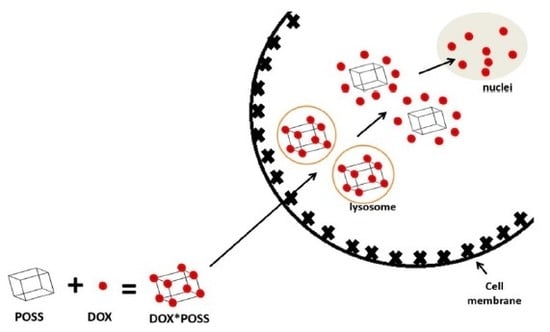
Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS)
Abstract
:1. Introduction
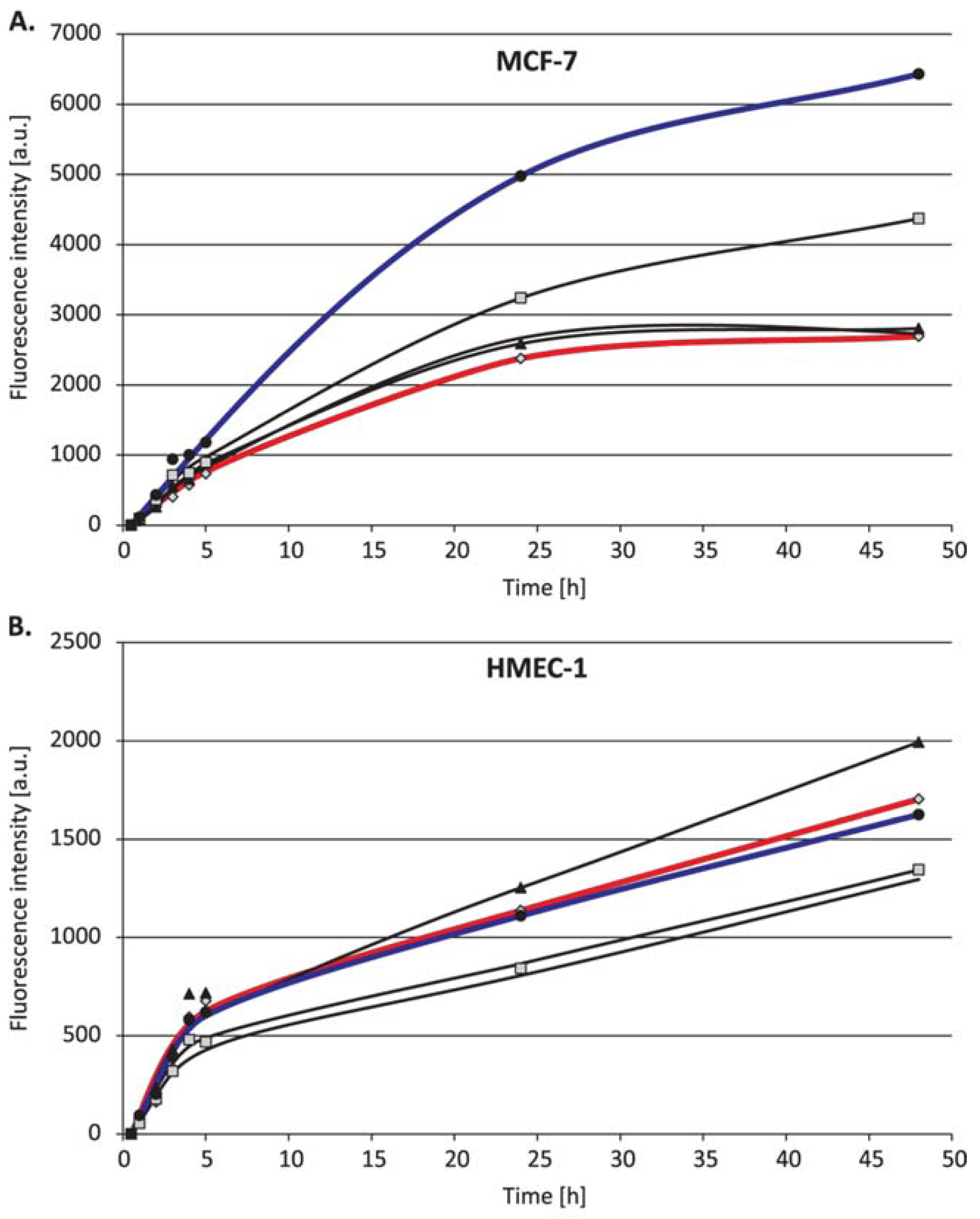
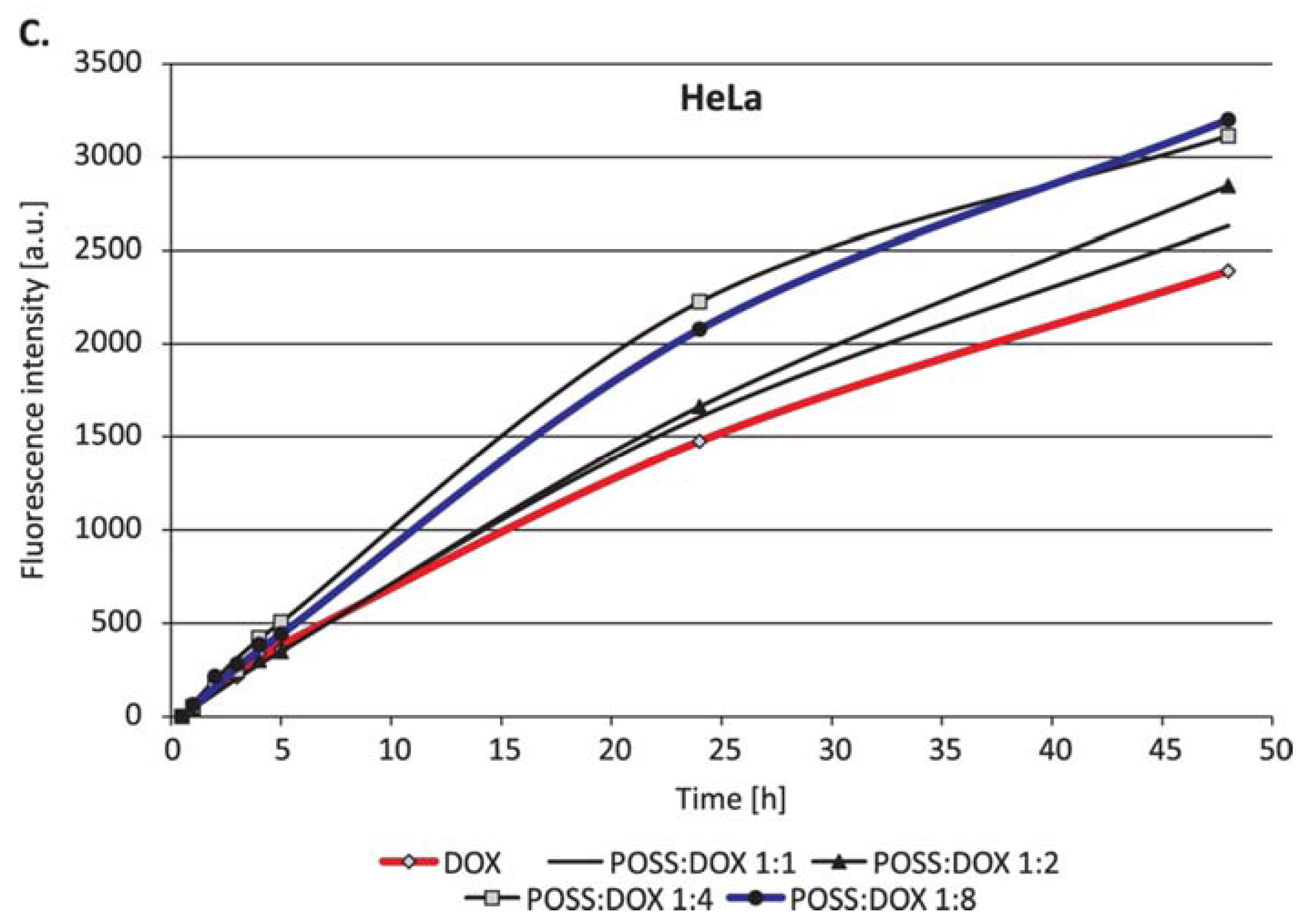
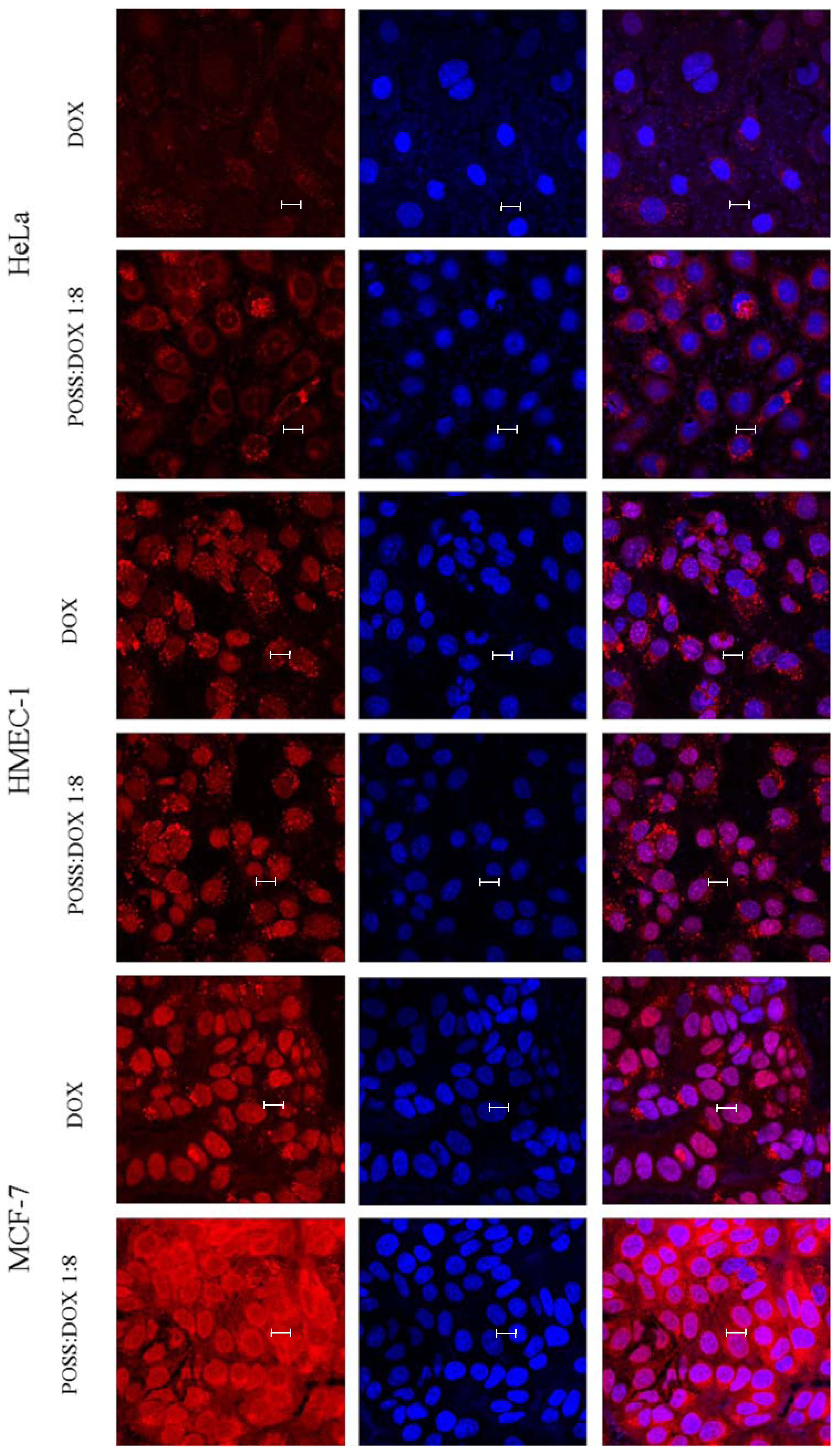
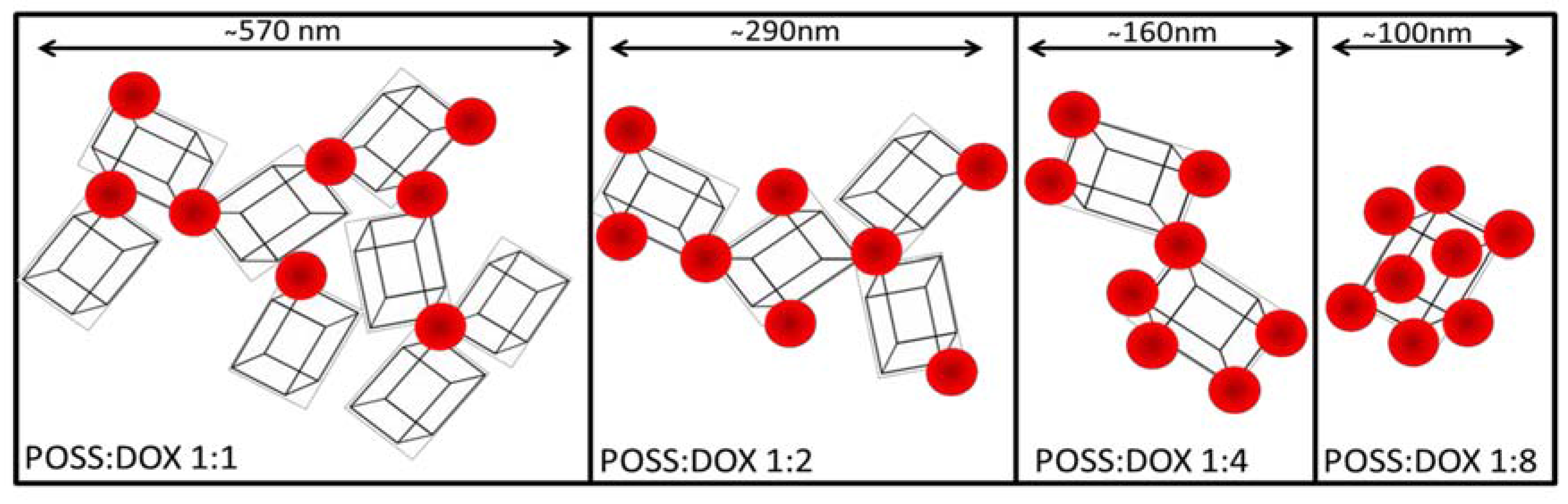
2. Results
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. In Vitro Toxicity
4.4. Uptake Detection
4.5. Confocal Microscopy
4.6. Hydrodynamic Diameter
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, L.; Lu, B.; Fan, Q.; Wu, J.; Wei, L.; Hou, J.; Guo, X.; Liu, Z. Synthesis and self-assembly behavior of pH-responsive star-shaped POSS-(PCL-P(DMAEMA-co-PEGMA))16 inorganic/organic hybrid block copolymer for the controlled intracellular delivery of doxorubicin. RSC Adv. 2016, 6, 61630–61640. [Google Scholar] [CrossRef]
- Wilczewska, A.Z.; Niemirowicz, K.; Markiewicz, K.H.; Car, H. Nanoparticles as drug delivery systems. Pharmacol. Rep. 2012, 64, 1020–1037. [Google Scholar] [CrossRef]
- McCusker, C.; Carroll, J.B.; Rotello, V.M. Cationic polyhedral oligomeric silsesquioxane (POSS) units as carriers for drug delivery processes. Chem. Commun. 2005, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral oligomeric silsesquioxane (POSS)-containing polymer nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef] [PubMed]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent developments in the chemistry of cubic polyhedral oligosilsesquioxanes. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Abate, L.; Antonelli, M.L.; Bottino, F.A.; Bottino, P. Phenyl hepta cyclopentyl–Polyhedral oligomeric silsesquioxane (ph,hcp-POSS)/Polystyrene (PS) nanocomposites: the influence of substituents in the phenyl group on the thermal stability. Express Polym. Lett. 2012, 6, 997–1006. [Google Scholar] [CrossRef]
- Blanco, I.; Abate, L.; Bottino, F.A. Variously substituted phenyl hepta cyclopentyl-polyhedral oligomeric silsesquioxane (ph,hcp-POSS)/polystyrene (PS) nanocomposites. The influence of substituents on the thermal stability. J. Therm. Anal. Calorim. 2013, 112, 421–428. [Google Scholar] [CrossRef]
- Kim, S.K.; Heo, S.J.; Koak, J.Y.; Lee, J.H.; Lee, Y.M.; Chung, D.J.; Lee, J.I.; Hong, S.D. A biocompatibility study of a reinforced acrylic-based hybrid denture composite resin with polyhedraloligosilsesquioxane. J. Oral Rehabil. 2007, 34, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Gradzinska, K.; Labecka, K.; Kowalewska, A.; Stanczyk, W.A. Silsesquioxane nanocarriers in diagnostics and biomedicine. Polimery 2016, 61, 229–304. [Google Scholar] [CrossRef]
- Yuan, K.; Luo, Y.; Pu, Y.; He, B.; Wang, G. A novel poly (l-glutamic acid) dendrimer based drug delivry system with pH-sensitive and targeting functions. Mol. Pharm. 2010, 7, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.K.; Lu, X.; He, C. Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J. Mater. Chem. 2011, 21, 2775–2782. [Google Scholar] [CrossRef]
- Ghanbari, H.; Mel, A.; Seifalian, M.A. Cardiovascular application of polyhedral oligomeric silsesquioxane nanomaterials: A glimpse into prospective horizons. Int. J. Nanomed. 2011, 6, 775–786. [Google Scholar] [CrossRef]
- Rozga-Wijas, K.; Michalski, A. An efficient synthetic route for soluble silsesquioxane-daunorubicin conjugate. Eur. Polym. J. 2016, 84, 490–501. [Google Scholar] [CrossRef]
- Piorecka, K.; Radzikowska, E.; Kurjata, J.; Rozga-Wijas, K.; Stanczyk, W.A.; Wielgus, E. Synthesis of the first POSS cage-anthracycline conjugates via amide bonds. New J. Chem. 2016, 40, 5997–6000. [Google Scholar] [CrossRef]
- Janaszewska, A.; Gradzinska, K.; Marcinkowska, M.; Klajnert-Maculewicz, B.; Stanczyk, W. In vitro studies of polyhedral oligo silsesquioxanes: evidence for their low cytotoxicity. Materials 2015, 8, 6062–6070. [Google Scholar] [CrossRef]
- Waddon, A.J.; Coughlin, E.B. Crystal structure of polyhedral oligomeric silsequioxane (POSS) nano-materials: A study by X-ray diffraction and electron microscopy. Chem. Mater. 2003, 15, 4555–4561. [Google Scholar] [CrossRef]
- Matejka, L.; Janata, M.; Plestil, J.; Zhigunov, A.; Slouf, M. Self-assembly of POSS-containing block copolymers: Fixing the hierarchical structure in networks. Polymer 2014, 55, 126–136. [Google Scholar] [CrossRef]
- Tan, A.; Farhatania, Y.; Goh, D.; Mel, A.; Lim, J.; Teoh, S.; Malkovskiy, A.; Chawla, R.; Rajadas, J.; Cousins, B.; et al. Surface modification of a polyhedral oligomeric silsesquioxane poly(carbhonate-urea) urethane (POSS-PCU) nanocomposite polymer as a stent coating for enhanced capture ofendothelial progenitor cells. Biointerphases 2013, 8, 23. [Google Scholar] [CrossRef]
- Kannan, R.; Salacinski, H.; Butler, P.; Seifalian, A. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Hong, L. Polyhedral oligomeric silsesquioxane (POSS) bioconjugates. In Chemistry of Bioconjugates: Synthesis, Characterization and Biomedical Applications; Narain, R., Ed.; Wiley: New York, NY, USA, 2013; pp. 344–356. [Google Scholar] [CrossRef]
- Kaneshiro, L.; Lu, Z.R. Targeted intracellular co-delivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nano-globular carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Chang, S.; Yuan, H.; Wang, G.; He, B.; Gu, Z. The anti-tumor efficiency of poly (l-glutamic acid) dendrimers with polyhedral oligomeric silsesquioxane cores. Biomaterials 2013, 34, 3658–3666. [Google Scholar] [CrossRef] [PubMed]
- Gravel, M.C.; Zhang, C.; Dinderman, M.; Laine, R.M. Octa(3-chloroammoniumpropyl) octasilsesquioxane. Appl. Organomet. Chem. 1999, 13, 329–336. [Google Scholar] [CrossRef]
- Morgan, D.M.L. Tetrazolium (MTT) Assay for Cellular Viability and Activity. In Methods in Molecular Biology, Polyamine Protocols; Morgan, D.M.L, Ed.; Humana Press Inc.: Totowa, NJ, USA, 1998; Volume 79, pp. 179–184. [Google Scholar]
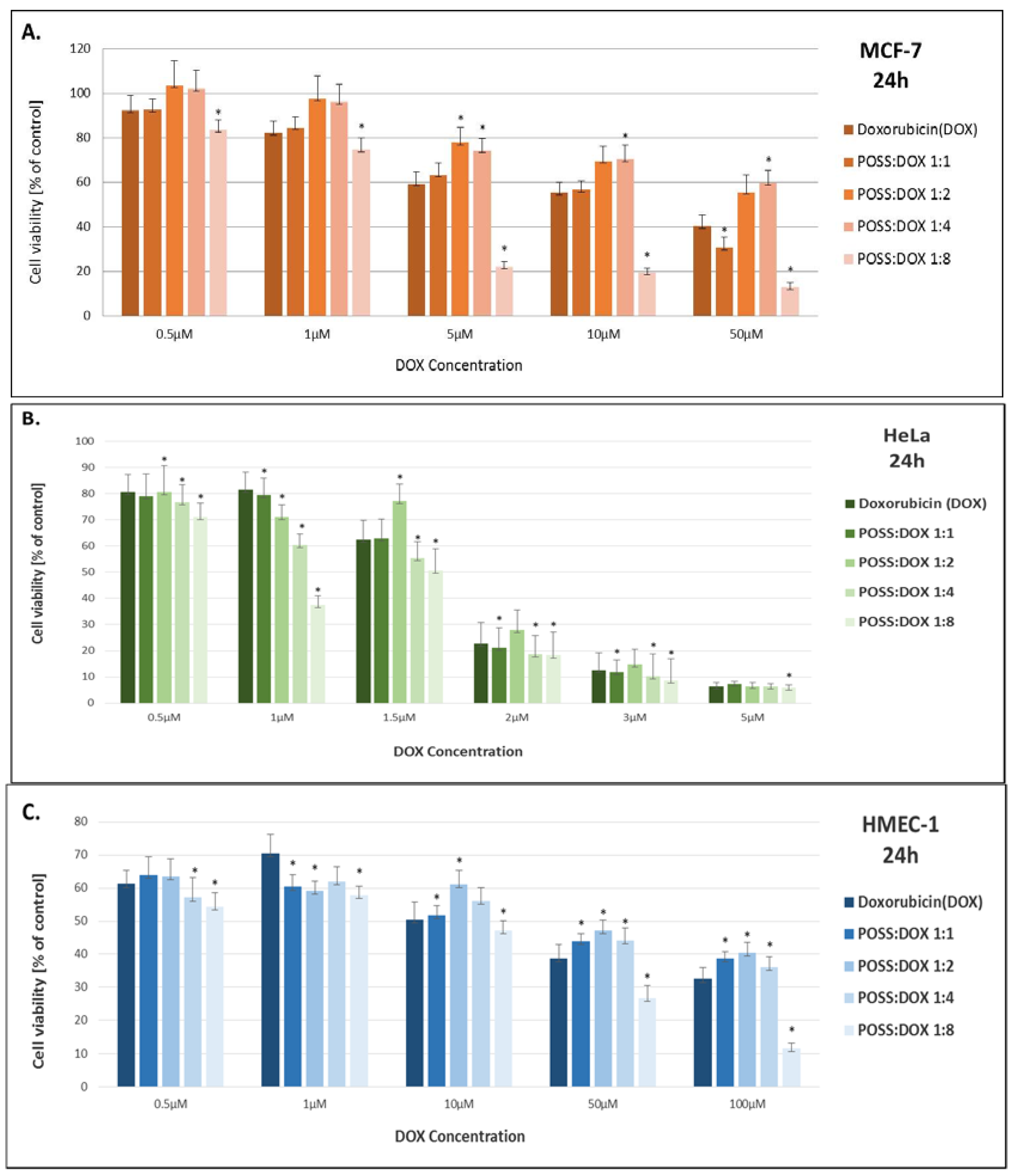

| Sample | IC50 [µM/L] | ||
|---|---|---|---|
| MCF-7 | HeLa | HMEC-1 | |
| Doxorubicin (DOX) | 17.44 ± 5.23 | 1.45 ± 0.15 | 10.33 ±4.63 |
| POSS:DOX 1:1 | 13.65 ± 4.03 | 1.44 ± 0.12 | 10.92 ± 2.26 |
| POSS:DOX 1:2 | 76.97 ± 35.11 | 1.61 ± 0.26 | 14.81 ± 12.10 |
| POSS:DOX 1:4 | 109.10 ± 55.88 | 1.25 ± 0.08 | 9.11 ± 4.56 |
| POSS:DOX 1:8 | 2.69 ± 0.15 | 0.92 ± 0.09 | 2.51 ± 0.42 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobierajska, E.; Konopka, M.; Janaszewska, A.; Piorecka, K.; Blauz, A.; Klajnert-Maculewicz, B.; Stanczyk, M.; Stanczyk, W.A. Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS). Materials 2017, 10, 559. https://doi.org/10.3390/ma10050559
Sobierajska E, Konopka M, Janaszewska A, Piorecka K, Blauz A, Klajnert-Maculewicz B, Stanczyk M, Stanczyk WA. Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS). Materials. 2017; 10(5):559. https://doi.org/10.3390/ma10050559
Chicago/Turabian StyleSobierajska, Ewelina, Malgorzata Konopka, Anna Janaszewska, Kinga Piorecka, Andrzej Blauz, Barbara Klajnert-Maculewicz, Maciej Stanczyk, and Wlodzimierz A. Stanczyk. 2017. "Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS)" Materials 10, no. 5: 559. https://doi.org/10.3390/ma10050559
APA StyleSobierajska, E., Konopka, M., Janaszewska, A., Piorecka, K., Blauz, A., Klajnert-Maculewicz, B., Stanczyk, M., & Stanczyk, W. A. (2017). Unusual Enhancement of Doxorubicin Activity on Co-Delivery with Polyhedral Oligomeric Silsesquioxane (POSS). Materials, 10(5), 559. https://doi.org/10.3390/ma10050559