Synthesis and Study of the Effect of Ba2+ Cations Substitution with Sr2+ Cations on Structural and Optical Properties of Ba2−xSrxZnWO6 Double Perovskite Oxides (x = 0.00, 0.25, 0.50, 0.75, 1.00)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structural Characterization
2.1.1. Scanning Electron Microscopy
2.1.2. X-ray Powder Diffraction
2.2. Optical Studies
2.2.1. FTIR Spectroscopy
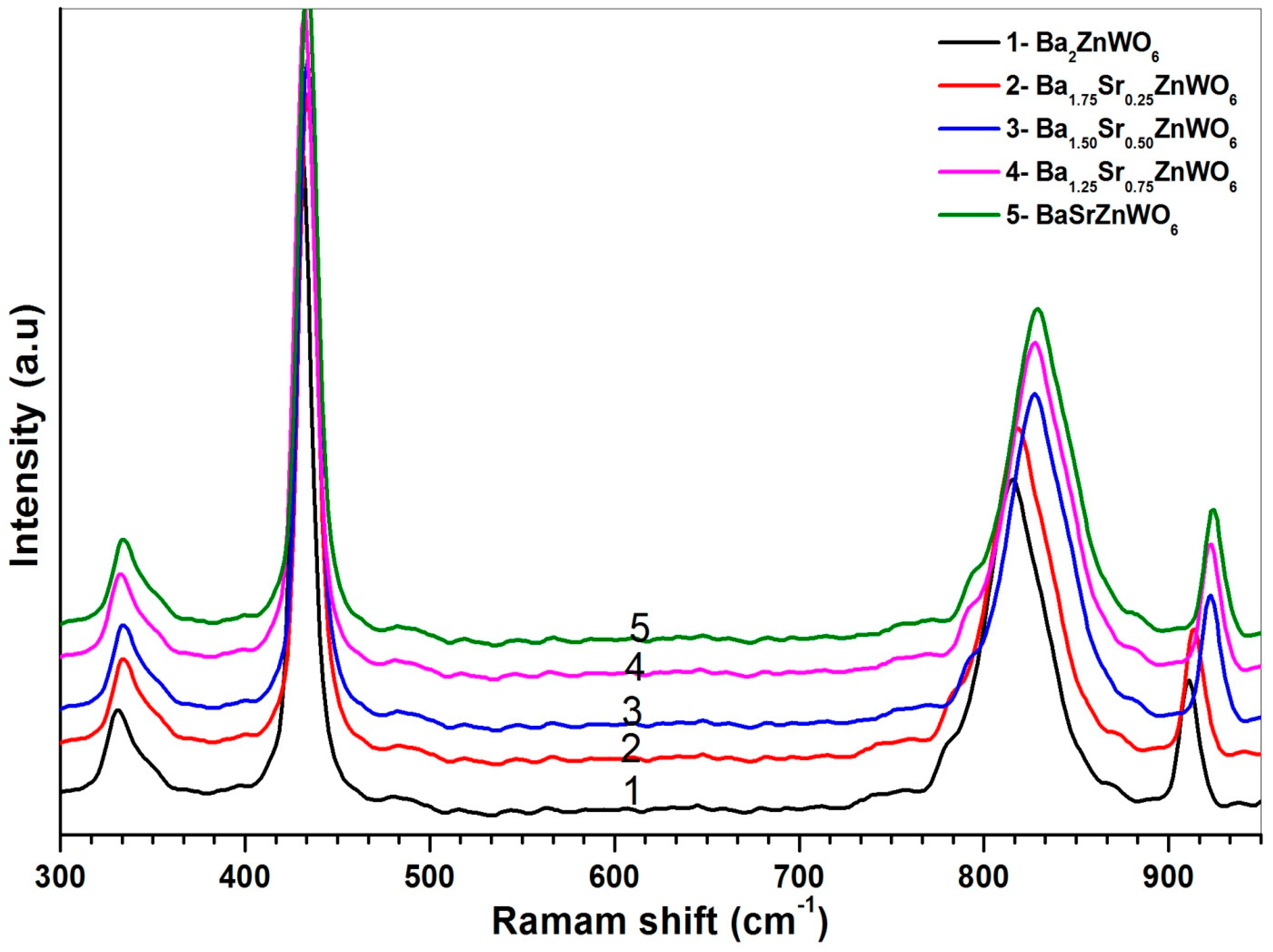
2.2.2. Raman Spectroscopy
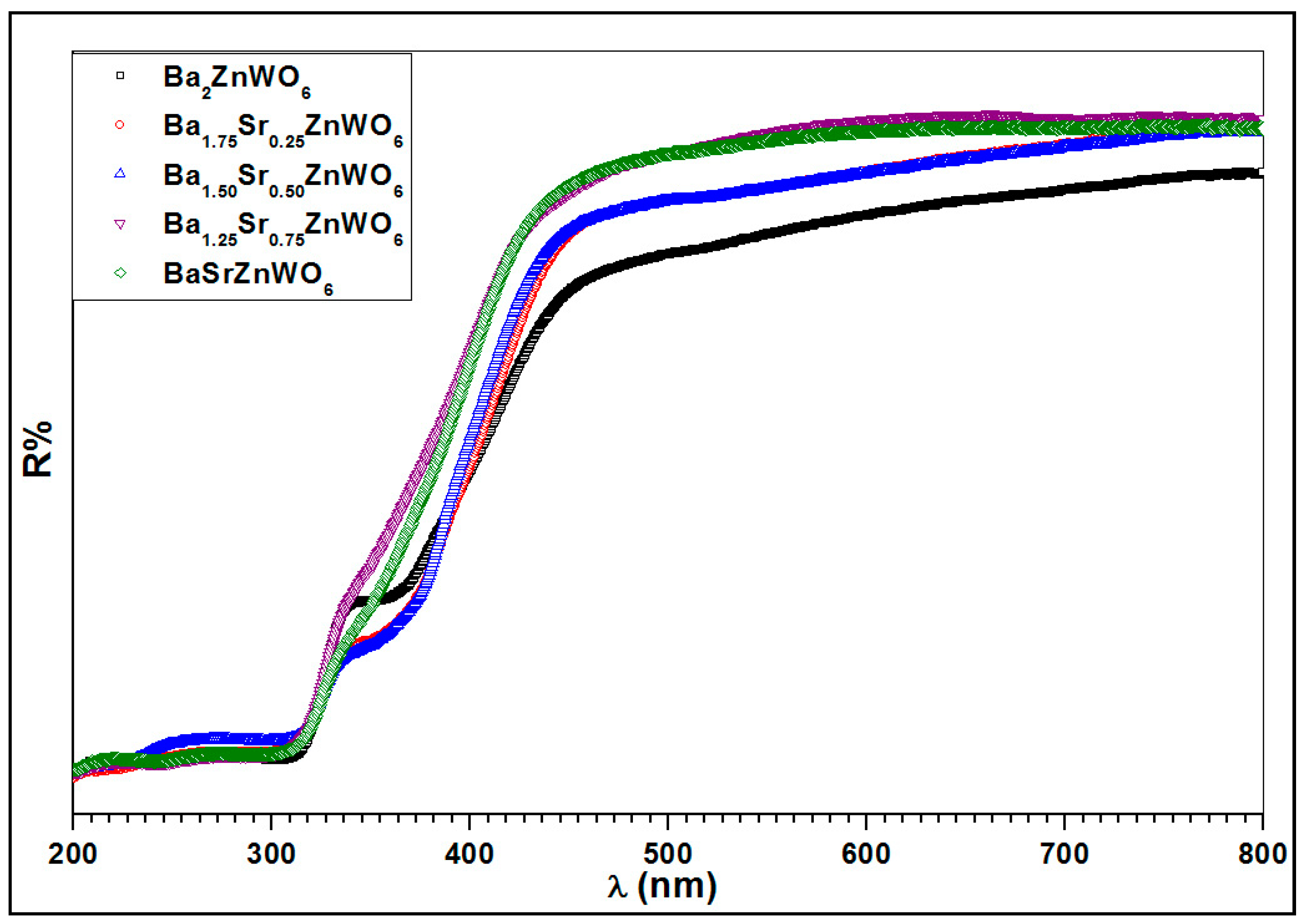
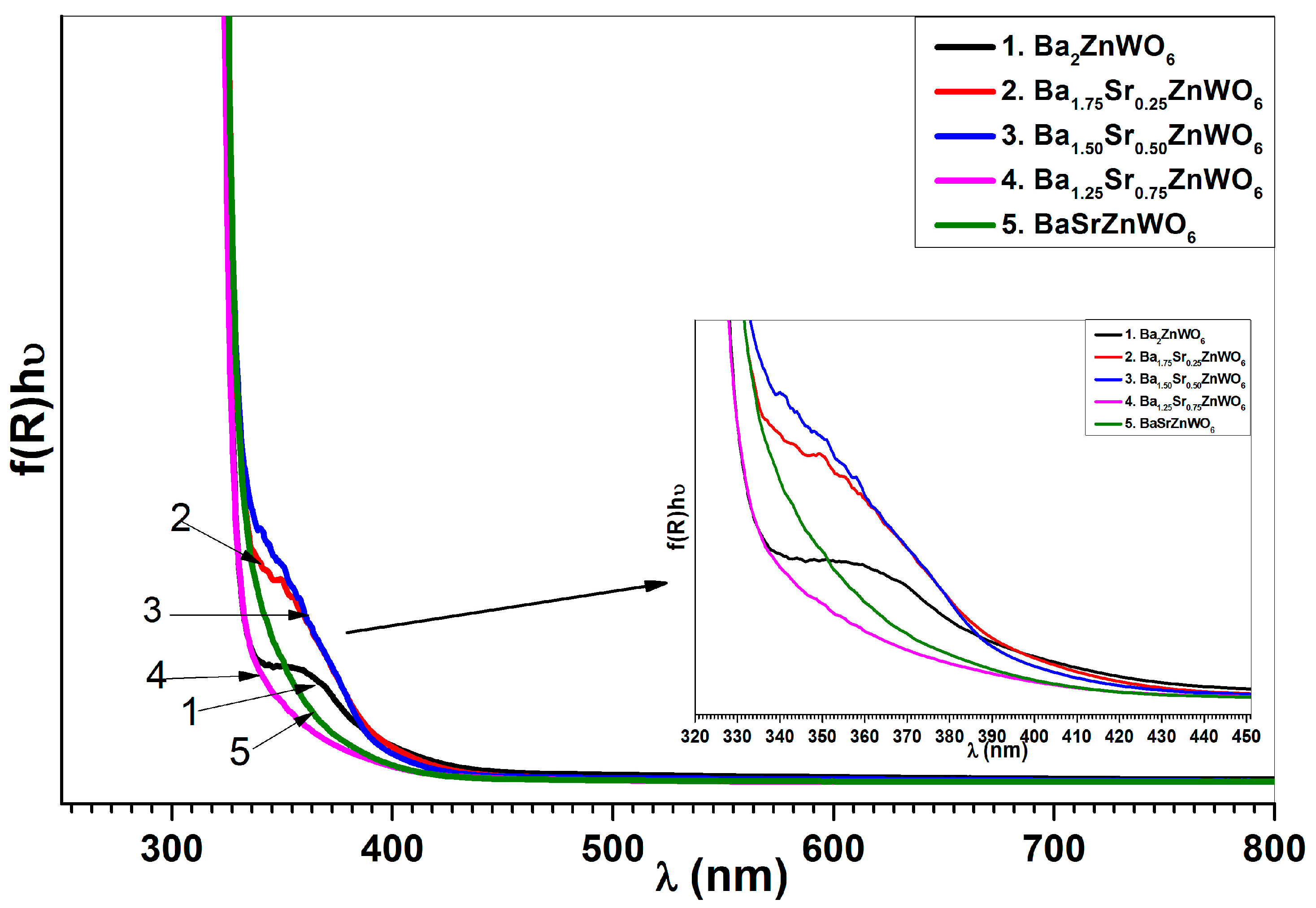
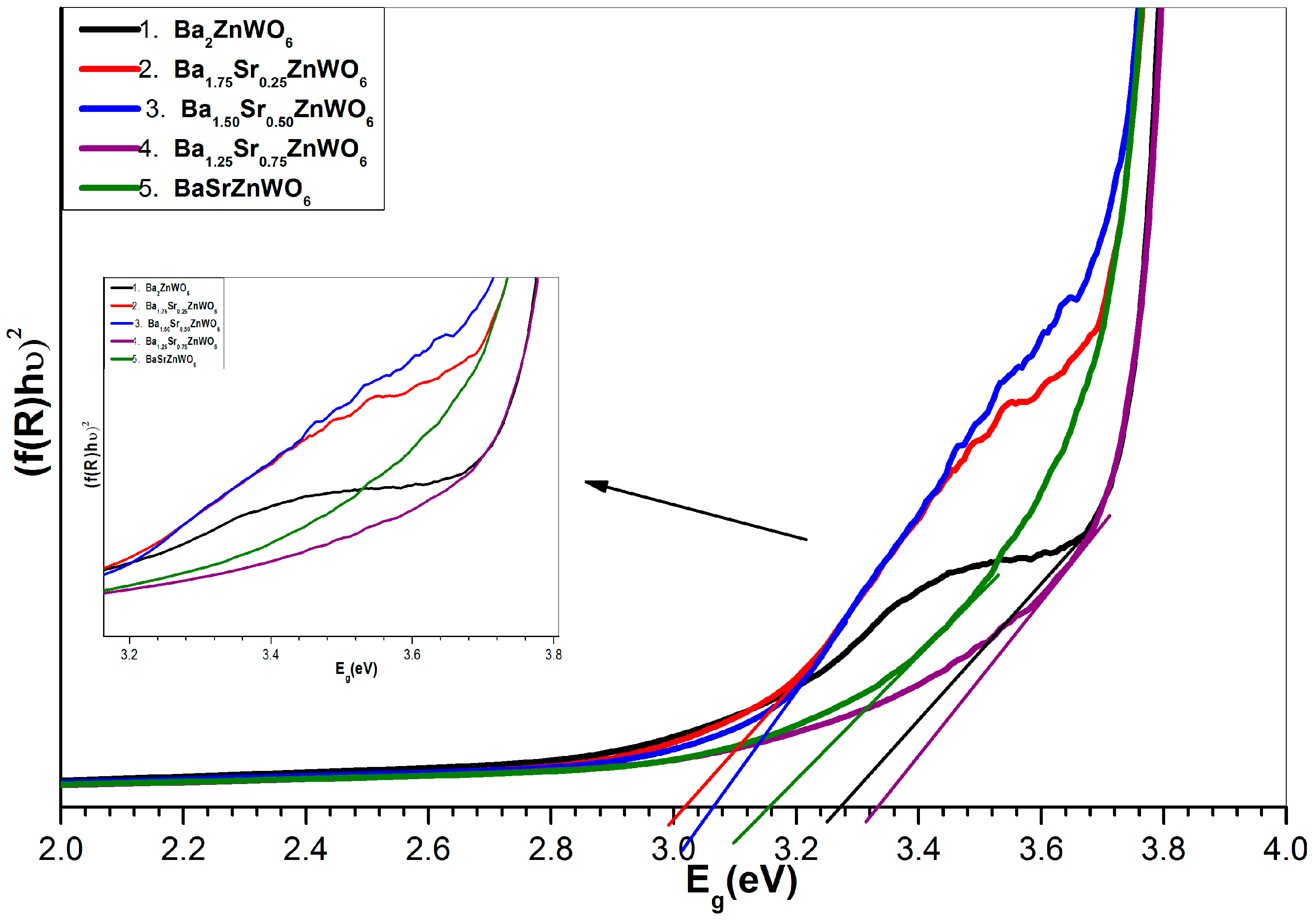
2.2.3. UV-Visible Diffuse Reflectance Spectroscopy
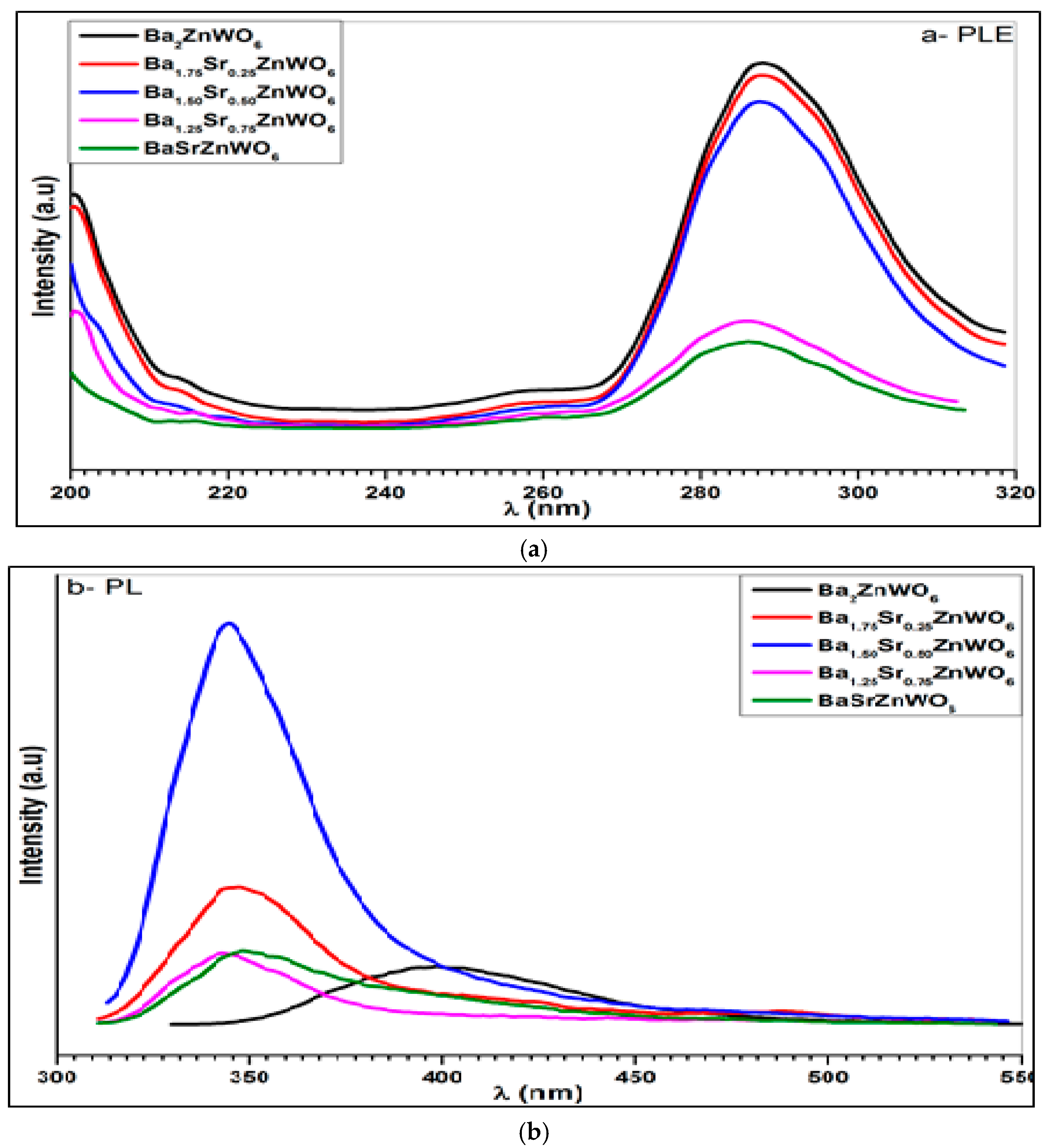
2.2.4. Photoluminescence Spectroscopy
3. Materials and Methods
3.1. Samples Preparation
3.2. Sample Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zaraq, A.; Orayech, B.; Faik, A.; Igartua, J.; Jouanneaux, A.; El Bouari, A. High temperature induced phase transitions in SrCaCoTeO6 and SrCaNiTeO6 ordered double perovskites. Polyhedron 2016, 110, 119–124. [Google Scholar] [CrossRef]
- Lan, C.; Zhao, S.; Xu, T.; Ma, J.; Hayase, S.; Ma, T. Investigation on structures, band gaps, and electronic structures of lead free La2NiMnO6 double perovskite materials for potential application of solar cell. J. Alloys Compd. 2016, 655, 208–214. [Google Scholar] [CrossRef]
- Paiva, D.; Silva, M.; Sombra, A.; Fechine, P. Dielectric investigation of the Sr3WO6 double perovskite at RF/microwave frequencies. RSC Adv. 2016, 6, 42502–42509. [Google Scholar] [CrossRef]
- Xie, H.D.; Chen, C.; Xi, H.H.; Tian, R.; Wang, X.C. Synthesis, low temperature co-firing, and microwave dielectric properties of two ceramics BiM2VO6 (M = Cu, Ca). Ceram. Int. 2016, 42, 989–995. [Google Scholar] [CrossRef]
- Orlandi, F.; Righi, L.; Mezzadri, F.; Manuel, P.; Khalyavin, D.D.; Delmonte, D.; Pernechele, C.; Cabassi, R.; Bolzoni, F.; Solzi, M.; et al. Improper Ferroelectric Contributions in the Double Perovskite Pb2Mn0.6Co0.4WO6 System with a Collinear Magnetic Structure. Inorg. Chem. 2016, 55, 4381–4390. [Google Scholar] [CrossRef] [PubMed]
- Shimakawa, Y.; Azuma, M.; Ichikawa, N. Multiferroic compounds with double-perovskite structures. Materials 2011, 4, 153–168. [Google Scholar] [CrossRef]
- DeMarco, M.; Blackstead, H.; Dow, J.D.; Wu, M.; Chen, D.Y.; Chen, D.F.; Haka, M.; Steve, T.; Joel, F. Magnetic phase transition in superconducting Sr2YRu0.95Cu0.05O6 observed by the 99 Ru Mössbauer effect. Phys. Rev. B 2000, 62, 14301–14303. [Google Scholar] [CrossRef]
- Harnagea, L.; Jurca, B.; Berthet, P. Low-field magnetoresistance up to 400 K in double perovskite Sr2FeMoO6 synthesized by a citrate route. J. Solid State Chem. 2014, 211, 219–226. [Google Scholar] [CrossRef]
- Gorodea, I.; Goanta, M.; Toma, M. Impact of A cation size of double perovskite A2AlTaO6 (A = Ca, Sr, Ba) on dielectric and catalytic properties. J. Alloys Compd. 2015, 632, 805–809. [Google Scholar] [CrossRef]
- Djerdj, I.; Popović, J.; Mal, S.; Weller, T.; Nuskol, M.; Jagličić, Z.S.; Damir, P.; Christian, S.; Pascal, V.; Roland, M.; et al. Aqueous Sol–Gel Route toward Selected Quaternary Metal Oxides with Single and Double Perovskite-Type Structure Containing Tellurium. Cryst. Growth Des. 2016, 16, 2535–2541. [Google Scholar] [CrossRef]
- Wang, Z.L.; Kang, Z.C. Perovskite and Related Structure Systems. In Functional and Smart Materials: Functional and Smart Materials; Springer: New York, NY, USA, 1998; Volume 3, pp. 93–149. [Google Scholar]
- Iranmanesh, M.; Lingg, M.; Stir, M.; Hulliger, J. Sol gel and ceramic synthesis of Sr2FeMo1−xWxO6 (0 ≤ x ≤ 1) double perovskites series. RSC Adv. 2016, 6, 42069–42075. [Google Scholar] [CrossRef]
- Wu, J.Y.; Bian, J.J. Structure stability and microwave dielectric properties of double perovskite ceramics Ba2Mg1−xCaxWO6 (0.0 ≤ x ≤ 0.15). Ceram. Int. 2012, 38, 3217–3225. [Google Scholar] [CrossRef]
- Xu, D.; Yang, Z.; Sun, J.; Gao, X.; Du, J. Synthesis and luminescence properties of double-perovskite white emitting phosphor Ca3WO6:Dy3+. J. Mater. Sci. Mater. Electron. 2016, 27, 8370–8377. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, J.; Qiao, C.; Ji, X.; Jiang, B. Structure and properties of the ordered double perovskites Sr2MWO6 (M = Co, Ni) by sol-gel route. Mater. Lett. 2006, 60, 2747–2750. [Google Scholar] [CrossRef]
- Xia, Z.; Sun, J.; Du, H.; Chen, D.; Sun, J. Luminescence properties of double-perovskite Sr2Ca1−2xEuxNaxMoO6 red-emitting phosphors prepared by the citric acid-assisted sol–gel method. J. Mater. Sci. 2010, 45, 1553–1559. [Google Scholar] [CrossRef]
- Elbadawi, A.A.; Yassin, O.; Siddig, M.A. Effect of the Cation Size Disorder at the A-Site on the Structural Properties of SrAFeTiO6 Double Perovskites (A = La, Pr or Nd). J. Mater. Sci. Chem. Eng. 2015, 3, 21–29. [Google Scholar]
- Manoun, B.; Ezzahi, A.; Benmokhtar, S.; Ider, A.; Lazor, P.; Bih, L.; Igartuae, J.M. X-ray diffraction and Raman spectroscopy studies of temperature and composition induced phase transitions in Ba2−xSrxZnWO6 (0 ≤ x ≤ 2) double perovskite oxides. J. Alloys Compd. 2012, 533, 43–52. [Google Scholar] [CrossRef]
- Bugaris, D.E.; Hodges, J.P.; Huq, A.; Zur-Loye, H.C. Crystal growth, structures, and optical properties of the cubic double perovskites Ba2MgWO6 and Ba2ZnWO6. J. Solid State Chem. 2011, 184, 2293–2298. [Google Scholar] [CrossRef]
- Tamraoui, Y.; Manoun, B.; Mirinioui, F.; Haloui, R.; Lazor, P. X-ray diffraction and Raman spectroscopy studies of temperature and composition induced phase transitions in Ba2−xSrxMgTeO6 (0 ≤ x ≤ 2). J. Alloys Compd. 2014, 603, 86–94. [Google Scholar] [CrossRef]
- Mostafa, M.F.; Ata-Allah, S.S.; Youssef, A.A.A.; Refai, H.S. Electric and AC magnetic investigation of the manganites La0.7Ca0.3Mn0.96In0.04xAl(1−x)0.04O3; (0.0 ≤ x ≤ 1.0). J. Magn. Magn. Mater. 2008, 320, 344–353. [Google Scholar] [CrossRef]
- Liegeois-Duyckaerts, M.; Tarte, P. Vibrational studies of molybdates, tungstates and related c compounds—III. Ordered cubic perovskites A2BIIBVIO6. Spectrochim. Acta Part A Mol. Spectrosc. 1974, 30, 1771–1786. [Google Scholar] [CrossRef]
- Baldinozzi, G.; Sciau, P.; Bulou, A. Raman study of the structural phase transition in the ordered perovskite Pb2MgWO6. J. Phys. Condens. Matter 1995, 7, 8109. [Google Scholar] [CrossRef]
- Xiao, N.; Shen, J.; Xiao, T.; Wu, B.; Luo, X.; Li, L.; Wang, Z.; zhou, X. Sr2CaWxMo1−xO6:Eu3+, Li+: An emission tunable phosphor through site symmetry and excitation wavelength. Mater. Res. Bull. 2015, 70, 684–690. [Google Scholar] [CrossRef]
- Dutta, A.; Mukhopadhyay, P.; Sinha, T.; Shannigrahi, S.; Himanshu, A.; Sen, P.; Bandyopadhyay, S.K. Sr2SmNbO6 perovskite: Synthesis, characterization and density functional theory calculations. Mater. Chem. Phys. 2016, 179, 55–64. [Google Scholar] [CrossRef]
- Chen, F.; Niu, C.; Yang, Q.; Li, X.; Zeng, G. Facile synthesis of visible-light-active BiOI modified Bi2MoO6 photocatalysts with highly enhanced photocatalytic activity. Ceram. Int. 2016, 42, 2515–2525. [Google Scholar] [CrossRef]
- Kittel, C.; Holcomb, D.F. Introduction to solid state physics. Am. J. Phys. 1967, 35, 547–548. [Google Scholar] [CrossRef]
- Manoun, B.; Igartua, J.M.; Lazor, P.; Ezzahi, A. High temperature induced phase transitions in Sr2ZnWO6 and Sr2CoWO6 double perovskite oxides: Raman spectroscopy as a tool. J. Mol. Struct. 2012, 1029, 81–85. [Google Scholar] [CrossRef]
- Eng, H.W. The Crystal and Electronic Structures of Oxides Containing d0 Transition Metals in Octahedral Coordination. Ph.D. Thesis, The Ohio State University, Columbus, OH, USA, 2003. [Google Scholar]
- Klein, P.; Nwagwu, U.; Edgar, J.H.; Freitas, J.A., Jr. Photoluminescence investigation of the indirect band gap and shallow impurities in icosahedral B12As2. J. Appl. Phys. 2012, 112, 013508. [Google Scholar] [CrossRef]
- Suryanarayana, C.; Norton, M.G. X-rays and Diffraction. In X-ray Diffraction; Springer: New York, NY, USA, 1998; Volume 1, pp. 3–19. [Google Scholar]
- Alsabah, Y.A.; Elbadawi, A.A.; Mustafa, E.M.; Siddig, M.A. The Effect of Replacement of Zn2+ Cation with Ni2+ Cation on the Structural Properties of Ba2Zn1−xNixWO6 Double Perovskite Oxides (X = 0, 0.25, 0.50, 0.75, 1). J. Mater. Sci. Chem. Eng. 2016, 4, 61–70. [Google Scholar]
- Kavitha, V.T.; Jose, R.; Ramakrishna, S.; Wariar, P.R.S.; Koshy, J. Combustion synthesis and characterization of Ba2NdSbO6 nanocrystalsm. Bull. Mater. Sci. 2011, 34, 661–665. [Google Scholar] [CrossRef]
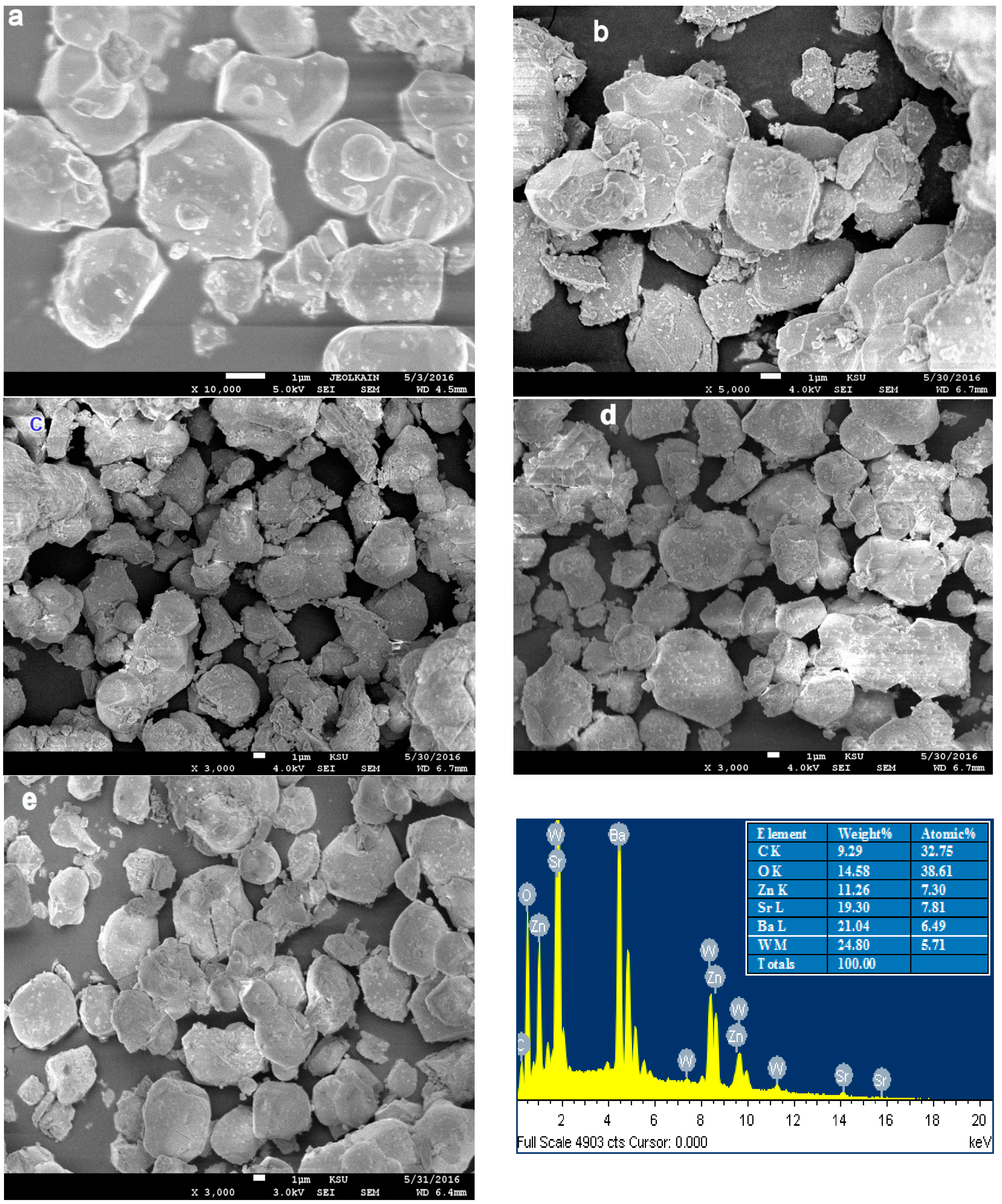
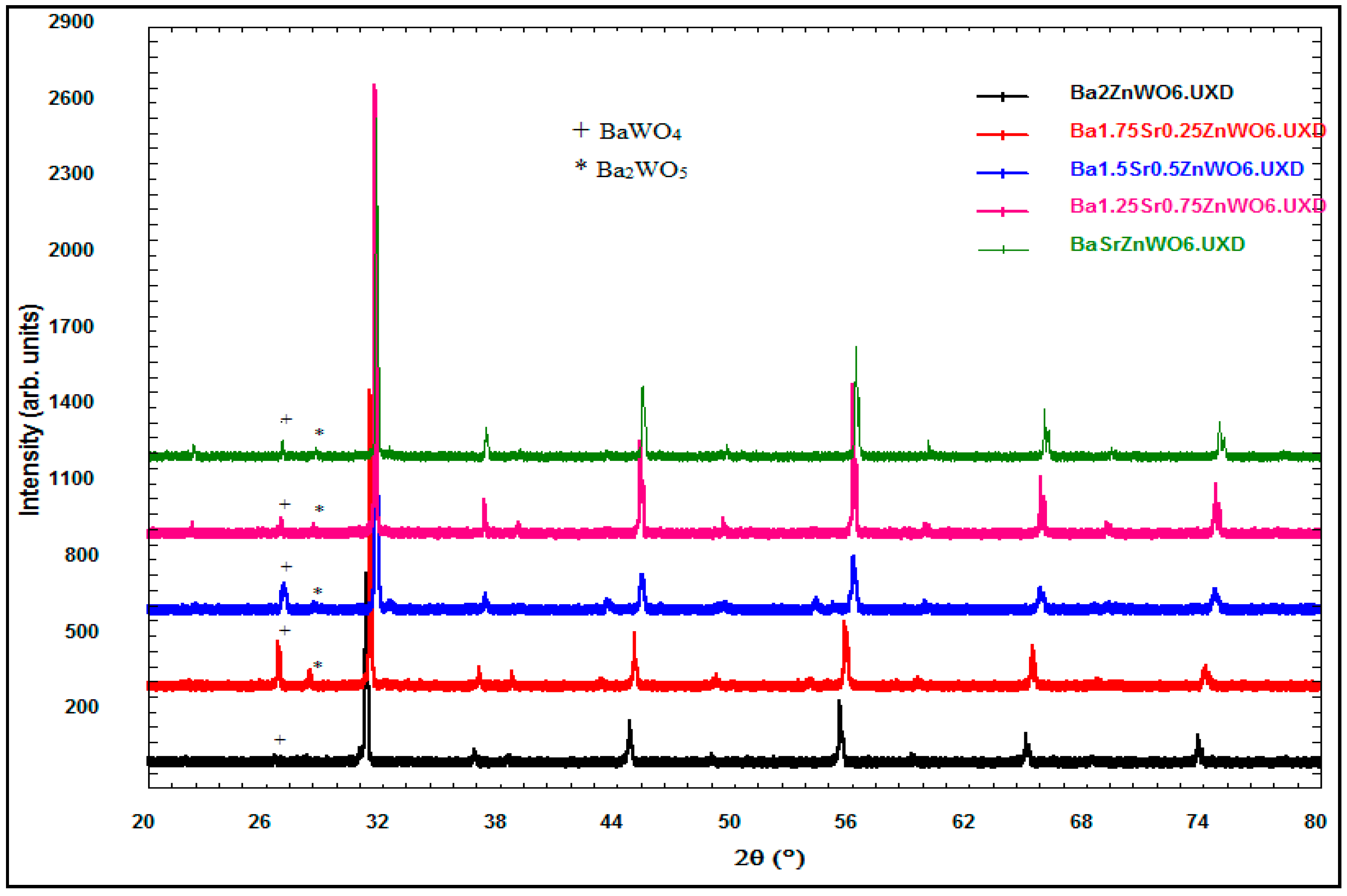
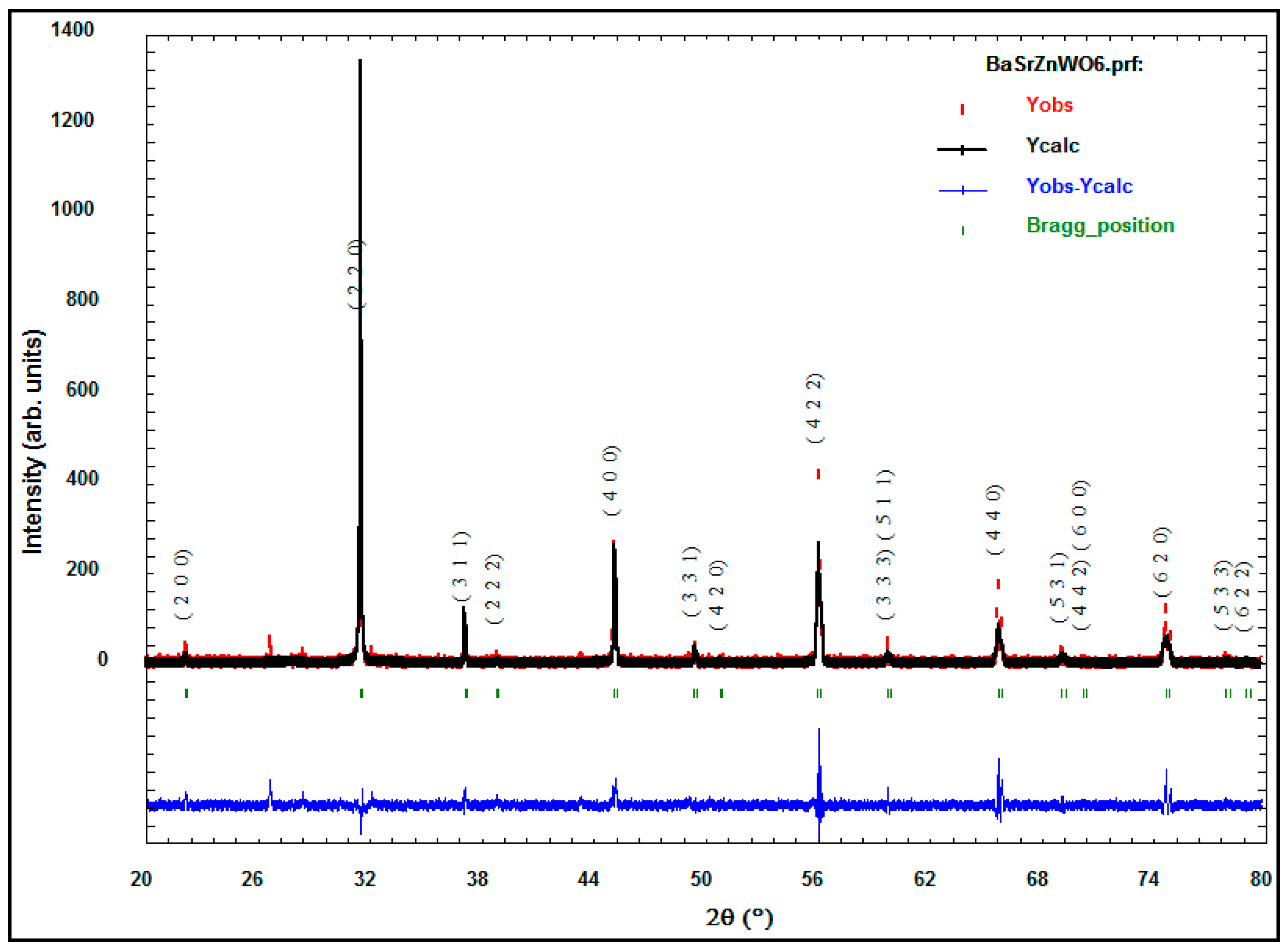
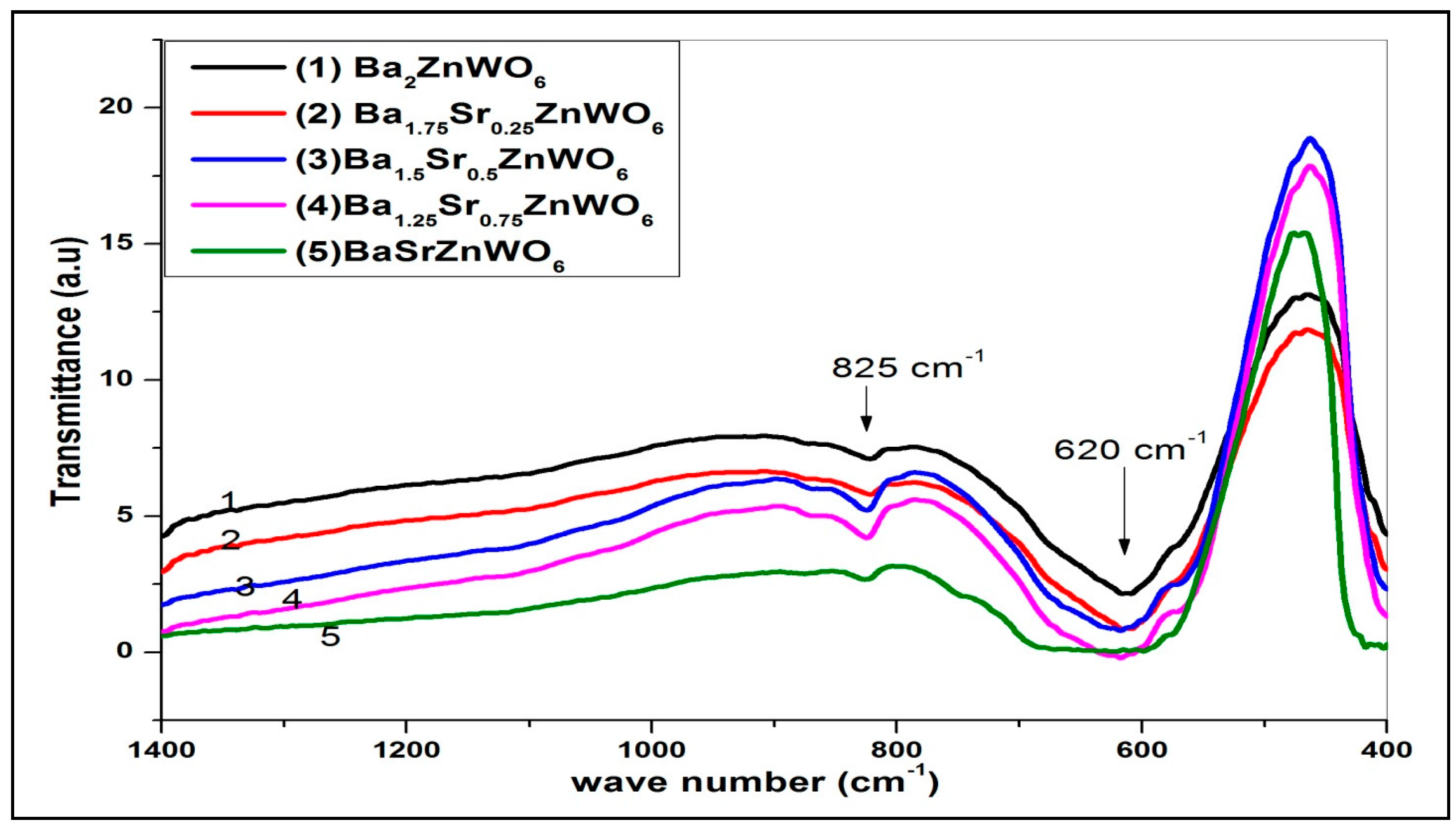
| Cation/Anion | Coordinates | Ba2ZnWO6 | Ba1.75Sr0.25ZnWO6 | Ba1.5Sr0.5ZnWO6 | Ba1.25Sr0.75ZnWO6 | BaSrZnWO6 |
|---|---|---|---|---|---|---|
| Ba+2 | X | 0.2500 | 0.2500 | 0.2500 | 0.2500 | 0.2500 |
| Y | 0.2500 | 0.2500 | 0.2500 | 0.2500 | 0.2500 | |
| Z | 0.2500 | 0.2500 | 0.2500 | 0.2500 | 0.2500 | |
| Sr+2 | --------- | 0.2500 | 0.2500 | 0.2500 | 0.2500 | |
| --------- | 0.2500 | 0.2500 | 0.2500 | 0.2500 | ||
| --------- | 0.2500 | 0.2500 | 0.2500 | 0.2500 | ||
| Zn+2 | X | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 |
| Y | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | |
| Z | 0.500 | 0.500 | 0.500 | 0.500 | 0.500 | |
| W+6 | X | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Y | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Z | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| O1−2/O2−2 | X | 0.23990 | 0.25815 | 0.24414 | 0.22556 | 0.24414 |
| Y | 0 | 0 | 0 | 0 | 0 | |
| Z | 0 | 0 | 0 | 0 | 0 |
| Empirical Formula | Ba2ZnWO6 | Ba1.75Sr0.25ZnWO6 | Ba1.5Sr0.5ZnWO6 | Ba1.25Sr0.75ZnWO6 | BaSrZnWO6 |
|---|---|---|---|---|---|
| Space group | Fm-3m | Fm-3m | Fm-3m | Fm-3m | Fm-3m |
| α (Å) | 8.11834 | 8.100679 | 8.076869 | 8.060842 | 8.039361 |
| α/β/γ | 90 | 90 | 90 | 90 | 90 |
| V (Å3) | 535.0590 | 531.57465 | 526.9001 | 523.7707 | 519.5751 |
| D (nm) | 105.09 | 78.38 | 47.41 | 88.03 | 84.59 |
| T | 1.007 | 1.00 | 0.992 | 0.990 | 0.998 |
| RWP | 9.78 | 9.71 | 8.87 | 13.6 | 11.3 |
| RP | 8.75 | 10.2 | 8.15 | 10.3 | 10.8 |
| 1.7738 | 1.886 | 1.681 | 2.813 | 2.356 |
| Bang Gap Energy | Ba2ZnWO6 | Ba1.75Sr0.25ZnWO6 | Ba1.5Sr0.5ZnWO6 | Ba1.25Sr0.75ZnWO6 | BaSrZnWO6 |
|---|---|---|---|---|---|
| Cut-off wavelength (nm) | 380 | 410 | 405 | 370 | 389 |
| Eg (eV) by cutoff wavelength | 3.26 | 3.02 | 3.06 | 3.35 | 3.18 |
| Eg (eV) by Tauc plot | 3.27 | 3.02 | 3.07 | 3.34 | 3.16 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsabah, Y.A.; AlSalhi, M.S.; Elbadawi, A.A.; Mustafa, E.M. Synthesis and Study of the Effect of Ba2+ Cations Substitution with Sr2+ Cations on Structural and Optical Properties of Ba2−xSrxZnWO6 Double Perovskite Oxides (x = 0.00, 0.25, 0.50, 0.75, 1.00). Materials 2017, 10, 469. https://doi.org/10.3390/ma10050469
Alsabah YA, AlSalhi MS, Elbadawi AA, Mustafa EM. Synthesis and Study of the Effect of Ba2+ Cations Substitution with Sr2+ Cations on Structural and Optical Properties of Ba2−xSrxZnWO6 Double Perovskite Oxides (x = 0.00, 0.25, 0.50, 0.75, 1.00). Materials. 2017; 10(5):469. https://doi.org/10.3390/ma10050469
Chicago/Turabian StyleAlsabah, Yousef A., Mohamad S. AlSalhi, Abdelrahman A. Elbadawi, and Eltayeb M. Mustafa. 2017. "Synthesis and Study of the Effect of Ba2+ Cations Substitution with Sr2+ Cations on Structural and Optical Properties of Ba2−xSrxZnWO6 Double Perovskite Oxides (x = 0.00, 0.25, 0.50, 0.75, 1.00)" Materials 10, no. 5: 469. https://doi.org/10.3390/ma10050469







