Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells
Abstract
:1. Introduction
2. Results and Discussion
2.1. X-ray Powder Diffraction
2.2. TEM Microscopy
2.3. ICP-OES Analysis
2.4. Cytotoxicity
3. Materials and Methods
3.1. Apparatus
3.2. Synthesis of Ca10(PO4)6(OH)2 Nanoparticles (nHAP)
3.3. Cell Culture
3.4. Suspension Preparation
3.5. Cytotoxicity Tests
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nassar, E.J.; Ciuffi, K.J.; Calefi, P.S.; Rocha, L.A.; De Faria, E.H.; e Silva, M.L.A.; Luz, P.P.; Bandeira, L.C.; Cestari, A.; Fernandes, C.N. Biomaterials and Sol-Gel Process: A Methodology for the Preparation of Functional Materials. In Bomaterial, Science and Engineering, 1st ed.; Rosario, P., Ed.; InTech: Rijeka, Croatia, 2007; pp. 3–30. [Google Scholar]
- Sadat-Shojai, M.; Khorasani, M.-T.; Dinpanah-Khoshdargi, E.; Jamshidi, A. Synthesis methods for nanosized hydroxyapatite with diverse structures. Acta Biomater. 2013, 9, 7591–7621. [Google Scholar] [CrossRef] [PubMed]
- Szymonowicz, M.; Rybak, Z.; Witkiewicz, W.; Pezowicz, C.; Filipiak, J. In vitro hemocompatibility studies of (poly(L-lactide) and poly(L-lactide-co-glycolide) as materials for bioresorbable stents manufacture. Acta Bioeng. Biomech. 2014, 16, 131–139. [Google Scholar] [PubMed]
- Chesnutt, B.M.; Yuan, Y.; Buddington, K.; Haggard, W.O.; Bumgardner, J.D. Composite chitosan/nano-hydroxyapatite scaffolds induce osteocalcin production by osteoblasts in vitro and support bone formation in vivo. Tissue Eng. A 2009, 15, 2571–2579. [Google Scholar] [CrossRef] [PubMed]
- Filová, E.; Suchý, T.; Sucharda, Z.; Supová, M.; Zaloudková, M.; Balík, K.; Lisá, V.; Slouf, M.; Bačáková, L. Support for the initial attachment, growth and differentiation of MG-63 cells: A comparison between nano-size hydroxyapatite and micro-size hydroxyapatite in composites. Int. J. Nanomedicine 2014, 9, 3687–3706. [Google Scholar] [CrossRef] [PubMed]
- Breding, K.; Jimbo, R.; Hayashi, M.; Xue, Y.; Mustafa, K.; Andersson, M. The Effect of Hydroxyapatite Nanocrystals on Osseointegration of Titanium Implants: An In Vivo Rabbit Study. Int. J. Dent. 2014. [Google Scholar] [CrossRef] [PubMed]
- Wiglusz, R.J.; Pozniak, B.; Zawisza, K.; Pazik, R. An up-converting HAP@β-TCP nanocomposite activated with Er3+/Yb3+ ion pairs for bio-related applications. RSC Adv. 2015, 5, 27610–27622. [Google Scholar] [CrossRef]
- Ashok, M.; Meenakshi Sundaram, N.; Kalkuta, S.N. Crystallization of hydroxyapatite at physiological temperature. Mater. Lett. 2003, 57, 2066–2070. [Google Scholar] [CrossRef]
- Kikuchi, M.; Itoh, S.; Ichinose, S.; Shinomiya, K.; Tanaka, J. Self-organization mechanism in a bone-like hydroxyapatite/collagen nanocomposite synthesized in vitro and its biological reaction in vivo. Biomaterials 2001, 22, 1705–1711. [Google Scholar] [CrossRef]
- Nandi, S.K.; Kundu, B.; Ghosh, S.K.; De, D.K.; Basu, D. Efficacy of nano-hydroxyapatite prepared by an aqueous solution combustion technique in healing bone defects of goat. J. Vet. Sci. 2008, 9, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Tang, S.; Tan, H.; Lin, W.; Wang, Y.; Wei, J.; Zhao, L.; Tang, T. Preparation, characterization, and in vitro osteoblast functions of a nano-hydroxyapatite/polyetheretherketone biocomposite as orthopedic implant material. Int. J. Nanomedicine 2014, 9, 3949–3961. [Google Scholar] [PubMed]
- ISO E N. 10993-5. Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- Szymonowicz, M.; Rybak, Z.; Frączek-Szczypta, A.; Paluch, D.; Rusak, A.; Nowicka, K.; Błażewicz, M. Haemocompatibility and cytotoxic studies of non-metallic composite materials modified with magnetic nano and microparticles. Acta Bioeng. Biomech. 2015, 17, 49–58. [Google Scholar] [PubMed]
- Szymonowicz, M.; Pielka, S.; Marcinkowska, A.; Żywicka, B.; Gamian, A.; Haznar, D.; Pluta, J. Cellular response after stimulation of the gelatin-alginate matrixes. Macromol. Symp. 2008, 272, 58–62. [Google Scholar] [CrossRef]
- Chlopek, J.; Morawska-Chochol, A.; Bajor, G.; Adwent, M.; Cieslik-Bielecka, A.; Cieslik, M.; Sabat, D. The influence of carbon fibres on the resorption time and mechanical properties of the lactide—Glycolide co-polymer. J. Biomater. Sci. 2007, 18, 1355–1368. [Google Scholar] [CrossRef] [PubMed]
- Haberko, K.; Bućko, M.M.; Brzezińska-Miecznik, J.; Haberko, M.; Mozgawa, W.; Panz, T.; Pyda, A.; Zarębski, J. Natural hydroxyapatite—Its behaviour during heat treatment. J. Eur. Ceram. Soc. 2006, 26.4–5, 537–542. [Google Scholar] [CrossRef]
- Schulte, P.A.; Geraci, C.L.; Murashov, V.; Kuempel, E.D.; Zumwalde, R.D.; Castranova, V.; Hoover, M.D.; Hodson, L.; Martinez, K.F. Occupational safety and health criteria for responsible development of nanotechnology. J. Nanopart. Res. 2014, 16, 2153. [Google Scholar] [CrossRef] [PubMed]
- Sudarsanan, K.; Young, R.A. Significant precision in crystal structural details. Holly Springs hydroxyapatite. Acta Cryst. B 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- Klimek, K.; Belcarz, A.; Pazik, R.; Sobierajska, P.; Han, T.; Wiglusz, R.J.; Ginalska, G. “False” cytotoxicity of ions-adsorbing hydroxyapatite—Corrected method of cytotoxicity evaluation for ceramics of high specific surface area. Mater. Sci. Eng. C 2016, 65, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, H.M.; Jang, J.E.; Kim, C.M.; Kim, E.Y.; Lee, D.; Khang, G. Osteogenic differentiation of bone marrow stem cell in poly (Lactic-co-Glycolic Acid) scaffold loaded various ratio of hydroxyapatite. Int. J. Stem Cells 2013, 6, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mccrate, J.M.; Lee, J.C.-M.; Li, H. The role of surface charge on the uptake and biocompatibity of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22.10, 997–1003. [Google Scholar] [CrossRef]









| Sample Mass (g) | Sample nHAP | Ca (mg/mL) | P (mg/mL) | Ca (mol) | P (mol) | Ca/P |
|---|---|---|---|---|---|---|
| 0.1 | 800 °C | 382.5 | 176.5 | 0.9544 | 0.5698 | 1.675 |
| 900 °C | 380.6 | 175.6 | 0.9496 | 0.5669 | 1.675 | |
| 1000 °C | 378.8 | 174.8 | 0.9452 | 0.5643 | 1.674 |
| Control | Suspension | Cell Viability (%) | Cell Morphology in Culture | Grade |
|---|---|---|---|---|
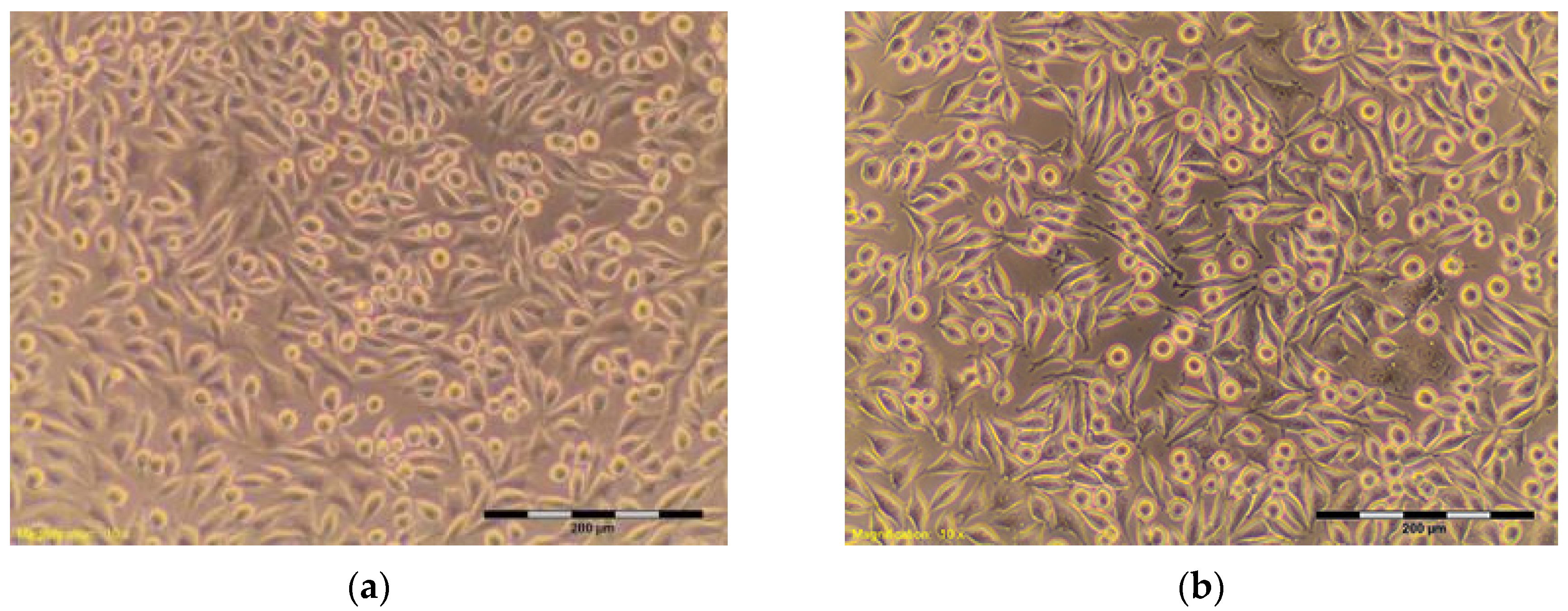
| HDPE ** negative | 100% | 95.59 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. The cells cover the entire pit. Many cells in divisions (Figure 4a,b) | 0 |
| SLS *** positive | 0.10 mg/mL | 75.40 | More than 20% of cells rounded, shrunk, separating from the substrate without densities of cytoplasm, single cells disrupted. Empty spaces between cells (Figure 5b) | 2 |
| 0.15 mg/mL | 12.60 | Completely destroyed cell culture. Extensive cell lysis (Figure 5c) | 4 | |
| 0.20 mg/mL | 12.50 | Completely destroyed cell culture. Extensive cell lysis (Figure 5d) | 4 |
| Sample | Suspension (%) | Cell Viability (%) | Cell Morphology in Culture | Grade |
|---|---|---|---|---|
| nHAP-800 | 100 | 87.64 | About 10% of the cells shrunk, separating from the substrate, visible intracytoplasmic granules (Figure 6a) | 1 |
| 50 | 87.35 | Approximately 15% of cells shrunk, separating from the substrate, visible intracytoplasmic granules (Figure 6b) | 1 | |
| 25 | 91.79 | Discrete intracytoplasmic granules. Single cells shrunk. Lysis of the cells was not observed (Figure 6c). | 0 | |
| 12.5 | 93.37 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of control culture, and there were many cells in divisions (Figure 6d). | 0 | |
| nHAP-900 | 100 | 88.87 | Approximately 15% of cells shrunk, separating from the substrate, visible intracytoplasmic granules (Figure 7a) | 1 |
| 50 | 95.59 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of negative control culture (Figure 7b). | 0 | |
| 25 | 95.00 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of negative control culture (Figure 7c). | 0 | |
| 12.5 | 92.48 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of control culture (Figure 7d). | 0 | |
| nHAP-1000 | 100 | 88.30 | Approximately 10% of cells shrunk, separating from the substrate, visible intracytoplasmic granules (Figure 8a) | 1 |
| 50 | 94.76 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of negative control culture (Figure 8b). | 0 | |
| 25 | 100.10 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of negative control culture (Figure 8c). | 0 | |
| 12.5 | 104.40 | Discrete intracytoplasmic granules. Lysis of the cells was not observed. Culture density was comparable to the density of control culture (Figure 8d). | 0 |
| Grade | Toxicity | Cell Morphology |
|---|---|---|
| 0 | lack | Discrete intracytoplasmic granules, no evidence of cell lysis, lack of inhibition of cell growth |
| 1 | weak | No more than 20% of rounded, shrunk cells, separating from the substrate without densities of cytoplasm, individual cells disrupted |
| 2 | moderate | No more than 50% of rounded cells, no evidence of granules, vast cell lysis and empty spaces between cells |
| 3 | average | No more than 70% of rounded cells, cells underwent lysis |
| 4 | strong | Almost completely or completely damaged cell culture |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials 2017, 10, 590. https://doi.org/10.3390/ma10060590
Szymonowicz M, Korczynski M, Dobrzynski M, Zawisza K, Mikulewicz M, Karuga-Kuzniewska E, Zywickab B, Rybak Z, Wiglusz RJ. Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials. 2017; 10(6):590. https://doi.org/10.3390/ma10060590
Chicago/Turabian StyleSzymonowicz, Maria, Mariusz Korczynski, Maciej Dobrzynski, Katarzyna Zawisza, Marcin Mikulewicz, Ewa Karuga-Kuzniewska, Boguslawa Zywickab, Zbigniew Rybak, and Rafal J. Wiglusz. 2017. "Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells" Materials 10, no. 6: 590. https://doi.org/10.3390/ma10060590
APA StyleSzymonowicz, M., Korczynski, M., Dobrzynski, M., Zawisza, K., Mikulewicz, M., Karuga-Kuzniewska, E., Zywickab, B., Rybak, Z., & Wiglusz, R. J. (2017). Cytotoxicity Evaluation of High-Temperature Annealed Nanohydroxyapatite in Contact with Fibroblast Cells. Materials, 10(6), 590. https://doi.org/10.3390/ma10060590