Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques
Abstract
:1. Introduction
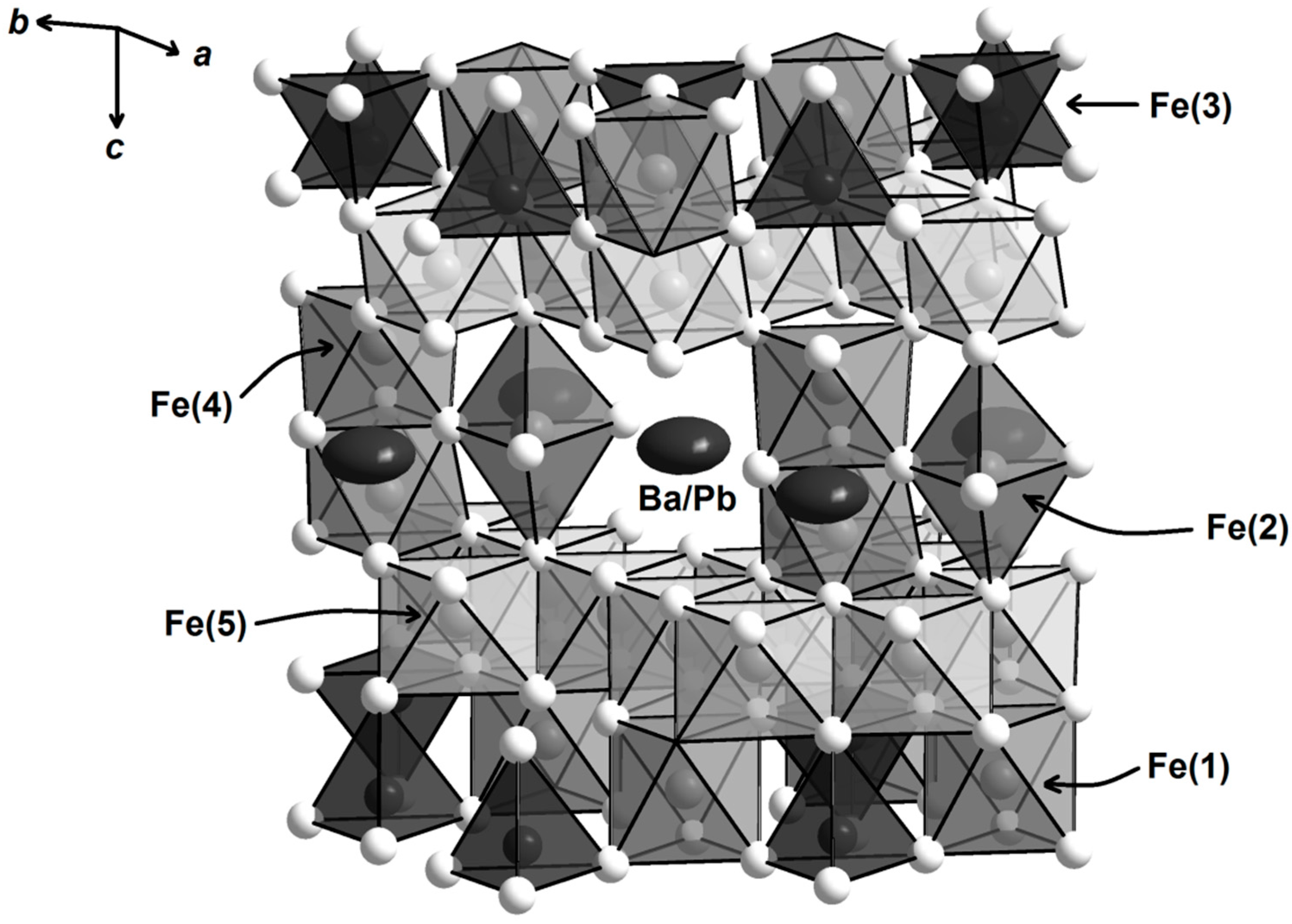
2. Materials and Methods
2.1. Synthesis and Crystal Growth
2.2. Characterization
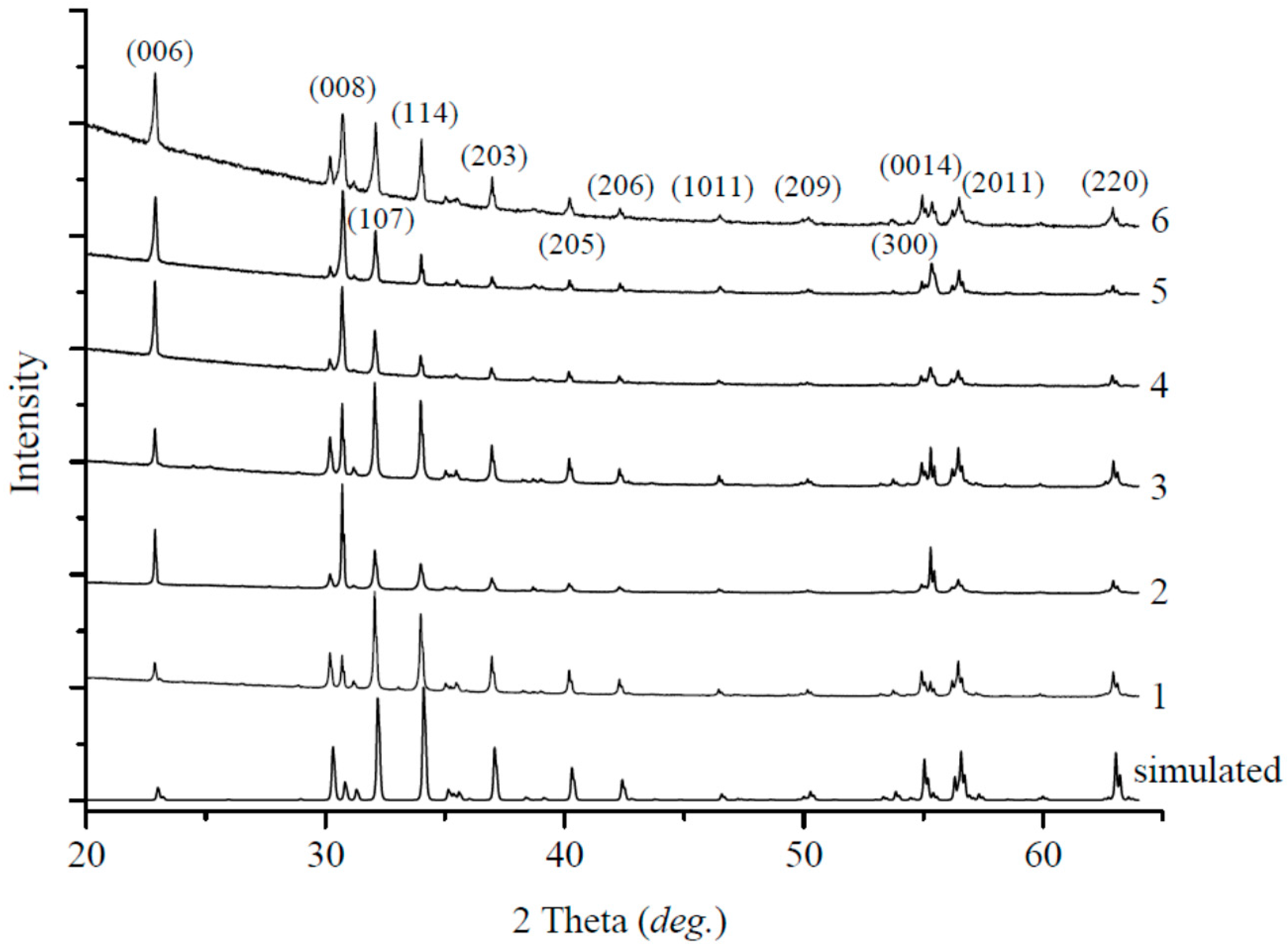
3. Results
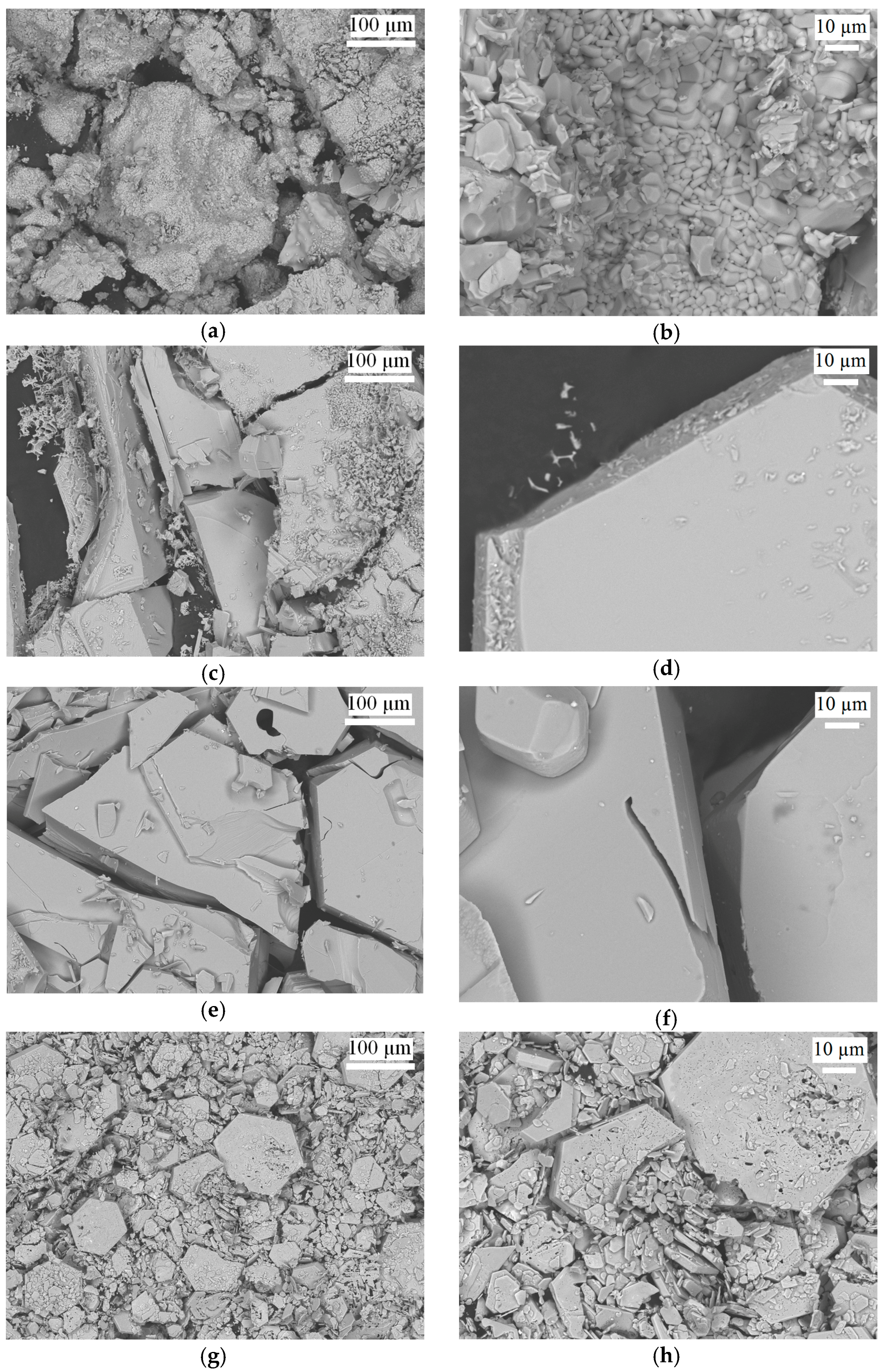
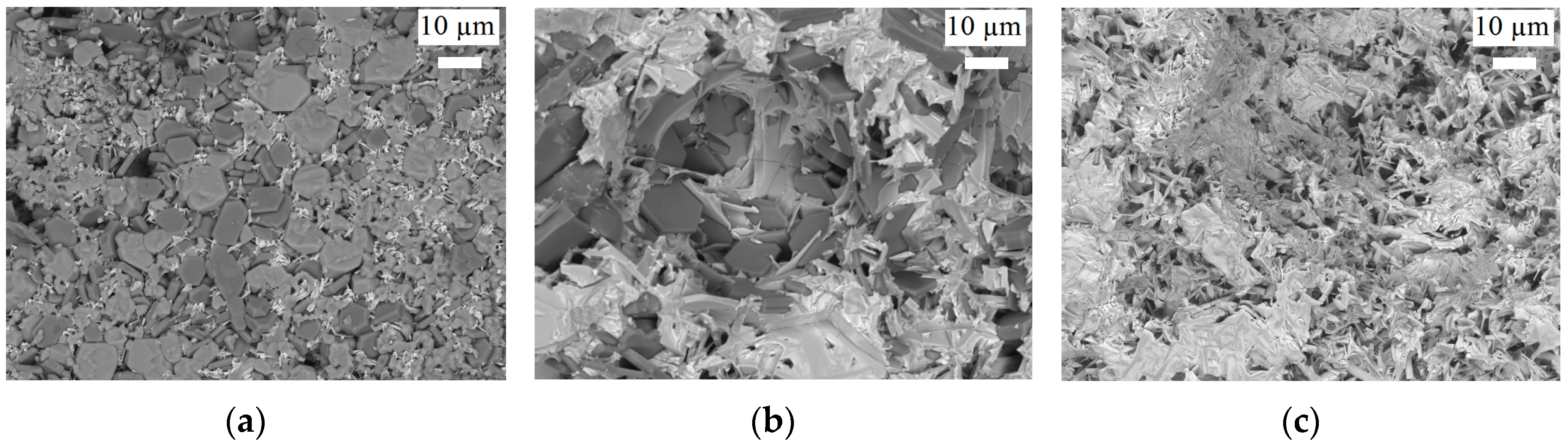
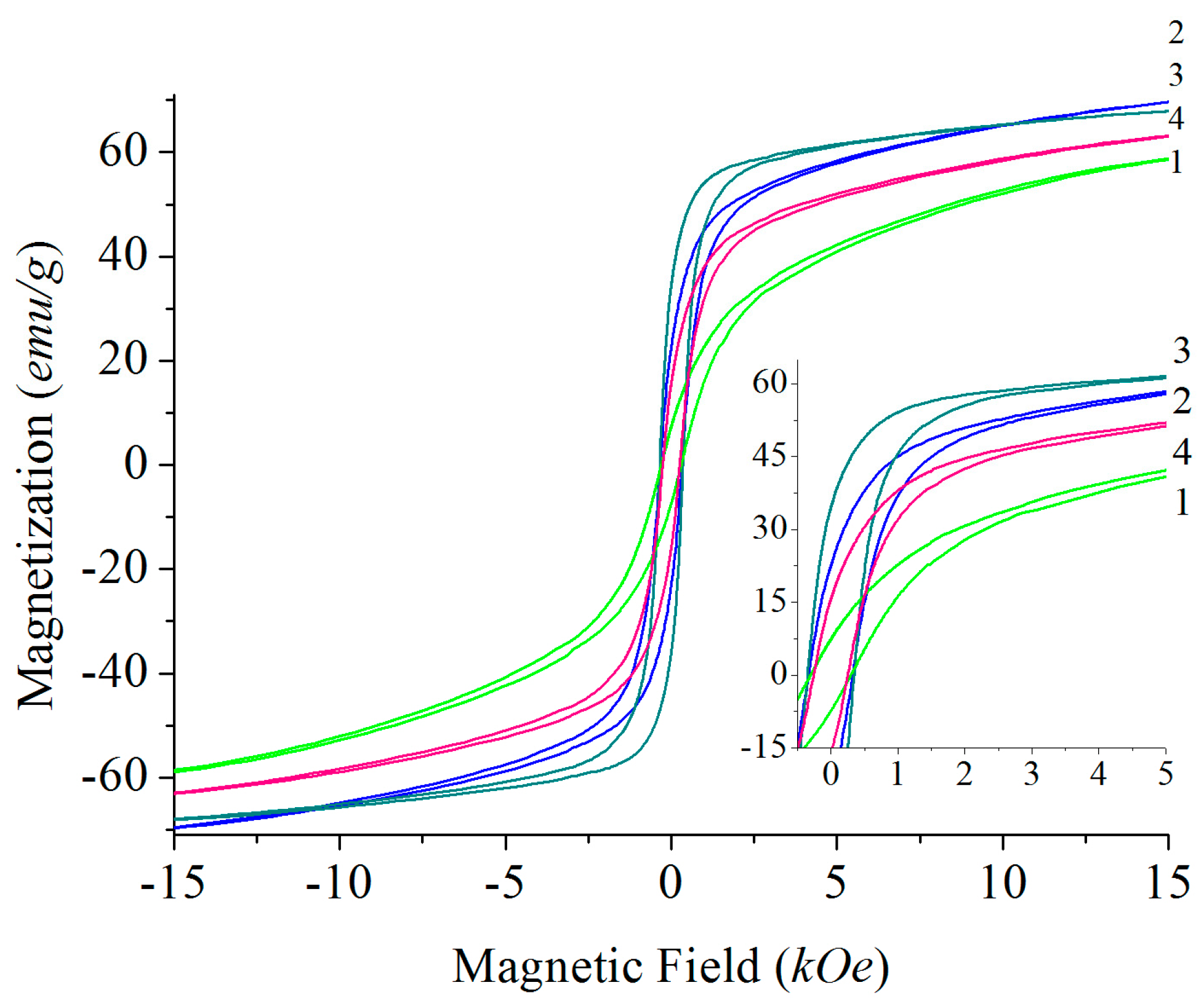
Solid-State Sintering
4. Discussion
4.1. Solid-State Sintering
4.2. Carbonate and Borate Flux Growth
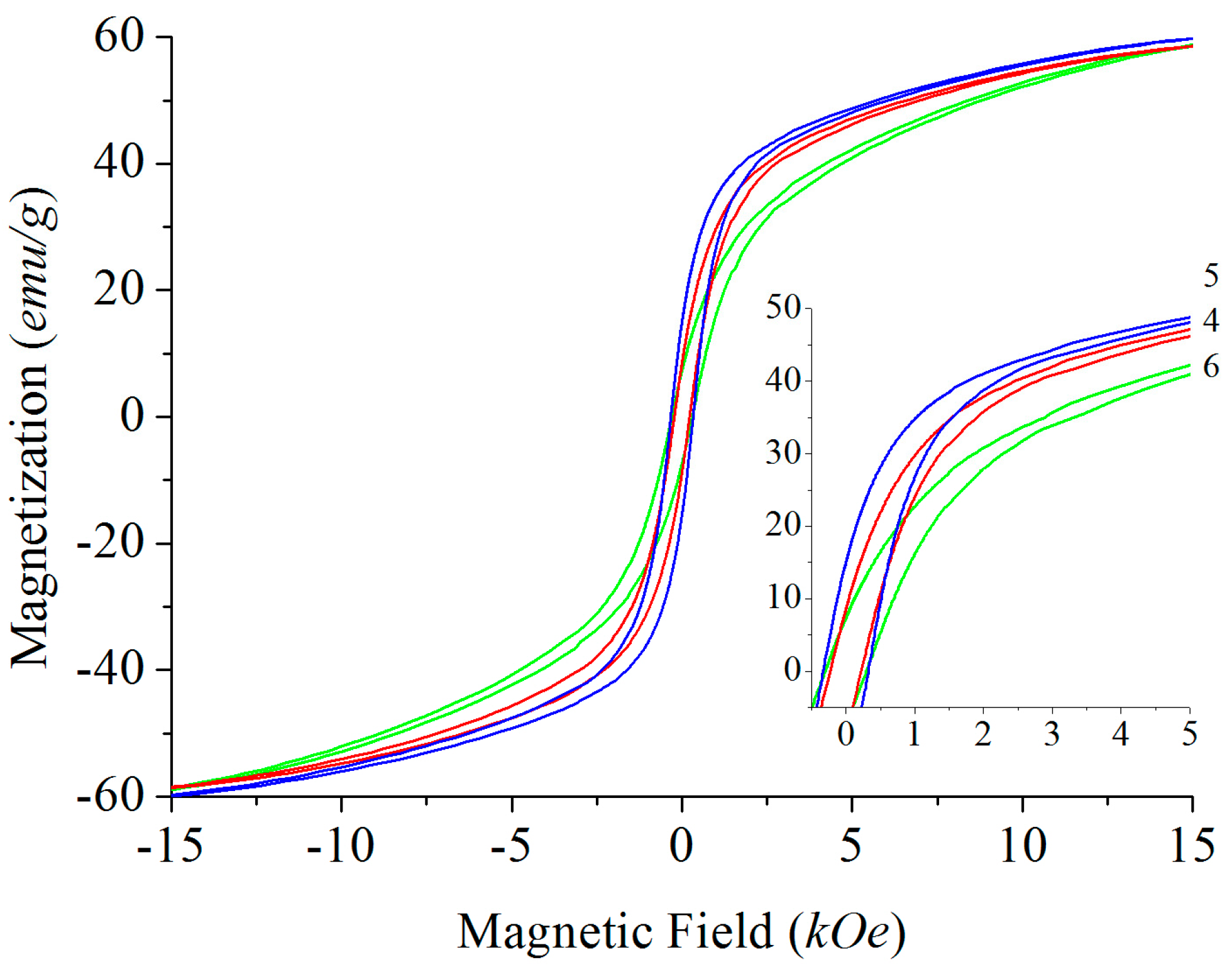
4.3. PbO Flux Growth
4.4. Magnetic Properties
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Afghahi, S.S.S.; Jafarian, M.; Salehi, M.; Atassi, Y. Improvement of the Performance of Microwave X Band Absorbers Based on Pure and Doped Ba-hexaferrite. J. Magn. Magn. Mater. 2017, 421, 340–348. [Google Scholar] [CrossRef]
- Lou, H.; Wang, J.; Xu, B.; Wang, G.; Hou, Y.; Gao, H.; Ye, W. Effects of Mg or Sr Doping on the Intrinsic Characteristics and Absorption Properties of Micro-nano BaFe12O19 Hollow Multiphase Ceramic Microspheres. J. Magn. Magn. Mater. 2015, 374, 530–538. [Google Scholar]
- Cheng, K.B.; Ramakrishna, S.; Lee, K.C. Electromagnetic Shielding Effectiveness of Copper/Glass Fiber Knitted Fabric Reinforced Polypropylene Composites. Compos. Part A 2000, 31, 1039–1045. [Google Scholar] [CrossRef]
- Das, N.C.; Khastgir, D.; Chaki, T.K.; Chakraborty, A. Electromagnetic Interference Shielding Effectiveness of Carbon Black and Carbon Fibre Filled EVA and NR Based Composites. Compos. Part A 2000, 31, 1069–1081. [Google Scholar] [CrossRef]
- Kwon, H.J.; Shin, J.Y.; Oh, T.H. The Microwave Absorbing and Resonance Phenomena of Y-Type Hexagonal Ferrite Microwave Absorbers. J. Appl. Phys. 1994, 75, 6109–6111. [Google Scholar] [CrossRef]
- Kotsuka, Y.; Yamazaki, H. Fundamental Investigation on a Weakly Magnetized Ferrite Absorber. IEEE Trans. Electr. Comat. 2000, 42, 116–124. [Google Scholar] [CrossRef]
- Singh, J.; Singh, C.; Kaur, D.; Narang, S.B.; Joshi, R.; Mishra, S.R.; Jotania, R.; Ghimire, M.; Chauhan, C.C. Tunable Microwave Absorption in Co/Al Substituted M-Type Ba/Sr Hexagonal Ferrite. Mater. Des. 2016, 110, 749–761. [Google Scholar] [CrossRef]
- Shams, M.H.; Rozatian, A.S.H.; Yousefi, M.H.; Valíček, J.; Šepelák, V. Effect of Mg2+ and Ti4+ Dopants on the Structural, Magnetic and High-Frequency Ferromagnetic Properties of Barium Hexaferrite. J. Magn. Magn. Mater. 2016, 399, 10–18. [Google Scholar] [CrossRef]
- Trukhanov, A.V.; Trukhanov, S.V.; Turchenko, V.A.; Oleinik, V.V.; Yakovenko, E.S.; Matsui, L.Y.; Vovchenko, L.L.; Launets, V.L.; Kazakevich, I.S.; Dzhabarov, S.G. Crystal Structure, Magnetic, and Microwave Properties of Solid Solutions BaFe12–xGaxO19 (0.1 ≤ x ≤ 1.2). Phys. Solid-State 2016, 58, 1792–1797. [Google Scholar] [CrossRef]
- Auwal, I.A.; Erdemi, H.; Sözeri, H.; Güngüneş, H.; Baykal, A. Magnetic and Dielectric Properties of Bi3+ Substituted SrFe12O19 Hexaferrite. J. Magn. Magn. Mater. 2016, 412, 69–82. [Google Scholar] [CrossRef]
- Liu, J.; Zeng, Y.; Su, Z.; Geiler, M.; Chen, Y.; Harris, V.G. Magnetic Properties of a Highly Textured Barium Hexaferrite Quasi-Single Crystal and Its Application in Low-Field Biased Circulators. J. Electr. Mater. 2016, 45, 5069–5073. [Google Scholar] [CrossRef]
- Li, Z.W.; Chen, L.; Ong, C.K. Studies of Static and High-Frequency Magnetic Properties for M-Type Ferrite BaFe12−2xCoxZrxO19. J. Appl. Phys. 2002, 92, 3902–3907. [Google Scholar] [CrossRef]
- Trukhanov, S.V.; Trukhanov, A.V.; Turchenko, V.O.; Kostishyn, V.G.; Panina, L.V.; Kazakevich, I.S.; Balagurov, A.M. Structure and Magnetic Properties of BaFe11.9In0.1O19 Hexaferrite in a Wide Temperature Range. J. Alloys Compd. 2016, 689, 383–393. [Google Scholar] [CrossRef]
- Li, Z.W.; Yang, Z.H.; Kong, L.B. High-Frequency Properties and Electromagnetic Wave Attenuation for Hexaferrite Composites. Procedia Eng. 2014, 75, 19–23. [Google Scholar] [CrossRef]
- Karmakar, M.; Mondal, B.; Pal, M.; Mukherjee, K. Acetone and Ethanol Sensing of Barium Hexaferrite Particles: A Case Study Considering the Possibilities of Non-Conventional Hexaferrite Sensor. Sens. Actuators B Chem. 2014, 190, 627–633. [Google Scholar] [CrossRef]
- Sugimoto, S.; Kondo, S.; Okayama, K.; Nakamura, H.; Book, D.; Kagotani, T.; Homma, M.; Ota, H.; Kimura, M.; Sato, R. M-Type Ferrite Composite as a Microwave Absorber with Wide Bandwidth in the Ghz Range. IEEE Trans. Magn. 1999, 35, 3154–3156. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Z.; Ma, C.; Yao, X.; Zhang, L.; Wu, M. Complex Permittivity, Permeability, and Microwave Absorption of Zn- and Ti-Substituted Barium Ferrite by Citrate Sol–Gel Process. Mater. Sci. Eng. B 2002, 96, 289–295. [Google Scholar]
- Fisher, J.G.; Sun, H.; Kook, Y.-G.; Kim, J.-S.; Le, P.G. Growth of Single Crystals of BaFe12O19 by Solid-State Crystal Growth. J. Magn. Magn. Mater. 2016, 416, 384–390. [Google Scholar] [CrossRef]
- Cho, H.-S.; Kim, S.-S. M-hexaferrites with Planar Magnetic Anisotropy and Their Application to High-Frequency Microwave Absorbers. IEEE Trans. Magn. 1999, 35, 3151–3153. [Google Scholar]
- Matsumoto, M.; Miyata, Y. A Gigahertz-Range Electromagnetic Wave Absorber with Wide Bandwidth Made of Hexagonal Ferrite. J. Appl. Phys. 1996, 79, 5486–5488. [Google Scholar] [CrossRef]
- Goto, Y.; Takada, T. On the Phase Diagram of the Condensed System BaO-Fe2O3. J. Jpn. Soc. Powder Powder Metall. 1960, 7, 35–40. [Google Scholar] [CrossRef]
- Tudorache, F.; Brinza, F.; Popa, P.D.; Petrila, I.; Grigoras, M.; Tascu, S. Comparison between Powders of Strontium Hexaferrite Processed by Dynamic Gas Heat Treatment and Re-Calcination. Acta Phys. Pol. A 2012, 121, 92–94. [Google Scholar] [CrossRef]
- Tudorache, F.; Popa, P.D.; Brinza, F.; Tascu, S. Structural Investigations and Magnetic Properties of BaFe12O19 Crystals. Acta Phys. Pol. A 2012, 121, 95–97. [Google Scholar] [CrossRef]
- Gambino, R.J.; Leonhard, F. Growth of Barium Ferrite Single Crystals. J. Am. Ceram. Soc. 1961, 44, 221–224. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Vinnik, D.A.; Gavrilova, T.A.; Gudkova, S.A.; Isaenko, L.I.; Jiang, X.; Pokrovsky, L.D.; Prosvirin, I.P.; Mashkovtseva, L.S.; Lin, Z. Flux Crystal Growth and the Electronic Structure of BaFe12O19 Hexaferrite. J. Phys. Chem. C 2016, 120, 5114–5123. [Google Scholar] [CrossRef]
- Aidelberg, J.; Flicstein, J.; Schieber, M. Cellular Growth in BaFe12O19 Crystals Solidified from Flux Solvent. J. Cryst. Growth 1974, 21, 195–202. [Google Scholar] [CrossRef]
- Licci, F.; Besagni, T.; Lábár, J. Growth and Characterization of Ba(Mn,Ti)xFe12-xO19 Crystals. J. Mater. Res. Bull. 1987, 22, 467–476. [Google Scholar] [CrossRef]
- Vinnik, D.A.; Gudkova, S.A.; Niewa, R. Growth of Lead and Aluminum Substituted Barium Hexaferrite Single Crystals from Lead Oxide Flux. Mater. Sci. Forum 2016, 843, 3–9. [Google Scholar] [CrossRef]
- Adelsköld, V. X-ray studies on magneto-plumbite, PbO·6Fe2O3, and other substances resembling ‘‘beta-alumina’’, Na2O·11Al2O3. Ark. Min. Geol. 1938, 12A, 1–9. [Google Scholar]
- Vinnik, D.A. Resistive Furnace for Single Crystal Growth. Butlerov Commun. 2014, 39, 153–154, In Russian. [Google Scholar]
- Vinnik, D.A.; Tarasova, A.Yu.; Gudkova, S.A.; Trofimov, E.A.; Zherebtsov, D.A.; Chernukha, A.S.; Galimov, D.M.; Zhivulin, V.E.; Kalandija, M.; Isaenko, L.I.; et al. Morphology and Magnetic Properties of Pressed Barium Hexaferrite BaFe12O19 Materials. J. Am. Ceram Soc. 2017. under review. [Google Scholar]
- Townes, W.D.; Fang, J.H.; Perrotta, A.J. The Crystal Structure and Refinement of Ferromagnetic Barium Ferrite, BaFe12O19. Z. Kristallogr. 1967, 125, 437–449. [Google Scholar] [CrossRef]
- Mou, F.-Z.; Guan, J.-G.; Sun, Z.-G.; Fan, X.-A.; Tong, G.-X. In Situ Generated Dense Shell-Engaged Ostwald Ripening: A Facile Controlled-Preparation for BaFe12O19 Hierarchical Hollow Fiber Arrays. J. Solid-State Chem. 2010, 183, 736–743. [Google Scholar] [CrossRef]
- Pillai, V.; Kumar, P.; Multani, M.S.; Shah, D.O. Structure and Magnetic Properties of Nanoparticles of Barium Ferrite Synthesized using Microemulsion Processing. J. Colloids Surf. A 1993, 80, 69–71. [Google Scholar] [CrossRef]
- Watanabe, K. Growth of Minute Barium Ferrite Single Crystals from a Na2O-B2O3 Flux System. J. Cryst. Growth 1996, 169, 509–518. [Google Scholar] [CrossRef]
- Moore, P.B.; Gupta, P.K.S.; Page, Y.L. Crystal Structure of Magnetoplumbite. Am. Mineral. 1989, 74, 1186–1194. [Google Scholar]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Dursun, S.; Topkaya, R.; Akdoğan, N.; Alkoy, S. Comparison of the Structural and Magnetic Properties of Submicron Barium Hexaferrite Powders Prepared by Molten Salt and Solid-State Calcination Routes. Ceram. Int. 2012, 38, 3801–3806. [Google Scholar] [CrossRef]
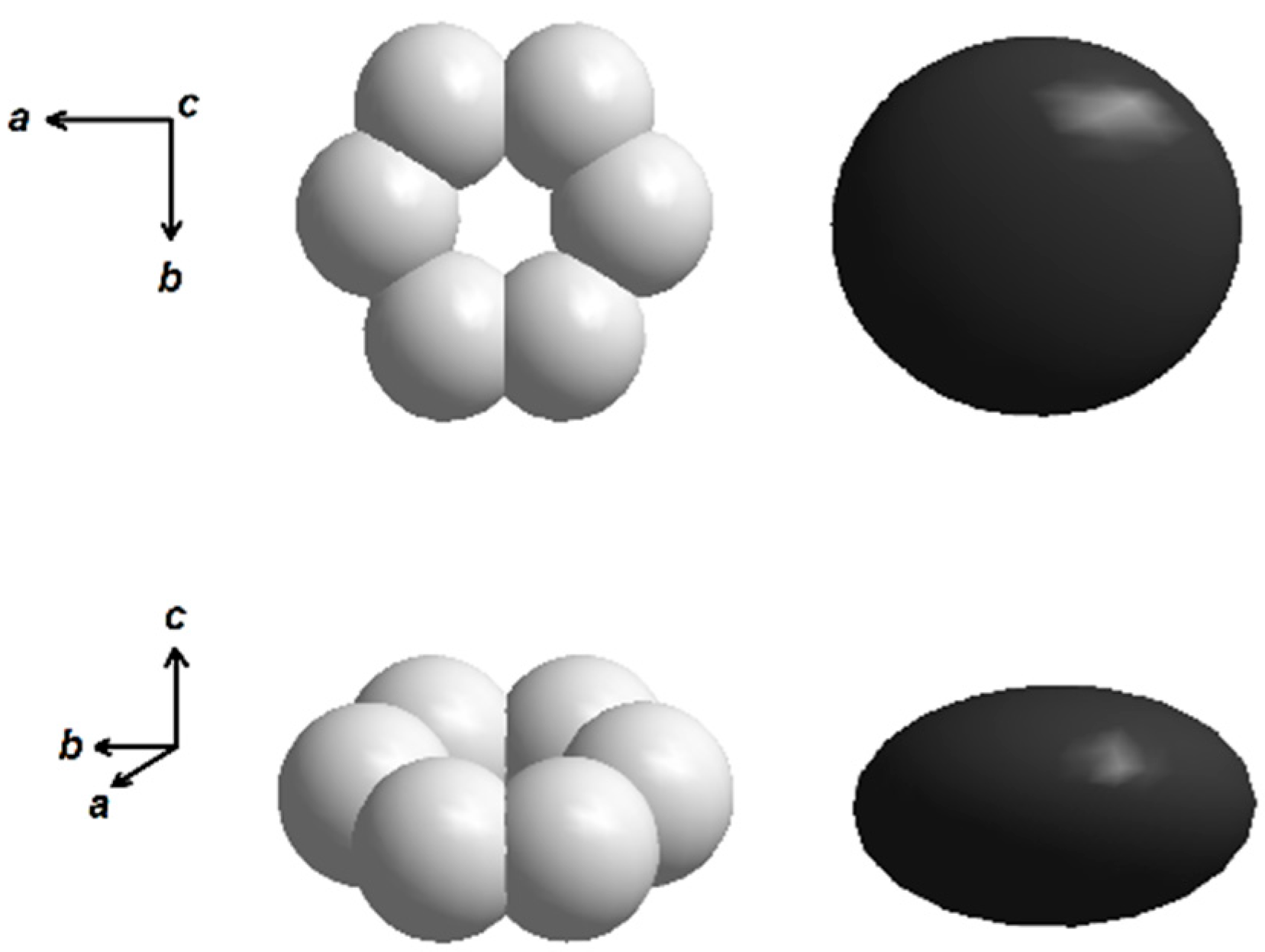
| No. | BaCO3 | Fe2O3 | Flux | Composition | T, °С |
|---|---|---|---|---|---|
| 1 | 14.3 | 85.7 | - | BaFe12O19 | 1350 |
| 2 | 10.5 | 63.2 | 26.3 Na2CO3 | BaFe12O19 | 1260 |
| 3 | 60.0 | 10.0 | 30.0 BaB2O4 | BaFe12O19 | 1260 |
| 4 | 5.7 | 34.3 | 60.0 PbO | Ba0.77(2)Pb0.23Fe12O19 | 1200 |
| 5 | 4.3 | 25.7 | 70.0 PbO | Ba0.56(2)Pb0.44Fe12O19 | 1025 |
| 6 | 2.9 | 17.1 | 80.0 PbO | Ba0.20(3)Pb0.80Fe12O19 | 950 |
| No. | Synthesis Method | Pb Content x | a (Å) | c (Å) | V (ų) | TC (K) | Ms (emu/g) | Hc (Oe) |
|---|---|---|---|---|---|---|---|---|
| [32] | 5.893 | 23.194 | 697.5 | - | - | - | ||
| [33] | - | - | - | 730 | - | - | ||
| [34] | - | - | - | - | 72.0 | 5395 | ||
| [35] | - | - | - | - | 59.0 | 360 | ||
| 1 | Solid-state | 0 | 5.8922(1) | 23.1953(6) | 697.40(2) | 726 | 63.5 | 254 |
| 2 | Na2CO3 flux | 0 | 5.8929(4) | 23.194(2) | 697.54(6) | 728 | 71.0 | 363 |
| 3 | BaB2O4 flux | 0 | 5.8915(2) | 23.1917(8) | 697.13(4) | 725 | 68.0 | 348 |
| 4 | 60 at % PbO flux | 0.23(2) | 5.8962(4) | 23.1927(1) | 698.28(6) | 721 | 59.3 | 299 |
| 5 | 70 at % PbO flux | 0.44(2) | 5.8948(3) | 23.1780(8) | 697.51(4) | 722 | 60.1 | 328 |
| 6 | 80 at % PbO flux | 0.80(2) | 5.8917(9) | 23.173(3) | 696.60(19) | 724 | 58.8 | 223 |
| [36] | PbFe12O19 | 1 | 5.873 | 23.007 | 687.24 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinnik, D.A.; Tarasova, A.Y.; Zherebtsov, D.A.; Gudkova, S.A.; Galimov, D.M.; Zhivulin, V.E.; Trofimov, E.A.; Nemrava, S.; Perov, N.S.; Isaenko, L.I.; et al. Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques. Materials 2017, 10, 578. https://doi.org/10.3390/ma10060578
Vinnik DA, Tarasova AY, Zherebtsov DA, Gudkova SA, Galimov DM, Zhivulin VE, Trofimov EA, Nemrava S, Perov NS, Isaenko LI, et al. Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques. Materials. 2017; 10(6):578. https://doi.org/10.3390/ma10060578
Chicago/Turabian StyleVinnik, Denis A., Aleksandra Yu. Tarasova, Dmitry A. Zherebtsov, Svetlana A. Gudkova, Damir M. Galimov, Vladimir E. Zhivulin, Evgeny A. Trofimov, Sandra Nemrava, Nikolai S. Perov, Ludmila I. Isaenko, and et al. 2017. "Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques" Materials 10, no. 6: 578. https://doi.org/10.3390/ma10060578
APA StyleVinnik, D. A., Tarasova, A. Y., Zherebtsov, D. A., Gudkova, S. A., Galimov, D. M., Zhivulin, V. E., Trofimov, E. A., Nemrava, S., Perov, N. S., Isaenko, L. I., & Niewa, R. (2017). Magnetic and Structural Properties of Barium Hexaferrite BaFe12O19 from Various Growth Techniques. Materials, 10(6), 578. https://doi.org/10.3390/ma10060578









