 eV). In addition, the band gap was found to be higher than that of diamond silicon by 0.23 eV using the same calculation method (diamond silicon: 1.28 eV [
11,
44]). Moreover, the band gap of diamond silicon calculated by HSE06 is very close to the experimental value (1.12 eV [
11,
30]). The charge effective mass, which is also a key physical property for applications, can be obtained by the formula m
* =
h2/(
d2E/
d2k) in which
h is the planck constant,
E is energy,
k is wave vector. (m
*n = 0.023m
0, m
*p = 0.019m
0.). The effective mass reflects the carrier mobility indirectly (
μ =
qτ/m
∗). The experimental effective mass at the
Γ valley for
a-GaN is 0.2m
0 [
46], while the effective mass for a hole in
a-GaN at the valence band is 0.6m
0. Besides, the effective mass at the
Γ valley for GaAs is 0.0635m
0 at the temperature 300 K [
47]. The effective mass at the
Γ valley for Ge is 0.038m
0 at the temperature 300 K. The averaged light-hole mass in [001] is 0.046m
0 [
48]. The calculated effective mass of
P2
/m-Si is much smaller than that of GaN. Therefore, the mobility of
P2/
m-Si is larger than that of GaN, because the mobility is inversely proportional to the effective mass (μ = qτ/m
∗). The electronic mobility of
P2/
m-Si is close to Ge. The density of states of
P2/
m-Si is displayed in
Figure 5b, and the inset figure illustrates the local features at the Fermi level in detail. The dashed line represents the Fermi level. The DOS is divided into three regions: −13.5 eV to −5 eV, −5 eV to 0 eV and 0 eV to 7 eV. The lowest band ranging from −13.5 eV to −5 eV is characterized by the Si1-
s, Si2-
s, Si3-
s and Si4-
s orbitals. The middle band ranging from −5 eV to 0 eV is characterized by the Si1-
p, Si2-
p, Si3-
p and Si4-
p orbitals, and the upper band is also characterized by the Si1-
p, Si2-
p, Si3-
p and Si4-
p orbitals. In addition, the energy of the upper band is above 0.86 eV.
The thermodynamic properties of semiconductor materials at higher temperatures and pressures are interesting [
38]. In this work, the highest pressure examined is 10 GPa, and the highest temperature is 1000 K. In the above conditions, the thermal expansion coefficient
α, Grüneisen parameter
γ and heat capacity were calculated.
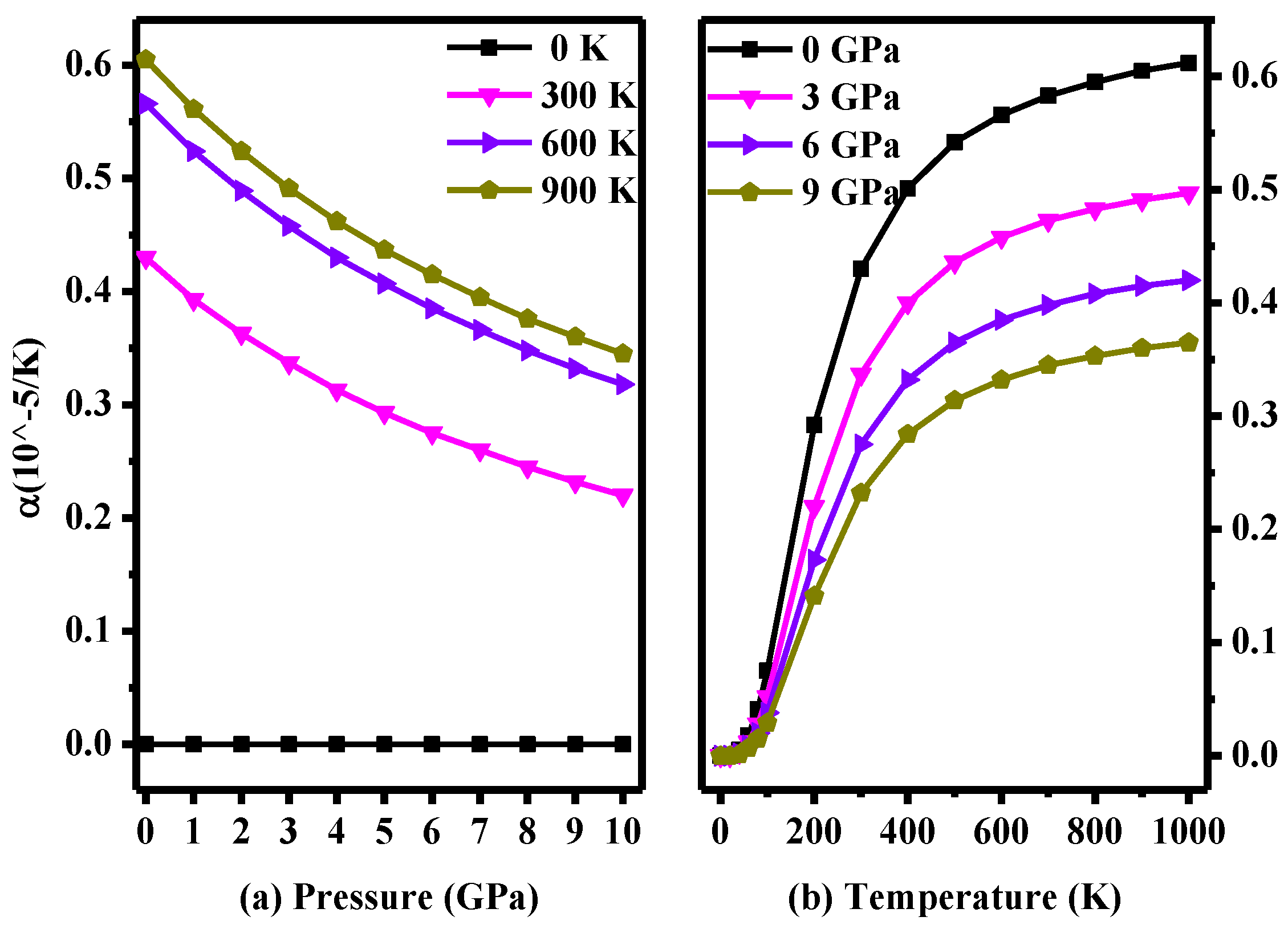
Figure 6 shows the thermal expansion coefficient
α for
P2/
m-Si as functions of temperature and pressure.
Figure 6a presents the relationship between the thermal expansion coefficient and pressure at different temperatures. The reduction in the amplitude decreases as the pressure increases, as shown in
Figure 6a, and the difference in
α is small at the same temperature. The reduction in amplitude at lower pressure is larger than that at higher pressure. However, the thermal expansion coefficient is constant when the temperature is 0 K (
α = 0 × 10
−5/K), and the difference in
α between 600 K and 900 K (0.04 × 10
−5/K at 0 GPa) is smaller than that between 300 K and 600 K (0.24 × 10
−5/K at 0 GPa). As seen in
Figure 6b, the thermal expansion coefficient increases quickly with increasing temperature, especially when the temperature is below 500 K. The increase in amplitude becomes very small when the temperature is over 500 K. Moreover, the thermal expansion coefficient increases less at increased pressure. Thus, by comparing
Figure 6a with
Figure 6b, it can be determine that the expansion coefficient
α is more sensitive to the temperature when the temperature is below 500 K. From
Figure 6, it is clear that the effect of pressure on the expansion coefficient
α is not as significant as the effect of temperature over calculated pressure and temperature ranges.
Figure 7 shows the change in the Grüneisen parameter
γ with temperature and pressure, where the Grüneisen parameter
γ is expressed as
γ = −(dlnΘ(V)/
dlnV) [
49], where
Θ is the Debye temperature and
V is the volume. This parameter describes the anharmonic effects in the lattice vibrations [
38]. As seen in
Figure 7a, the
γ parameter reduces by 0.045 per GPa, and the values are very close at different temperatures. In other words, the effect of pressure on the Grüneisen parameter
γ is greater than that of temperature. As seen in
Figure 7b, the
γ parameter is not very sensitive to temperature. As the temperature increases to 1000 K, the Grüneisen parameter
γ only increases by 0.02 at 6 GPa and 0.01 at 7 GPa or more.
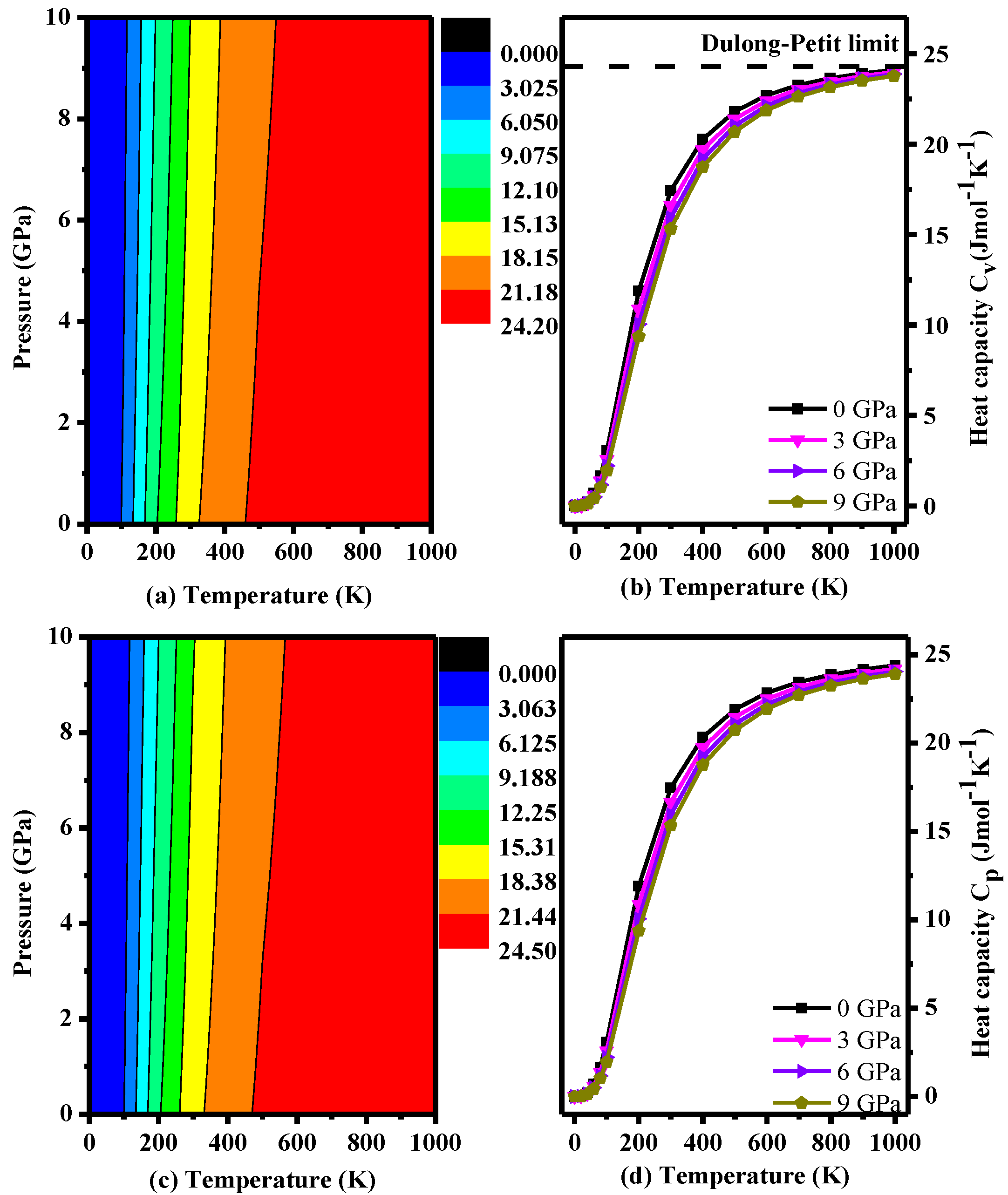
This study also examined the heat capacity at constant volume (
CV) and constant pressure (
CP) (
Figure 8), which were calculated by the following formulas [
38]:
where
n is the number of atoms per formula unit,
Θ is the Debye temperature,
k is Boltzmann constant and D (
Θ/T) represents the Debye integral [
38]. As seen in
Figure 8, the difference between
CP and
CV is very small. In addition, the trend is basically consistent with the trend at different pressures. Both plots increase as the temperature increases. It is obvious that the heat capacity is sensitive to temperature when
T < 500 K; however, the magnitude of the increasement becomes very small when the temperature is over 500 K. In addition, the effect of pressure on the heat capacity is very small.
CV stays close to constant at the Dulong-Petit limit (24.92 J·mol
−1·K
−1) [
38] at high temperature and high pressure. Thus, the heat capacity of
P2/
m-Si is mainly affected by the temperature, and the effect of the pressure is small compared to that of the temperature.