RETRACTED: Epoxy Resins Toughened with Surface Modified Epoxidized Natural Rubber Fibers by One-Step Electrospinning
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. Materials
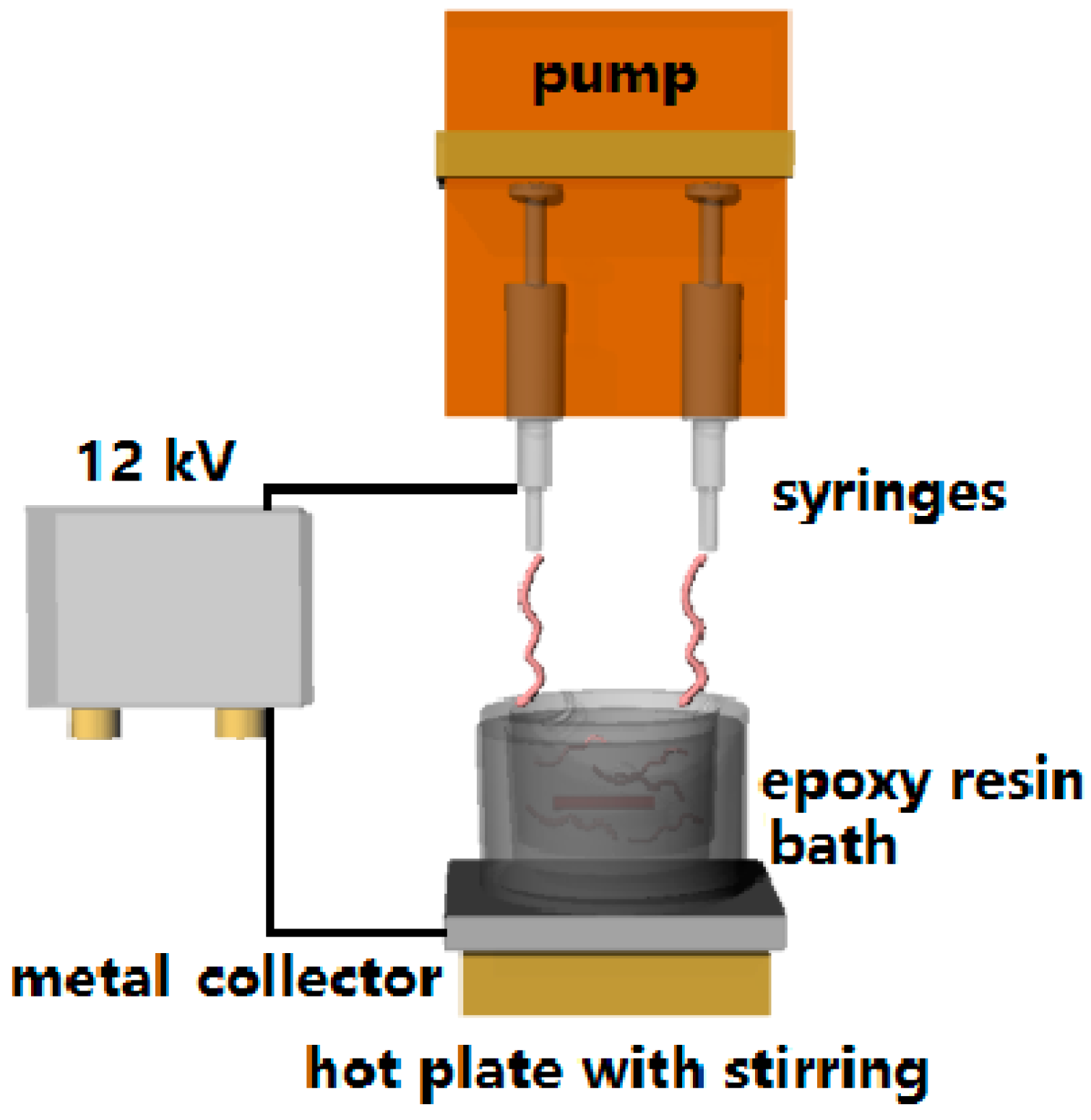
3.2. Electrospinning of ENR Fibers
3.3. Fabrication Process of Epoxy Composites at Different Loadings of ERFs
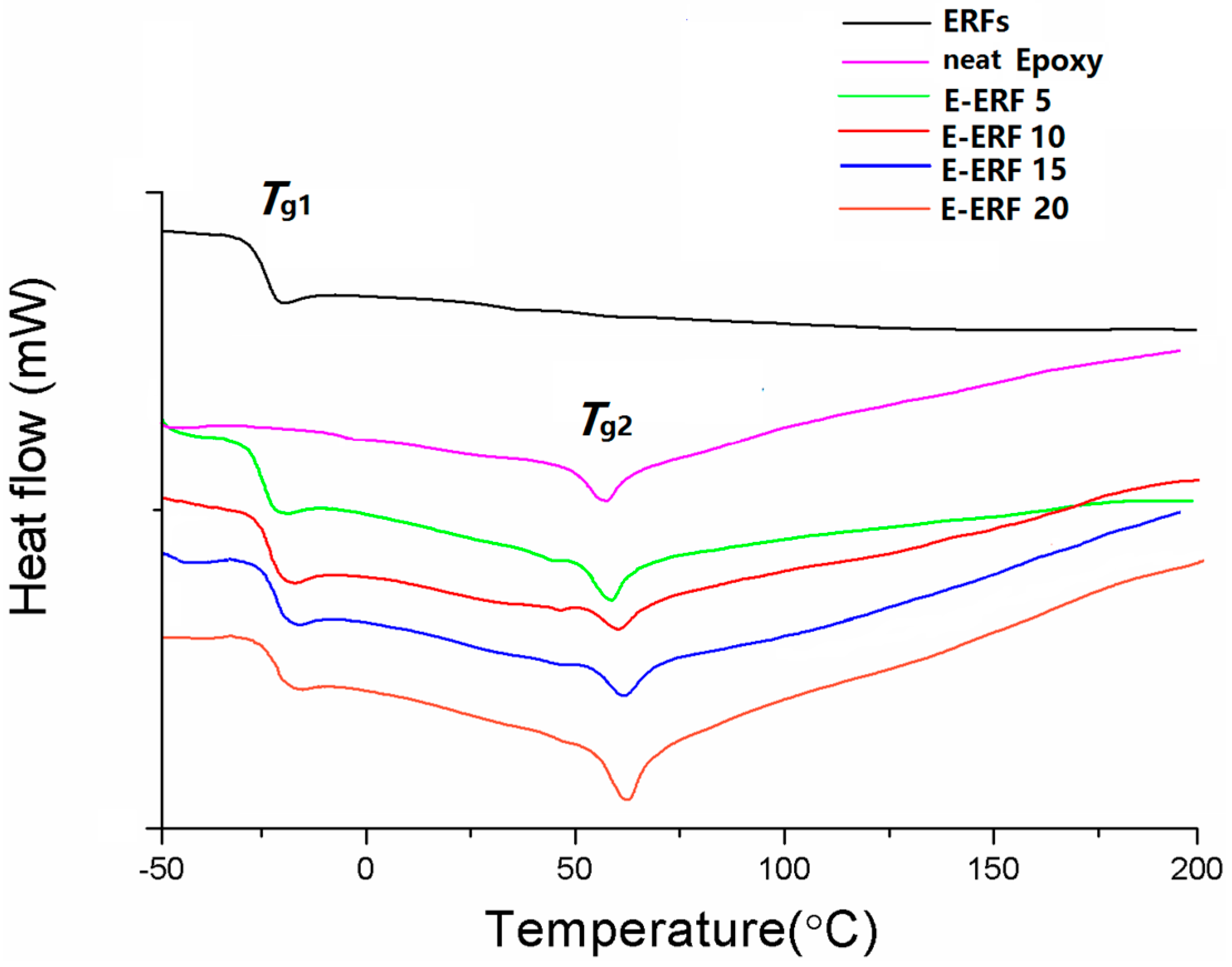
3.4. Thermal Analysis
3.5. Tensile Properties of E-ERF Resins
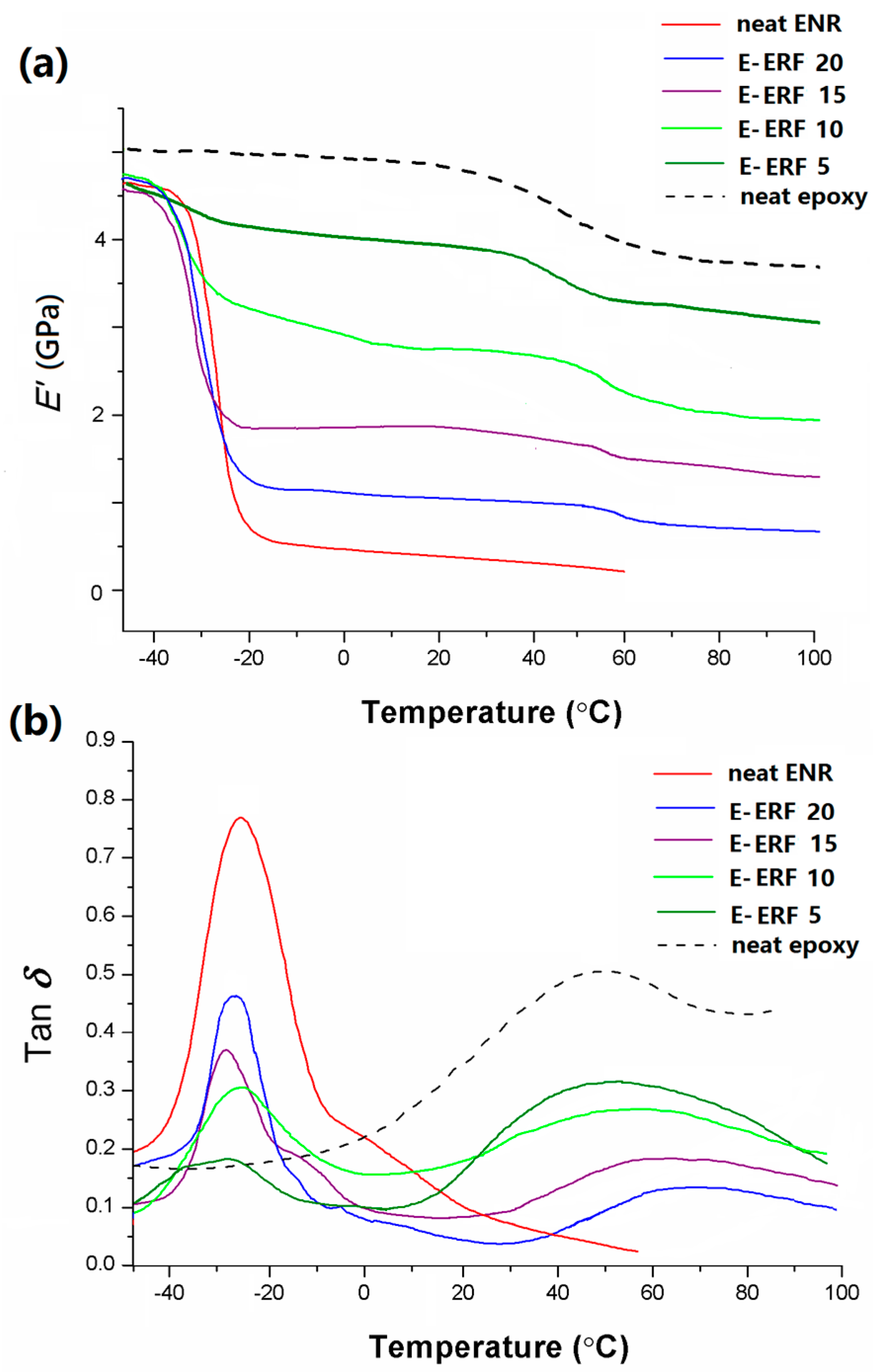
3.6. Dynamic Mechanical Analysis of E-ERF Resins
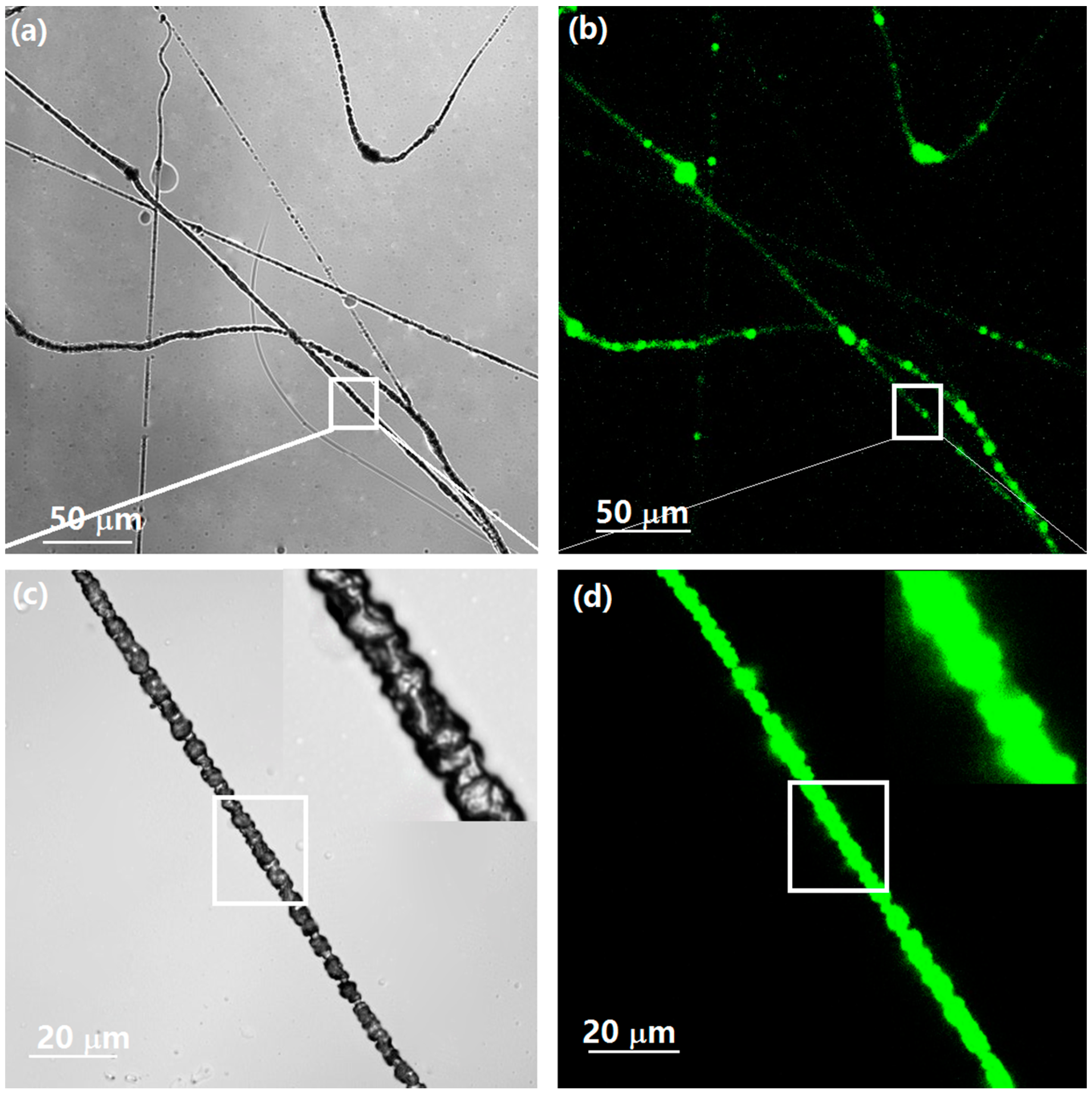
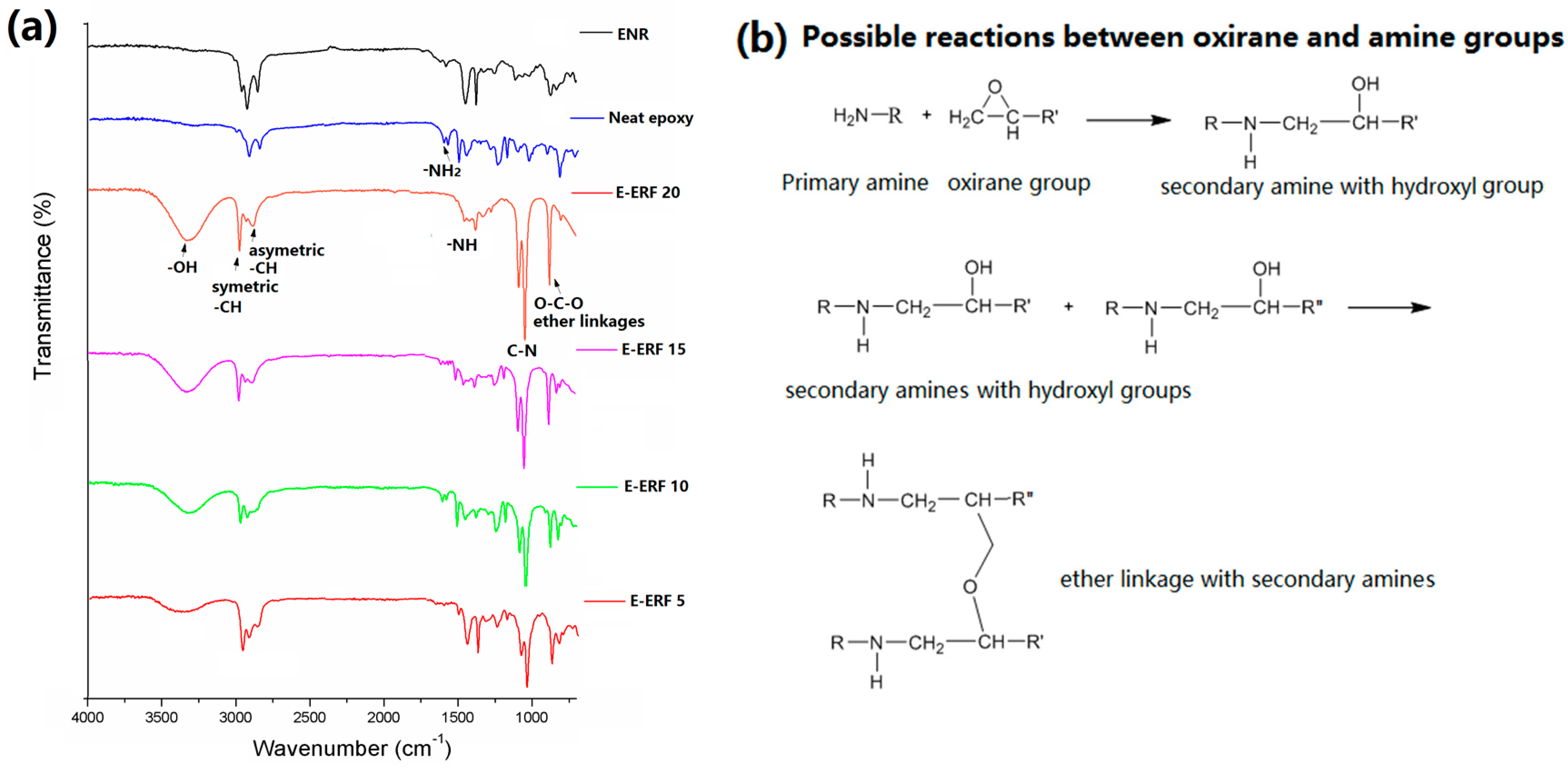
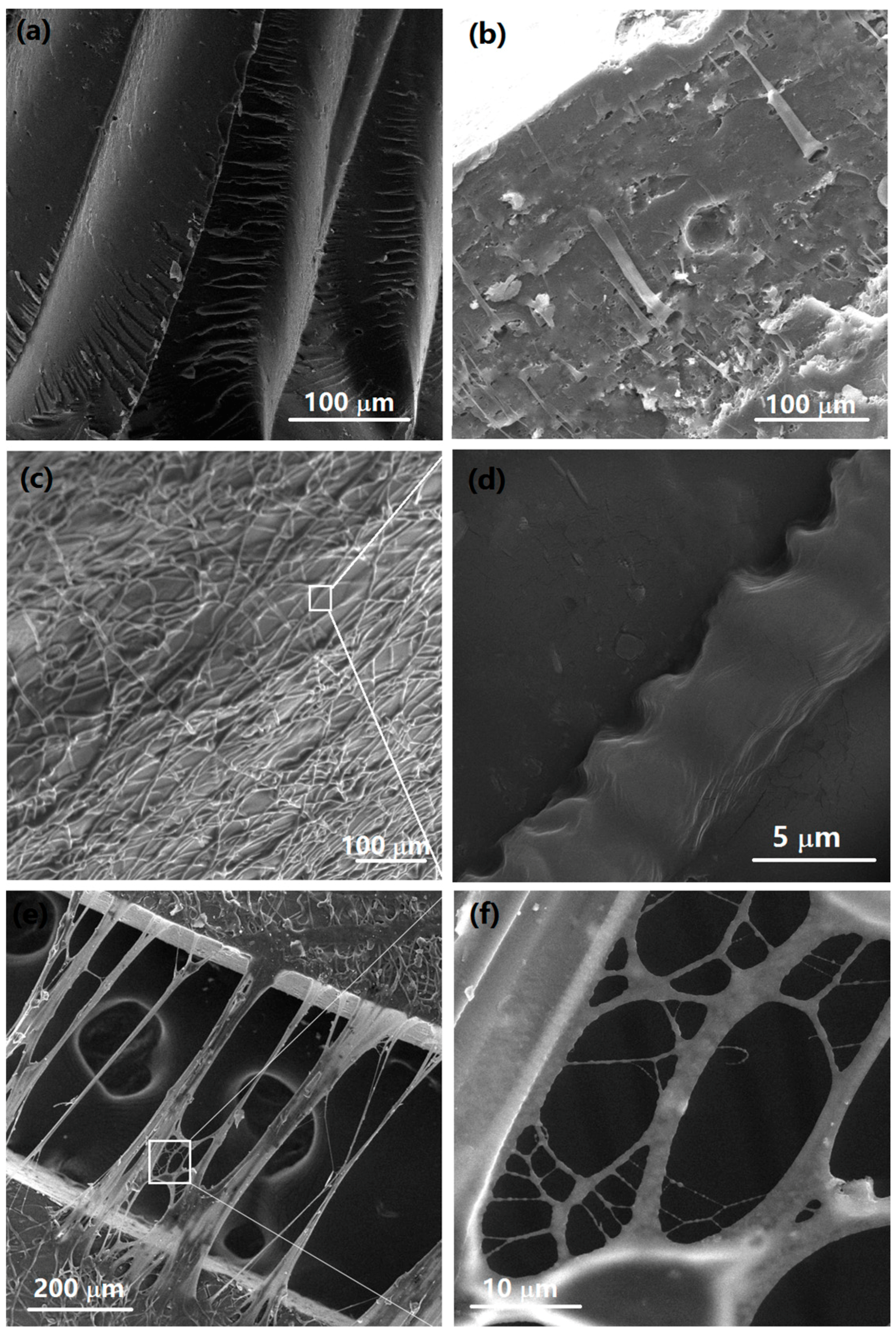
3.7. Morphological and Chemical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Moghe, A.; Gupta, B. Co-axial electrospinning for nanofiber structures: Preparation and applications. Polym. Rev. 2008, 48, 353–377. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef] [PubMed]
- Deitzel, J.; Kosik, W.; McKnight, S.; Tan, N.B.; DeSimone, J.M.; Crette, S. Electrospinning of polymer nanofibers with specific surface chemistry. Polymer 2002, 43, 1025–1029. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Theron, A.; Zussman, E.; Yarin, A. Electrostatic field-assisted alignment of electrospun nanofibres. Nanotechnology 2001, 12, 384. [Google Scholar] [CrossRef]
- Zussman, E.; Yarin, A.; Weihs, D. A micro-aerodynamic decelerator based on permeable surfaces of nanofiber mats. Exp. Fluids 2002, 33, 315–320. [Google Scholar] [CrossRef]
- Xiang, C.; Frey, M.W. Increasing mechanical properties of 2-D-structured electrospun nylon 6 non-woven fiber mats. Materials 2016, 9, 270. [Google Scholar] [CrossRef]
- Magniez, K.; Chaffraix, T.; Fox, B. Toughening of a carbon-fibre composite using electrospun poly (hydroxyether of bisphenol a) nanofibrous membranes through inverse phase separation and inter-domain etherification. Materials 2011, 4, 1967–1984. [Google Scholar] [CrossRef]
- Kim, J.R.; Michielsen, S. Synthesis of antifungal agents from xanthene and thiazine dyes and analysis of their effects. Nanomaterials 2016, 6, 243. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, P.; Zhang, C.; Yu, Y.; Liu, H.; Zhang, S.; Jia, X.; Yang, X.; Xue, Z.; Ryu, S. Inhomogeneous toughening of carbon fiber/epoxy composite using electrospun polysulfone nanofibrous membranes by in situ phase separation. Compos. Sci. Technol. 2008, 68, 987–994. [Google Scholar] [CrossRef]
- Van der Heijden, S.; Daelemans, L.; De Schoenmaker, B.; De Baere, I.; Rahier, H.; Van Paepegem, W.; De Clerck, K. Interlaminar toughening of resin transfer moulded glass fibre epoxy laminates by polycaprolactone electrospun nanofibres. Compos. Sci. Technol. 2014, 104, 66–73. [Google Scholar] [CrossRef]
- Liang, Y.; Wen, S.; Ren, Y.; Liu, L. Fabrication of nanoprotrusion surface structured silica nanofibers for the improvement of the toughening of polypropylene. RSC Adv. 2015, 5, 31547–31553. [Google Scholar] [CrossRef]
- Guo, T.; Zhou, Z.; Guo, H.; Xiao, G.; Tang, X.; Peng, M. Toughening of epoxy resin with functionalized core-sheath structured pan/sbs electrospun fibers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- He, J.; Raghavan, D.; Hoffman, D.; Hunston, D. The influence of elastomer concentration on toughness in dispersions containing preformed acrylic elastomeric particles in an epoxy matrix. Polymer 1999, 40, 1923–1933. [Google Scholar] [CrossRef]
- Chikhi, N.; Fellahi, S.; Bakar, M. Modification of epoxy resin using reactive liquid (ATBN) rubber. Eur. Polym. J. 2002, 38, 251–264. [Google Scholar] [CrossRef]
- Kunz, S.; Sayre, J.; Assink, R. Morphology and toughness characterization of epoxy resins modified with amine and carboxyl terminated rubbers. Polymer 1982, 23, 1897–1906. [Google Scholar] [CrossRef]
- Yamanaka, K.; Takagi, Y.; Inoue, T. Reaction-induced phase separation in rubber-modified epoxy resins. Polymer 1989, 30, 1839–1844. [Google Scholar] [CrossRef]
- Mo, H.; Yang, K.; Li, S.; Jiang, P. High thermal conductivity and high impact strength of epoxy nanodielectrics with functionalized halloysite nanotubes. RSC Adv. 2016, 6, 69569–69579. [Google Scholar] [CrossRef]
- Zou, Z.-P.; Liu, X.-B.; Wu, Y.-P.; Tang, B.; Chen, M.; Zhao, X.-L. Hyperbranched polyurethane as a highly efficient toughener in epoxy thermosets with reaction-induced microphase separation. RSC Adv. 2016, 6, 18060–18070. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, E.; Li, T.; Chen, S.; Zhang, J.; Cheng, X.; Zhou, J.; Zhang, A. Environment-friendly synthesis and performance of a novel hyperbranched epoxy resin with a silicone skeleton. RSC Adv. 2013, 3, 3095–3102. [Google Scholar] [CrossRef]
- Fei, X.; Wei, W.; Zhao, F.; Zhu, Y.; Luo, J.; Chen, M.; Liu, X. Efficient toughening of epoxy-anhydride thermosets with a bio-based tannic acid derivative. ACS Sustain. Chem. Eng. 2017, 5, 596–603. [Google Scholar] [CrossRef]
- Coativy, G.; Misra, M.; Mohanty, A.K. Microwave synthesis and melt blending of glycerol based toughening agent with poly (lactic acid). ACS Sustain. Chem. Eng. 2016, 4, 2142–2149. [Google Scholar] [CrossRef]
- Tan, S.; Ahmad, S.; Chia, C.; Mamun, A.; Heim, H. A comparison study of liquid natural rubber (LNR) and liquid epoxidized natural rubber (LENR) as the toughening agent for epoxy. Am. J. Mater. Sci. 2013, 3, 55–61. [Google Scholar]
- Kim, J.R.; Netravali, A.N. Comparison of thermoset soy protein resin toughening by natural rubber and epoxidized natural rubber. J. Appl. Polym. Sci. 2017. [Google Scholar] [CrossRef]
- Salehabadi, A.; Bakar, M.A.; Bakar, N.H.H.A. Effect of organo-modified nanoclay on the thermal and bulk structural properties of poly (3-hydroxybutyrate)-epoxidized natural rubber blends: Formation of multi-components biobased nanohybrids. Materials 2014, 7, 4508–4523. [Google Scholar] [CrossRef]
- Cosme, J.G.; Silva, V.M.; Nunes, R.R.; Picciani, P.H. Development of biobased poly (lactic acid)/epoxidized natural rubber blends processed by electrospinning: Morphological, structural and thermal properties. Mater. Sci. Appl. 2016, 7, 210. [Google Scholar] [CrossRef]
- Akbari, A.; Jawaid, M.; Hassan, A.; Balakrishnan, H. Epoxidized natural rubber toughened polylactic acid/talc composites: Mechanical, thermal, and morphological properties. J. Compos. Mater. 2014, 48, 769–781. [Google Scholar] [CrossRef]
- Saito, T.; Klinklai, W.; Yamamoto, Y.; Kawahara, S.; Isono, Y.; Ohtake, Y. Quantitative analysis for reaction between epoxidized natural rubber and poly (l-lactide) through 1h-NMR spectroscopy. J. Appl. Polym. Sci. 2010, 115, 3598–3604. [Google Scholar] [CrossRef]
- Megelski, S.; Stephens, J.S.; Chase, D.B.; Rabolt, J.F. Micro-and nanostructured surface morphology on electrospun polymer fibers. Macromolecules 2002, 35, 8456–8466. [Google Scholar] [CrossRef]
- Moroni, L.; Licht, R.; de Boer, J.; de Wijn, J.R.; van Blitterswijk, C.A. Fiber diameter and texture of electrospun peot/pbt scaffolds influence human mesenchymal stem cell proliferation and morphology, and the release of incorporated compounds. Biomaterials 2006, 27, 4911–4922. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Zhan, X.; Zhang, Q.; Chen, F. Toughening of an epoxy thermoset with poly [styrene-alt-(maleic acid)]-block-polystyrene-block-poly (n-butyl acrylate) reactive core–shell particles. RSC Adv. 2016, 6, 35621–35627. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, L. Preparation and characterization of soy protein plastics plasticized with waterborne polyurethane. Polym. Int. 2005, 54, 233–239. [Google Scholar] [CrossRef]
- Acocella, M.; Corcione, C.E.; Giuri, A.; Maggio, M.; Maffezzoli, A.; Guerra, G. Graphene oxide as a catalyst for ring opening reactions in amine crosslinking of epoxy resins. RSC Adv. 2016, 6, 23858–23865. [Google Scholar] [CrossRef]
- Sam, S.; Ismail, H.; Ahmad, Z. Effect of epoxidized natural rubber on the processing behavior, tensile properties, morphology, and thermal properties of linear-low-density polyethylene/soya powder blends. J. Vinyl Add. Technol. 2010, 16, 238–245. [Google Scholar] [CrossRef]
- Nikolic, G.; Zlatkovic, S.; Cakic, M.; Cakic, S.; Lacnjevac, C.; Rajic, Z. Fast fourier transform ir characterization of epoxy GY systems crosslinked with aliphatic and cycloaliphatic eh polyamine adducts. Sensors 2010, 10, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Di Liello, V.; Martuscelli, E.; Musto, P.; Ragosta, G.; Scarinzi, G. Toughening of highly crosslinked thermosetting resins by blending with thermoplastic polyether imide. Die Angew. Makromol. Chem. 1993, 213, 93–111. [Google Scholar] [CrossRef]
- O'Driscoll, K.; Sanayei, R.A. Chain-length dependence of the glass transition temperature. Macromolecules 1991, 24, 4479–4480. [Google Scholar] [CrossRef]
- Thomas, R.; Yumei, D.; Yuelong, H.; Le, Y.; Moldenaers, P.; Weimin, Y.; Czigany, T.; Thomas, S. Miscibility, morphology, thermal, and mechanical properties of a dgeba based epoxy resin toughened with a liquid rubber. Polymer 2008, 49, 278–294. [Google Scholar] [CrossRef]
- Park, S.-J.; Jin, F.-L.; Lee, J.-R. Thermal and mechanical properties of tetrafunctional epoxy resin toughened with epoxidized soybean oil. Mater. Sci. Eng. A 2004, 374, 109–114. [Google Scholar] [CrossRef]
- Jong, L. Characterization of soy protein/styrene–butadiene rubber composites. Compos. A 2005, 36, 675–682. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Zhang, M.; Wang, B.; Wang, S. Miscibility, free volume behavior and properties of blends from cellulose acetate and castor oil-based polyurethane. Polymer 2003, 44, 1733–1739. [Google Scholar] [CrossRef]
- Liu, D.; Tian, H.; Zhang, L.; Chang, P.R. Structure and properties of blend films prepared from castor oil-based polyurethane/soy protein derivative. Ind. Eng. Chem. Res. 2008, 47, 9330–9336. [Google Scholar] [CrossRef]
- Lodha, P.; Netravali, A.N. Thermal and mechanical properties of environment-friendly ‘green’plastics from stearic acid modified-soy protein isolate. Ind. Crops Prod. 2005, 21, 49–64. [Google Scholar] [CrossRef]
- Rahman, M.; Netravali, A.; Tiimob, B.; Rangari, V. Bioderived “green” composite from soy protein and eggshell nanopowder. ACS Sustain. Chem. Eng. 2014, 2, 2329–2337. [Google Scholar] [CrossRef]
- Kinloch, A.; Shaw, S.; Tod, D.; Hunston, D. Deformation and fracture behaviour of a rubber-toughened epoxy: 1. Microstructure and fracture studies. Polymer 1983, 24, 1341–1354. [Google Scholar] [CrossRef]
- Mäder, E.; Pisanova, E. Interfacial design in fiber reinforced polymers. Macromol. Symp. 2001, 168, 189–212. [Google Scholar] [CrossRef]
- Brodowsky, H.; Mäder, E. Jute fibre/epoxy composites: Surface properties and interfacial adhesion. Compos. Sci. Technol. 2012, 72, 1160–1166. [Google Scholar]
- Liu, M.; Guo, B.; Du, M.; Chen, F.; Jia, D. Halloysite nanotubes as a novel β-nucleating agent for isotactic polypropylene. Polymer 2009, 50, 3022–3030. [Google Scholar] [CrossRef]
- Ding, B.; Li, C.; Miyauchi, Y.; Kuwaki, O.; Shiratori, S. Formation of novel 2D polymer nanowebs via electrospinning. Nanotechnology 2006, 17, 3685. [Google Scholar] [CrossRef]
- Rooj, S.; Das, A.; Thakur, V.; Mahaling, R.; Bhowmick, A.K.; Heinrich, G. Preparation and properties of natural nanocomposites based on natural rubber and naturally occurring halloysite nanotubes. Mater. Des. 2010, 31, 2151–2156. [Google Scholar] [CrossRef]
- Cizravi, J.C.; Subramaniam, K. Thermal and mechanical properties of epoxidized natural rubber modified epoxy matrices. Polym. Int. 1999, 48, 889–895. [Google Scholar] [CrossRef]
- Mathew, V.S.; George, S.C.; Parameswaranpillai, J.; Thomas, S. Epoxidized natural rubber/epoxy blends: Phase morphology and thermomechanical properties. J. Appl. Polym. Sci. 2013, 131. [Google Scholar] [CrossRef]
- Ondarcuhu, T.; Joachim, C. Drawing a single nanofibre over hundreds of microns. Europhys. Lett. 1998, 42, 215. [Google Scholar] [CrossRef]
- Wu, Q.; Selke, S.; Mohanty, A.K. Processing and properties of biobased blends from soy meal and natural rubber. Macromol. Mater. Eng. 2007, 292, 1149–1157. [Google Scholar] [CrossRef]
- Kim, J.R.; Sharma, S. The development and comparison of bio-thermoset plastics from epoxidized plant oils. Ind. Crops Prod. 2012, 36, 485–499. [Google Scholar] [CrossRef]


| Type of Resins | Epoxy/ERF Composition (w/w) | Tensile Strength (MPa) | Tensile Strain (%) | Toughness at Break (MPa) | Young’s Modulus (GPa) |
|---|---|---|---|---|---|
| E-ERF | 100/0 | 58 (1.6) * | 1.2 (0.1) | 0.3 (0.03) | 1.4 (0.09) |
| 95/5 | 52 (1.2) | 4.4 (0.3) | 0.8 (0.1) | 1.2 (0.05) | |
| 90/10 | 47 (1.0) | 7.5 (0.5) | 1.4 (0.1) | 1.1 (0.05) | |
| 85/15 | 39 (0.8) | 10.2 (0.4) | 1.8 (0.2) | 1.0 (0.04) | |
| 80/20 | 34 (1.1) | 12.8 (0.8) | 1.9 (0.1) | 0.9 (0.05) |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.R.; Kim, J.J. RETRACTED: Epoxy Resins Toughened with Surface Modified Epoxidized Natural Rubber Fibers by One-Step Electrospinning. Materials 2017, 10, 464. https://doi.org/10.3390/ma10050464
Kim JR, Kim JJ. RETRACTED: Epoxy Resins Toughened with Surface Modified Epoxidized Natural Rubber Fibers by One-Step Electrospinning. Materials. 2017; 10(5):464. https://doi.org/10.3390/ma10050464
Chicago/Turabian StyleKim, Joo Ran, and Jung J. Kim. 2017. "RETRACTED: Epoxy Resins Toughened with Surface Modified Epoxidized Natural Rubber Fibers by One-Step Electrospinning" Materials 10, no. 5: 464. https://doi.org/10.3390/ma10050464





