Effects of Wastes from the Brewing Industry in Lightweight Aggregates Manufactured with Clay for Green Roofs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Characterization of Raw Material
- X-ray diffractometry of clay and wastes was performed using an automatic Siemens D5000 diffractometer (Siemens AG, Munich, Germany), with Bragg-Brentano geometry (θ/2θ) and Kα1, 2 radiation, equipped with a graphite monochromator to eliminate Cu Kβ radiation.
- Chemical composition was determined by X-ray Fluorescence (XRF) using Philips Magix Pro (PW-2440) equipment (Philips, Amsterdam, The Netherlands).
- The total carbon, hydrogen, nitrogen and sulfur contents were determined by combustion of the samples in an O2 atmosphere using the CHNS-O Thermo Finnigan Flash Elementary Analyzer EA 1112 (Thermo Scientific, Waltham, MA, USA). The determination of the organic content was performed according to ASTM D-2974 [35].
- The Higher Heating Value (HHV) and Lower Heating Value (LHV) were determined by a Parr 1341 Plain Oxygen Bomb Calorimeter (Parr Instrument Company, Moline, IL, USA), following the UNE 32 006:1995 standard [36].
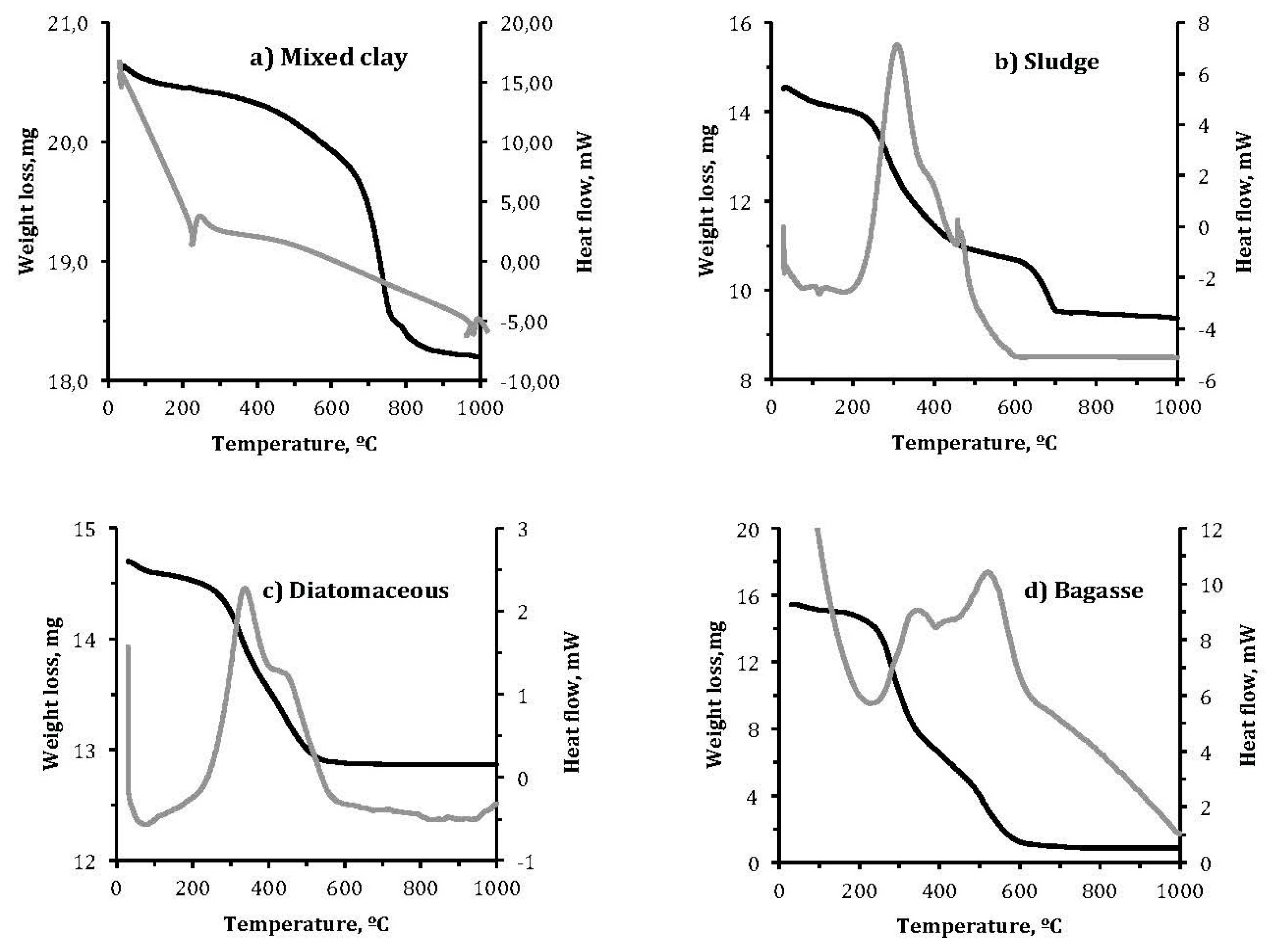
- Thermal behavior was determined by Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA) using a Mettler Toledo 850e balance (Mettler Toledo, Columbus, OH, USA). Samples of approximately 15–30 mg were placed in a platinum crucible and heated at a rate of 10 °C/min in the range from room temperature to 900 °C. Air flow the samples was maintained at a rate of 50 mL/min. The data shown reflect weight loss that depended on temperature and DTA diagram simultaneously.
2.3. Preparation of Samples: Sintered Materials
2.4. Characterization of Sintered Materials
- Weight loss from sintering was obtained by determining the difference between weight after the drying stage at 110 °C and after the firing stage at 900, 950 and 1000 °C.
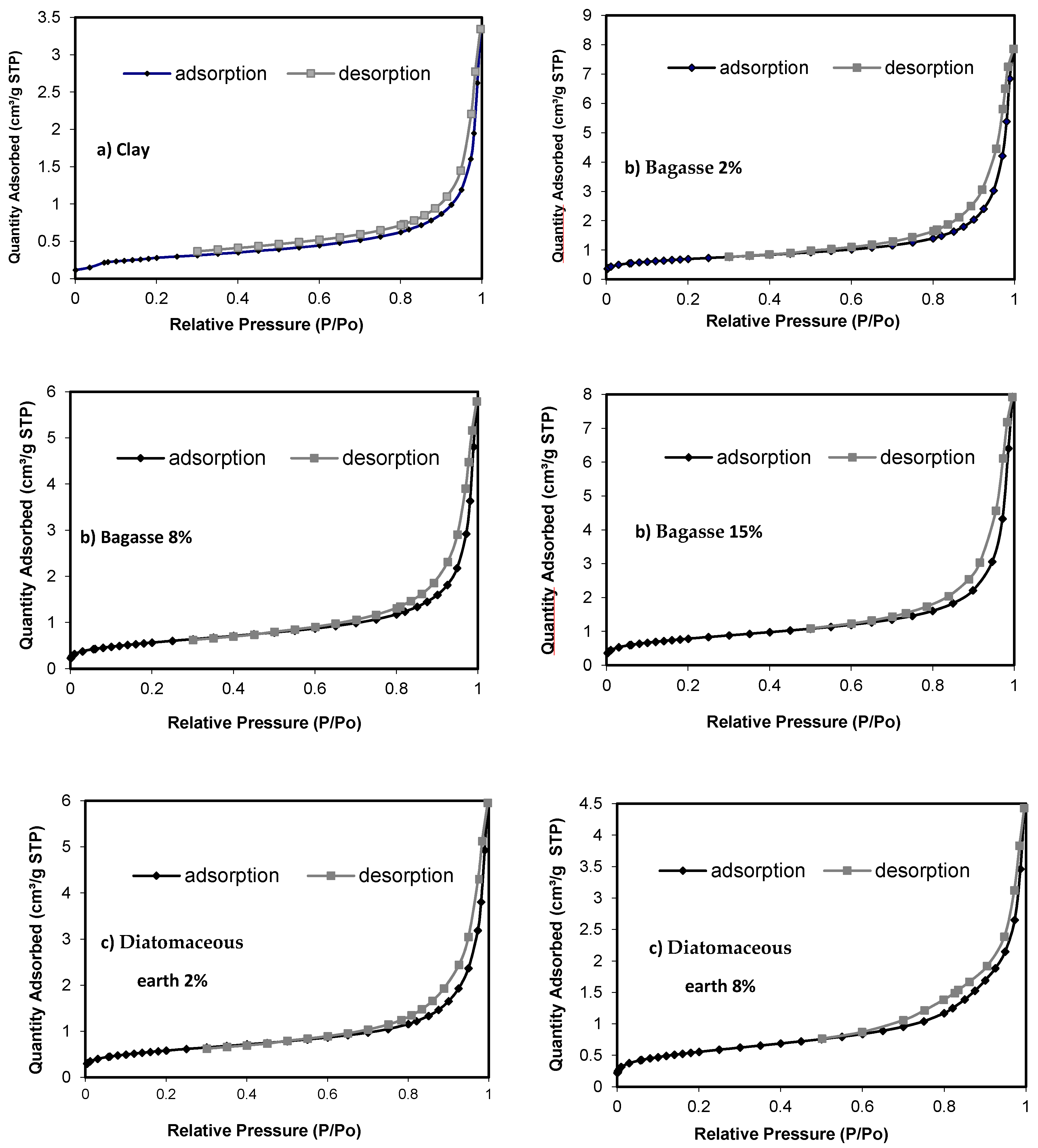
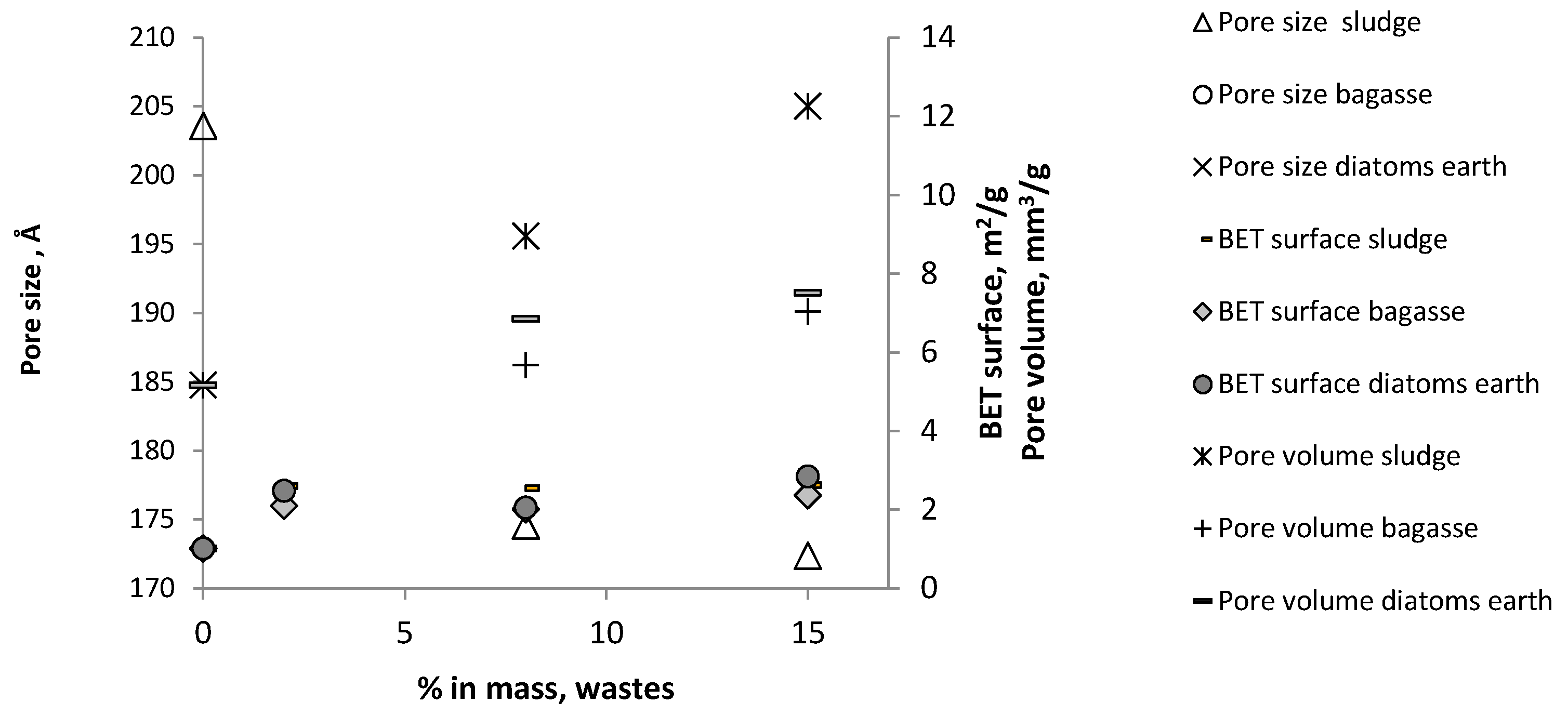
- Adsorption and desorption isotherms with N2 were determined and distribution of mesoporosity studied following the BJH (Barrett, Joyner, and Halenda) method, using an ASAP 2020 Accelerated Surface Area and Porosimetry System by Micromeritics (Micromeritics, Norcross, GA, USA). The following parameters were measured with the adsorption isotherm, using the BET surface area method (m2/g), pore volume (cm3/g) and pore size (Å).
- Water absorption capacity is defined as the measurement of moisture when a solid is completely immersed in water for a long time. The test to determine water absorption capacity was implemented according to Standard Procedure ASTM C373 [38].
- Absolute density was determined using a Gas (He) Displacement Pycnometry System AccupycTM II 1340 by Micromeritics (Micromeritics, Norcross, GA, USA).
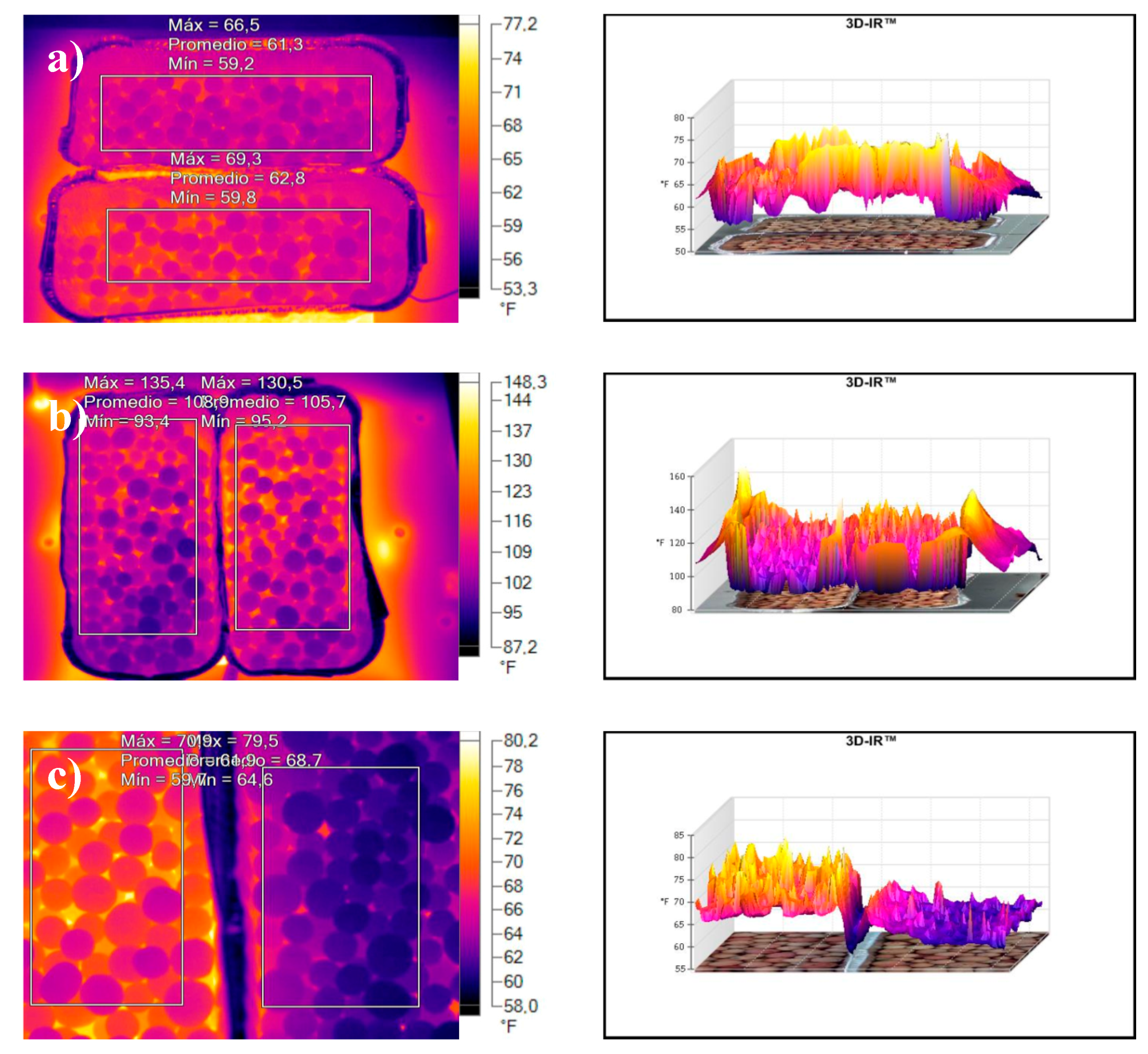
- Insulating properties were determined using the thermal imaging camera Fluke Ti-32 (Fluke, Everett, WA, USA).
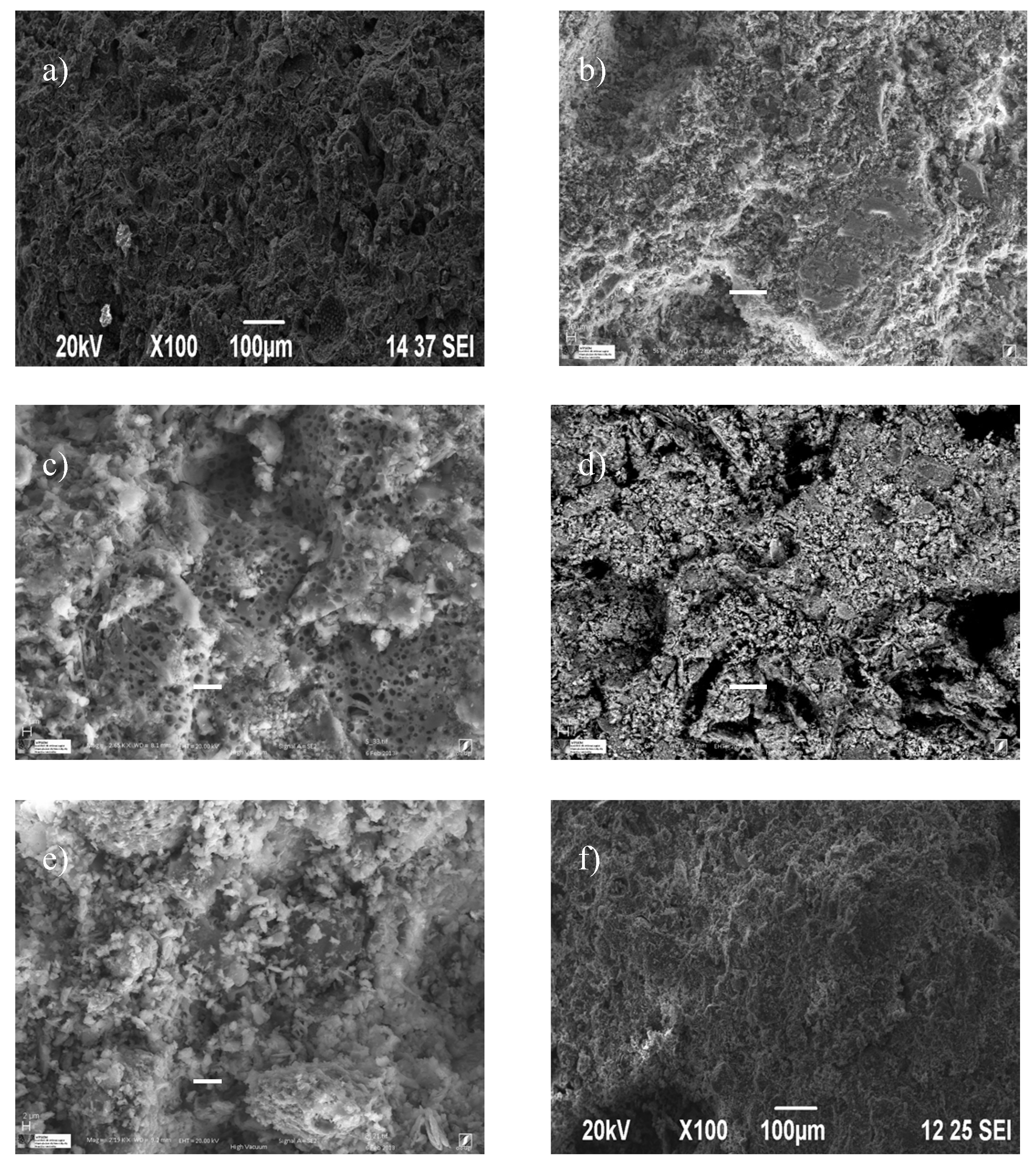
- The microstructure of the formed samples was observed by means of a scanning electron microscope (SEM), with a high-resolution transmission electron microscope JEOL SM 840 (JEOL, Solutions for Innovation, Peabody, MA, USA).
3. Results and Discussion
3.1. Raw Materials
3.1.1. Chemical Composition and Loss on Ignition
3.1.2. Total Content of Carbon, Hydrogen, Nitrogen and Sulfur: Heating Values
3.1.3. Thermogravimetric Analysis (TGA) and Differential Thermal Analysis (DTA)
3.2. Sintered Materials
3.2.1. Weight Loss
3.2.2. Adsorption and Desorption Isotherms
3.2.3. Technological Properties
3.2.4. Insulating Properties
3.2.5. Microstructure
3.2.6. Life Cycle Assessment (LCA)
- Goal and scope: Definition of system boundaries, functional unit used in the analysis and data sources description.
- LCI: Definition of inputs and outputs, data-based process of quantifying energy, raw material requirements, gas emissions, effluents, solid waste and every environmental release incurred throughout the life cycle processes.
- LCIA: A method, quantitative, and/or qualitative process to characterize and assess the effects of the environmental loadings identified in the inventory component.
- Life Cycle Improvement: A systematic assessment of the needs and opportunities to reduce environmental burdens associated with energy and raw materials use and waste emissions throughout the whole life-cycle of a product process, or activity.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, B.; Poon, C.S. Use of Furnace Bottom Ash for Producing Lightweight Aggregate Concrete with Thermal Insulation Properties. J. Clean. Prod. 2015, 99, 94–100. [Google Scholar] [CrossRef]
- Chiou, I.J.; Wang, K.S.; Chen, C.H.; Lin, Y.T. Lightweight aggregate made from sewage sludge and incinerated ash. Waste Manag. 2006, 26, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Wang, S.Y.; Tang, C.W. Reuse of incineration fly ashes and reaction ashes for manufacturing lightweight aggregate. Constr. Build. Mater. 2010, 24, 46–55. [Google Scholar] [CrossRef]
- Kourti, I.; Cheeseman, C.R. Properties and microstructure of lightweight aggregate produced from lignite coal fly ash and recycled glass. Resour. Conserv. Recycl. 2010, 54, 769–775. [Google Scholar] [CrossRef]
- Tan, W.; Wang, L.; Huang, C.; Liu, Y.Y.; Green, J.D.; Newport, D.; Green, T. Utilization of municipal solid waste incineration fly ash in lightweight aggregates. J. Cent. South Univ. Technol. 2012, 19, 835–841. [Google Scholar] [CrossRef]
- Quina, J.M.; Almeida, M.A.; Santos, R.; Bordado, J.M.; Quinta-Ferreira, R.M. Compatibility analysis of municipal solid waste incineration residues and clay for producing lightweight aggregates. Appl. Clay Sci. 2014, 102, 71–80. [Google Scholar] [CrossRef]
- Colangelo, F.; Messina, F.; Cioffi, R. Recycling of MSWI fly ash by means of cementitious double step cold bonding pelletization: Technological assessment for the production of lightweight artificial aggregates. J. Hazard. Mater. 2015, 299, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, F.; Messina, F.; Di Palma, L.; Cioffi, R. Recycling of non-metallic automotive shredder residues and coal fly-ash in cold-bonded aggregates for sustainable concrete. Compos. Part B Eng. 2017, 116, 46–52. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R. Use of cement kiln dust, blast furnace slag and marble sludge in the manufacture of sustainable artificial aggregates by means of cold bonding pelletization. Materials 2013, 6, 3139–3159. [Google Scholar] [CrossRef]
- Gennaro, R.D.; Cappelletti, P.; Cerri, G.; Gennaro, M.D.; Dondi, M.; Langella, A. Neapolitan yellow tuff as raw material for lightweight aggregates in lightweight structural concrete production. Appl. Clay Sci. 2005, 28, 309–319. [Google Scholar] [CrossRef]
- Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Rodas, M. Characterization of lightweight aggregates manufactured from washing aggregate sludge and fly ash. Resour. Conserv. Recycl. 2009, 53, 571–581. [Google Scholar] [CrossRef]
- Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Rodas, M.; Luque, F.J. Microstructure and mineralogy of lightweight aggregates manufactured from mining and industrial wastes. Constr. Build. Mater. 2011, 25, 3591–3602. [Google Scholar] [CrossRef]
- Gonzalez-Corrochano, B.; Alonso-Azcarate, J.; Rodas, M. Effect of thermal treatment on the retention of chemical elements in the structure of lightweight aggregates manufactured from contaminated mine soil and fly ash. Constr. Build. Mater. 2012, 35, 497–507. [Google Scholar] [CrossRef]
- Huang, S.C.; Chang, F.C.; Lo, S.L.; Lee, M.Y.; Wang, C.F.; Lin, J.D. Production of lightweight aggregates from mining residues, heavy metal sludge, and incinerator fly ash. J. Hazard. Mater. 2007, 144, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Shafigh, P.; Mahmud, H.B.; Jumaat, M.Z.B.; Ahmmad, R.; Bahri, S. Structural lightweight aggregate concrete using two types of waste from the palm oil industry as aggregate. J. Clean. Prod. 2014, 80, 187–196. [Google Scholar] [CrossRef]
- Bogas, J.A.; de Brito, J.; Figueiredo, J.M. Mechanical characterization of concrete produced with recycled lightweight expanded clay aggregate concrete. J. Clean. Prod. 2015, 89, 187–195. [Google Scholar] [CrossRef]
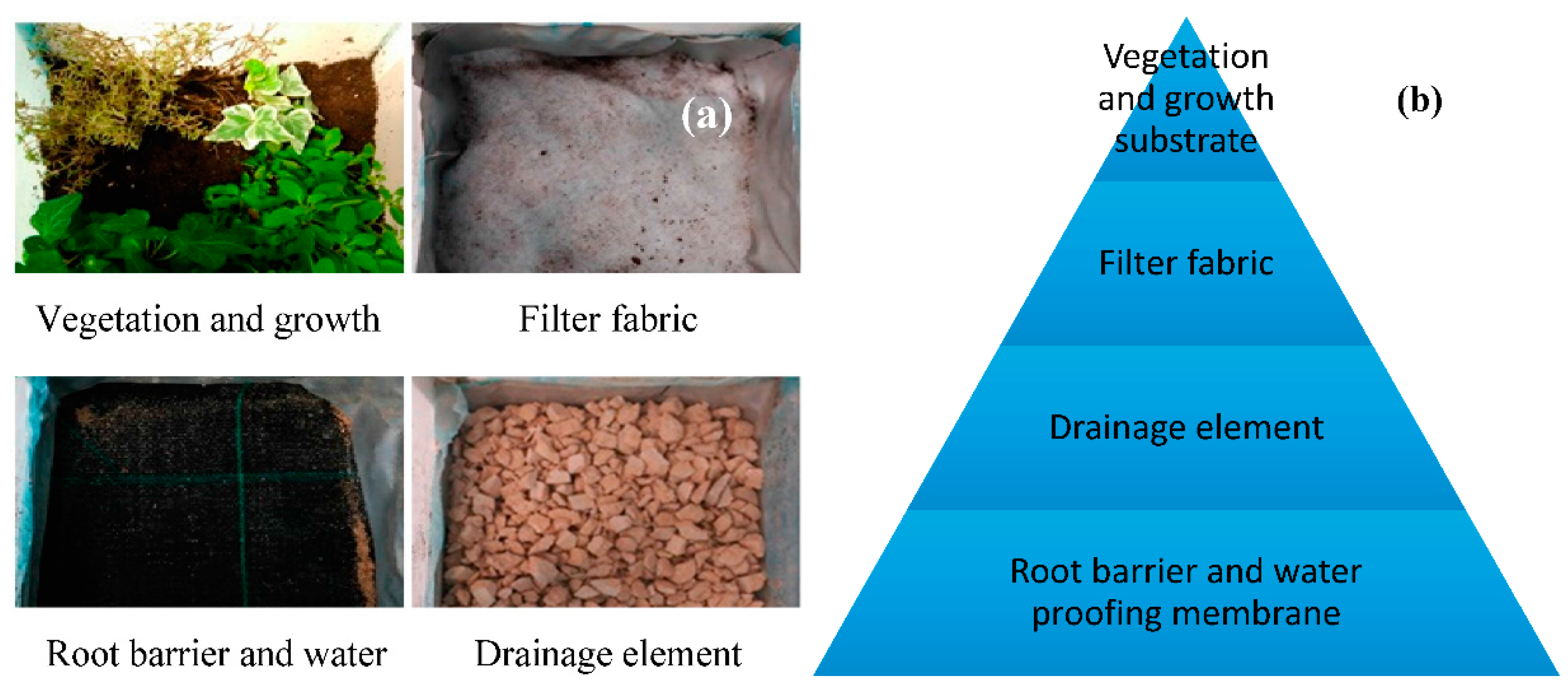
- Yang, W.; Wang, Z.; Cui, J.; Zhu, Z.; Zhao, X. Comparative study of the thermal performance of the novel green (planting) roofs against other existing roofs. Sustain. Cities Soc. 2015, 16, 1–12. [Google Scholar] [CrossRef]
- Mefteh, H.; Kebaïli, O.; Oucief, H.; Berredjem, L.; Nourredine Arabi, N. Influence of moisture conditioning of recycled aggregates on the properties of fresh and hardened concrete. J. Clean. Prod. 2013, 54, 282–288. [Google Scholar] [CrossRef]
- Pelisser, F.; Zavarise, N.; Longo, T.A.; Bernardin, A.M. Concrete made with recycled tire rubber: Effect of alkaline activation and silica fume addition. J. Clean. Prod. 2011, 19, 757–763. [Google Scholar] [CrossRef]
- Sengul, O.; Azizi, S.; Karaosmanoglu, F.; Tasdemir, M.A. Effect of expanded perlite on the mechanical properties and thermal conductivity of lightweight concrete. Energy Build. 2011, 2–3, 671–676. [Google Scholar] [CrossRef]
- Akçaözoğlu, S.; Akçaözoğlu, K.; Atiş, C.D. Thermal conductivity, compressive strength and ultrasonic wave velocity of cementitious composite containing waste PET lightweight aggregate (WPLA). Compos. Part B Eng. 2013, 1, 721–726. [Google Scholar] [CrossRef]
- Refahi, A.H.; Talkhabi, H. Investigating the effective factors on the reduction of energy consumption in residential buildings with green roofs. Renew. Energy 2015, 80, 595–603. [Google Scholar] [CrossRef]
- Jaffal, I.; Ouldboukhitine, S.E.; Belarbi, R. A comprehensive study of the impact of green roofs on building energy performance. Renew. Energy 2012, 43, 157–164. [Google Scholar] [CrossRef]
- Tabares-Velasco, P.C.; Jelena, S. Experimental quantification of heat and mass transfer process through vegetated roof samples in a new laboratory setup. Int. J. Heat Mass Transfer. 2011, 54, 5149–5162. [Google Scholar] [CrossRef]
- Castleton, H.F.; Stovin, V.; Beck, S.B.M.; Davison, J.B. Green roofs; building energy savings and the potential for retrofit. Energy Build. 2010, 42, 1582–1591. [Google Scholar] [CrossRef]
- Beer Statistics. 2015. Available online: http://www.brewersofeurope.org/uploads/mycms-files/documents/publications/2015/statistics_2015_v3.pdf (accessed on 3 January 2016).
- Ishiwaki, N.; Murayama, H.; Awayama, H.; Kanauchi, O.; Sato, T. Development of high value uses of spent grain by fractionation technology. MBAA Tech. Q. 2000, 37, 261–265. [Google Scholar]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Russ, W.; Mörtel, H.; Meyer-Pittroff, R. Application of spent grains to increase porosity in bricks. Constr. Build. Mater. 2005, 19, 117–126. [Google Scholar] [CrossRef]
- Cioffi, R.; Colangelo, F.; Montagnaro, F.; Santoro, L. Manufacture of artificial aggregate using MSWI bottom ash. Waste Manag. 2011, 31, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Rashad, A. Cementitious materials and agricultural wastes as natural fine aggregate replacement in conventional mortar and concrete. J. Build. Eng. 2016, 5, 119–141. [Google Scholar] [CrossRef]
- Bories, C.; Borredon, M.E.; Vedrenne, E.; Vilarem, G. Development of eco-friendly porous fired clay bricks using pore-forming agents: A review. J. Environ. Manag. 2014, 143, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Cheeseman, C.R.; Makinde, A.; Bethanis, S. Properties of lightweight aggregate produced by rapid sintering of incinerator bottom ash. Resour. Conserv. Recycl. 2005, 43, 147–162. [Google Scholar] [CrossRef]
- Cheeseman, C.R.; Virdi, G.S. Properties and microstructure of lightweight aggregate produced from sintered sewage sludge ash. Resour. Conserv. Recycl. 2005, 45, 18–30. [Google Scholar] [CrossRef]
- ASTM D-2974. Standard Test Method for Moisture, Ash, and Organic Matter of Peat and other Organic Soils; American Society for Testing and Materials International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Elías, X. Optimización de los Procesos Cerámicos Industriales Ponencias ID 57, 58, 59 y 60. 2001. Available online: http://www.cnpml.org/html/archivos/Ponencias (accessed on 10 December 2016).
- Colomer, F.J.; Gallardo, A.; Bodea, M.D.; Carlos, M.; Herrera, L. Characterization of different sludge from municipal and industrial sewages treatment plants. In Proceedings of the 13th International Congress Project Engineering, Badajoz, Spain, July 2009; pp. 235–245. [Google Scholar]
- ASTM-C373-14a. Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity and Apparent Specific Gravity on Fired Whiteware Products, Ceramic Tiles and Glass Tiles; American Society for Testing and Materials International: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Aranda, A.; López-Sabrión, A.M.; Ferreira, G.; Llera, E. Uses of alternative fuels and raw materials in the cement industry as sustainable waste management options. Renew. Sustain. Energy Rev. 2013, 23, 242–260. [Google Scholar] [CrossRef]
- Gregorová, E.; Pabst, W.; Stetina, J. Viscoelastic behavior of ceramic suspensions with carrageenn. J. Eur. Ceram. Soc. 2006, 26, 1185–1194. [Google Scholar] [CrossRef]
- Tancredi, N.; Medero, N.; Möller, F.; Píriz, J.; Plada, K.; Cordero, T.J. Phenol adsorption onto powdered and granular activated carbon, prepared from Eucalyptus wood. J. Colloid Interface Sci. 2004, 279, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Mikhail, R.Sh.; Brunauer, S.; Bodor, E.E. Investigations of a complete pore structure analysis: II. J. Colloid Interface Sci. 1968, 26, 54–61. [Google Scholar] [CrossRef]
- Mikhail, R.; Brunauer, S. Surface area measurements by nitrogen and argon adsorption. J. Colloid Interface Sci. 1975, 52, 572–577. [Google Scholar] [CrossRef]
- UNE-EN-ISO-14040:2006. Environmental Management—Life Cycle Assessment—Principles and Framework (ISO 14040:2006). Available online: www.aenor.es (accessed on 23 December 2016).
- UNE-EN-ISO-14044:2006. Environmental Management—Life Cycle Assessment—Requirements and Guidelines (ISO 14044:2006). Available online: www.aenor.es (accessed on 25 January 2017).
- UNE-EN ISO 14064-1:2012 (Corrected Version 2015-02-04). Greenhouse Gases—Part 1: Specification with Guidance at the Organization Level for Quantification and Reporting of Greenhouse Gas Emissions and Removals (ISO 14064-1:2006). Available online: www.aenor.es (accessed on 15 January 2017).
- Proietti, S.; Desideri, U.; Sdringola, P.; Zepparelli, F. Carbon footprint of a reflective foil and comparison with other solutions for thermal insulation in building envelope. Appl. Energy 2013, 112, 843–855. [Google Scholar] [CrossRef]
- Simion, I.M.; Ghinea, C.; Maxineasa, S.G.; Taranu, N.; Bonoli, A.; Gavrilescu, M. Ecological footprint applied in the assessment of construction and demolition waste integrated management. Environ. Eng. Manag. J. 2013, 12, 779–788. [Google Scholar]



| Component | SiO2 (%) | Al2O3 (%) | Fe2O3 (%) | MnO (%) | MgO (%) | CaO (%) | Na2O (%) | K2O (%) | TiO2 (%) | P2O5 (%) | Zr (ppm) | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red Clay | 50.15 | 19.67 | 8.21 | 0.12 | 3.57 | 2.80 | 0.02 | 6.37 | 0.83 | 0.16 | 135.5 | 7.86 |
| Yellow Clay | 60.40 | 11.46 | 4.02 | 0.03 | 1.39 | 8.73 | 0.38 | 2.73 | 0.64 | 0.10 | 270.6 | 9.54 |
| Black Clay | 55.98 | 9.80 | 3.72 | 0.03 | 2.55 | 11.26 | 0.21 | 2.57 | 0.61 | 0.11 | 301.3 | 12.74 |
| MC | 54.26 | 12.41 | 4.60 | 0.10 | 2.23 | 9.77 | 1.00 | 3.26 | 0.58 | 0.14 | 230.5 | 11.90 |
| Sludge Ash | 53.32 | 4.31 | 2.42 | 0.04 | 1.00 | 26.45 | 1.43 | 0.72 | 0.33 | 8.59 | -- | -- |
| Bagasse Ash | 44.33 | 0.29 | 0.97 | 0.13 | 4.60 | 6.30 | 0.24 | 2.89 | 0.05 | 21.29 | 0.10 | -- |
| Diatomaceous Earth Ash | 67.34 | 4.95 | 0.66 | -- | 0.11 | 0.43 | 1.20 | 1.31 | 0.27 | 0.35 | 0.09 | 0.04 |
| Wastes | % C | % H | % N | % S | LHV (kcal/kg) | HHV (kcal/kg) |
|---|---|---|---|---|---|---|
| MC | 2.140 ± 0.012 | 0.340 ± 0.001 | 0.025 ± 0.002 | --- | --- | -- |
| Bagasse | 48.110 ± 0.070 | 7.570 ± 0.019 | 4.800 ± 0.025 | --- | 4363.91 ± 47.13 | 4762.47 ± 47.13 |
| Sludge | 13.613 ± 0.192 | 1.973 ± 0.022 | 1.807 ± 0.046 | --- | 1000.40 ± 1.87 | 1242.59 ± 1.87 |
| Diatomaceous Earth | 6.460 ± 0.036 | 0.876 ± 0.015 | 1.117 ± 0.001 | --- | 583.19 ± 22.34 | 537.07 ± 22.34 |
| T a, °C | 900 °C | 950 °C | 1000 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Weight % | Loss on Ignition, % | Loss on Ignition, % | Loss on Ignition, % | ||||||
| Bagasse | Sludge | Diatomaceous | Bagasse | Sludge | Diatomaceous | Bagasse | Sludge | Diatomaceous | |
| 0 | 9.80 | 9.80 | 9.80 | 9.74 | 9.74 | 9.74 | 9.82 | 9.82 | 9.82 |
| 2 | 12.00 | 9.64 | 10.32 | 12.06 | 10.70 | 10.37 | 12.14 | 10.69 | 10.41 |
| 4 | 13.63 | 11.34 | 10.86 | 13.64 | 11.08 | 10.98 | 13.81 | 11.43 | 10.92 |
| 6 | 14.84 | 10.69 | 10.95 | 14.86 | 10.95 | 10.98 | 15.11 | 11.13 | 10.91 |
| 8 | 16.20 | 12.12 | 10.31 | 16.24 | 12.00 | 10.55 | 16.42 | 12.29 | 10.57 |
| 10 | 18.14 | 10.67 | 10.56 | 18.20 | 13.45 | 10.66 | 18.35 | 13.47 | 10.73 |
| 12.5 | 20.53 | 10.59 | 10.49 | 20.51 | 13.88 | 10.53 | 20.91 | 14.29 | 10.61 |
| 15 | 22.42 | 14.18 | 10.73 | 22.55 | 14.26 | 10.57 | 22.77 | 14.49 | 10.74 |
| Clay | 900 °C | 950 °C | 1000 °C | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | |
| 35.06 ± 0.12 | 20.58 ± 0.14 | 1703.5 ± 08.7 | 34.57 ± 0.27 | 20.24 ± 0.25 | 1708.1 ± 10.3 | 35.5435 ± 0.6836 | 20.9253 ± 0.5088 | 1698.8 ± 09.0 | |
| Brewery wastewater sludge | |||||||||
| % w/w | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 |
| 2.0 | 31.65 ± 1.68 | 18.02 ± 0.96 | 1757.2 ± 26.4 | 33.84 ± 1.22 | 20.11 ± 0.73 | 1690.0 ± 21.7 | 36.03 ± 0.75 | 22.20 ± 0.49 | 1622.9 ± 10.7 |
| 4.0 | 35.86 ± 0.47 | 22.46 ± 0.36 | 1596.8 ± 07.6 | 36.52 ± 0.28 | 22.83 ± 0.28 | 1599.3 ± 09.0 | 36.88 ± 0.43 | 23.32 ± 0.39 | 1582.1 ± 09.6 |
| 6.0 | 35.36 ± 0.95 | 20.48 ± 0.70 | 1726.8 ± 18.9 | 32.59 ± 0.58 | 18.90 ± 0.41 | 1724.0 ± 12.7 | 29.83 ± 0.20 | 17.33 ± 0.13 | 1721.2 ± 06.4 |
| 8.0 | 38.69 ± 0.27 | 25.61 ± 0.27 | 1510.1 ± 06.5 | 40.19 ± 0.50 | 26.74 ± 0.43 | 1502.9 ± 07.0 | 47.08 ± 0.41 | 34.70 ± 0.32 | 1357.0 ± 05.3 |
| 10.0 | 41.26 ± 0.27 | 27.92 ± 0.26 | 1478.4 ± 07.1 | 42.69 ± 0.24 | 29.10 ± 0.33 | 1467.1 ± 11.0 | 39.84 ± 0.30 | 26.74 ± 0.20 | 1489.6 ± 03.2 |
| 12.5 | 38.23 ± 0.29 | 23.45 ± 0.26 | 1630.3 ± 06.8 | 41.47 ± 0.32 | 26.90 ± 0.24 | 1541.5 ± 05.2 | 46.97 ± 0.39 | 32.30 ± 0.35 | 1453.8 ± 05.2 |
| 15.0 | 42.57 ± 0.27 | 29.17 ± 0.27 | 1459.2 ± 07.4 | 43.79 ± 0.47 | 30.56 ± 0.48 | 1434.4 ± 09.1 | 45.01 ± 0.72 | 31.94 ± 0.68 | 1409.5 ± 10.7 |
| Bagasse | |||||||||
| % w/w | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 |
| 2.0 | 36.77 ± 1.15 | 23.33 ± 0.81 | 1576.3 ± 13.9 | 37.20 ± 0.23 | 23.75 ± 0.30 | 1566.7 ± 12.9 | 37.63 ± 0.99 | 24.17 ± 0.79 | 1557.0 ± 12.7 |
| 4.0 | 42.41 ± 0.34 | 28.53 ± 0.36 | 1486.6 ± 08.5 | 42.86 ± 0.44 | 29.29 ± 0.39 | 1463.7 ± 09.6 | 43.30 ± 0.61 | 30.06 ± 0.49 | 1440.7 ± 04.3 |
| 6.0 | 45.36 ± 0.25 | 32.10 ± 0.32 | 1413.4 ± 06.8 | 46.33 ± 0.16 | 33,13 ± 0.31 | 1398.6 ± 08.5 | 49.98 ± 0.61 | 34.24 ± 0.73 | 1372.3 ± 12.4 |
| 8.0 | 48.29 ± 0.29 | 36.52 ± 0.35 | 1322.4 ± 06.7 | 49.29 ± 0.55 | 37.94 ± 0.46 | 1300.1 ± 14.3 | 50.29 ± 0.21 | 39.36 ± 0.28 | 1277.8 ± 08.0 |
| 10.0 | 51.33 ± 0.29 | 41.03 ± 0.41 | 1251.0 ± 06.2 | 52.24 ± 0.41 | 42.10 ± 0.62 | 1241.0 ± 08.6 | 51,61 ± 0,25 | 41.70 ± 0.31 | 1237.8 ± 07.5 |
| 12.5 | 54.73 ± 0.32 | 47.46 ± 0.39 | 1153.3 ± 11.2 | 55.01 ± 0.22 | 47.43 ± 0.36 | 1159.8 ± 05.7 | 54.46 ± 0.38 | 47.49 ± 0.43 | 1146.8 ± 08.9 |
| 15.0 | 56.25 ± 0.65 | 50.54 ± 1.14 | 1113.2 ± 13.3 | 56.54 ± 0.38 | 50.88 ± 0.30 | 1111.5 ± 10.2 | 56.84 ± 0.52 | 51.22 ± 0.61 | 1109.8 ± 04.2 |
| Diatomaceous earth | |||||||||
| % w/w | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 | Open porosity, % | Water abs, % | Bulk density, Kg/m3 |
| 2.0 | 36.77 ± 0.61 | 22.99 ± 0.36 | 1598.7 ± 05.8 | 36.68 ± 0.62 | 22.97 ± 0.40 | 1597.1 ± 07.1 | 36.59 ± 0.64 | 22.93 ± 0.44 | 1595.4 ± 08.3 |
| 4.0 | 40.32 ± 0.58 | 26.23 ± 0.45 | 1537.3 ± 09.0 | 40.14 ± 0.59 | 26.30 ± 0.48 | 1526.7 ± 09.2 | 39.97 ± 0.59 | 26.37 ± 0.50 | 1516.1 ± 09.3 |
| 6.0 | 41.29 ± 0.56 | 27.87 ± 0.59 | 1481.7 ± 11.5 | 42.56 ± 0.53 | 29.27 ± 0.62 | 1454.2 ± 12.5 | 44.11 ± 0.47 | 30.94 ± 0.52 | 1425.6 ± 09.0 |
| 8.0 | 43.77 ± 0.46 | 30.735 ± 0.48 | 1424.1 ± 08.0 | 44.73 ± 0.40 | 31.80 ± 0.46 | 1407.3 ± 08.4 | 45.70 ± 0.34 | 32.87 ± 0.43 | 1390.4 ± 08.7 |
| 10.0 | 44.59 ± 0.38 | 31.72 ± 0.42 | 1405.7 ± 07.1 | 45.61 ± 0.42 | 32.83 ± 0.43 | 1390.0 ± 07.4 | 46.63 ± 0.46 | 33.93 ± 0.44 | 1374.3 ± 07.6 |
| 12.5 | 47.37 ± 0.55 | 35.41 ± 0.73 | 1338.1 ± 12.5 | 47.96 ± 0.29 | 36.26 ± 0.45 | 1323.0 ± 08.6 | 48.10 ± 0.11 | 36.22 ± 0.23 | 1328.0 ± 06.3 |
| 15.0 | 50.13 ± 0.55 | 39.70 ± 0.86 | 1263.0 ± 14.5 | 50.37 ± 0.41 | 40.01 ± 0.63 | 1259.0 ± 11.4 | 50.61 ± 0.26 | 40.33 ± 0.41 | 1255.0 ± 08.2 |
| Impact Category | Unit | Sludge | Bagasse | Diatomaceous | Clay |
|---|---|---|---|---|---|
| IPCC GWP 20y | kg CO2 eq. | 3.992 | 3.280 | 4.007 | 5.611 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farías, R.D.; Martínez García, C.; Cotes Palomino, T.; Martínez Arellano, M. Effects of Wastes from the Brewing Industry in Lightweight Aggregates Manufactured with Clay for Green Roofs. Materials 2017, 10, 527. https://doi.org/10.3390/ma10050527
Farías RD, Martínez García C, Cotes Palomino T, Martínez Arellano M. Effects of Wastes from the Brewing Industry in Lightweight Aggregates Manufactured with Clay for Green Roofs. Materials. 2017; 10(5):527. https://doi.org/10.3390/ma10050527
Chicago/Turabian StyleFarías, Romina D., Carmen Martínez García, Teresa Cotes Palomino, and Myriam Martínez Arellano. 2017. "Effects of Wastes from the Brewing Industry in Lightweight Aggregates Manufactured with Clay for Green Roofs" Materials 10, no. 5: 527. https://doi.org/10.3390/ma10050527





