Preparation of Superhydrophobic Film on Ti Substrate and Its Anticorrosion Property
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
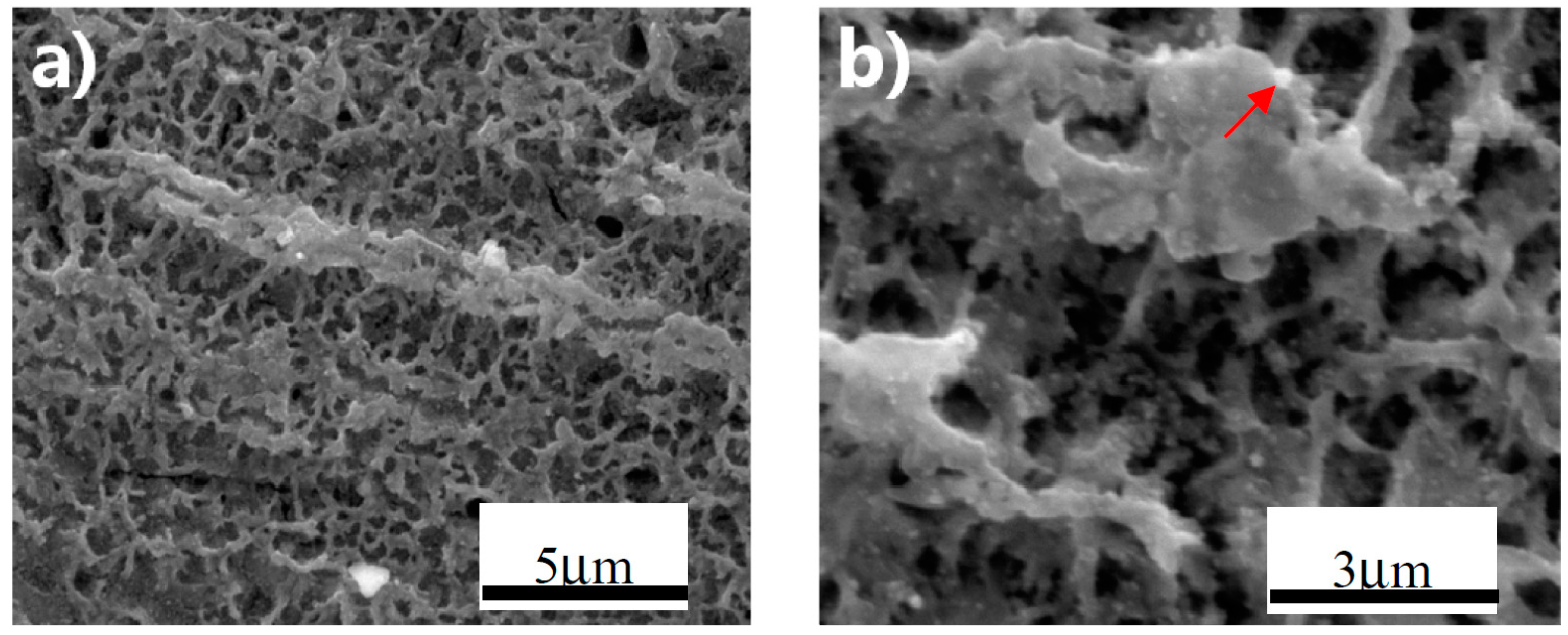
3.1. Superhydrophobic Film Fabricated by Anodizing
3.2. Superhydrophobic Film Fabricated without Anodic Oxidation
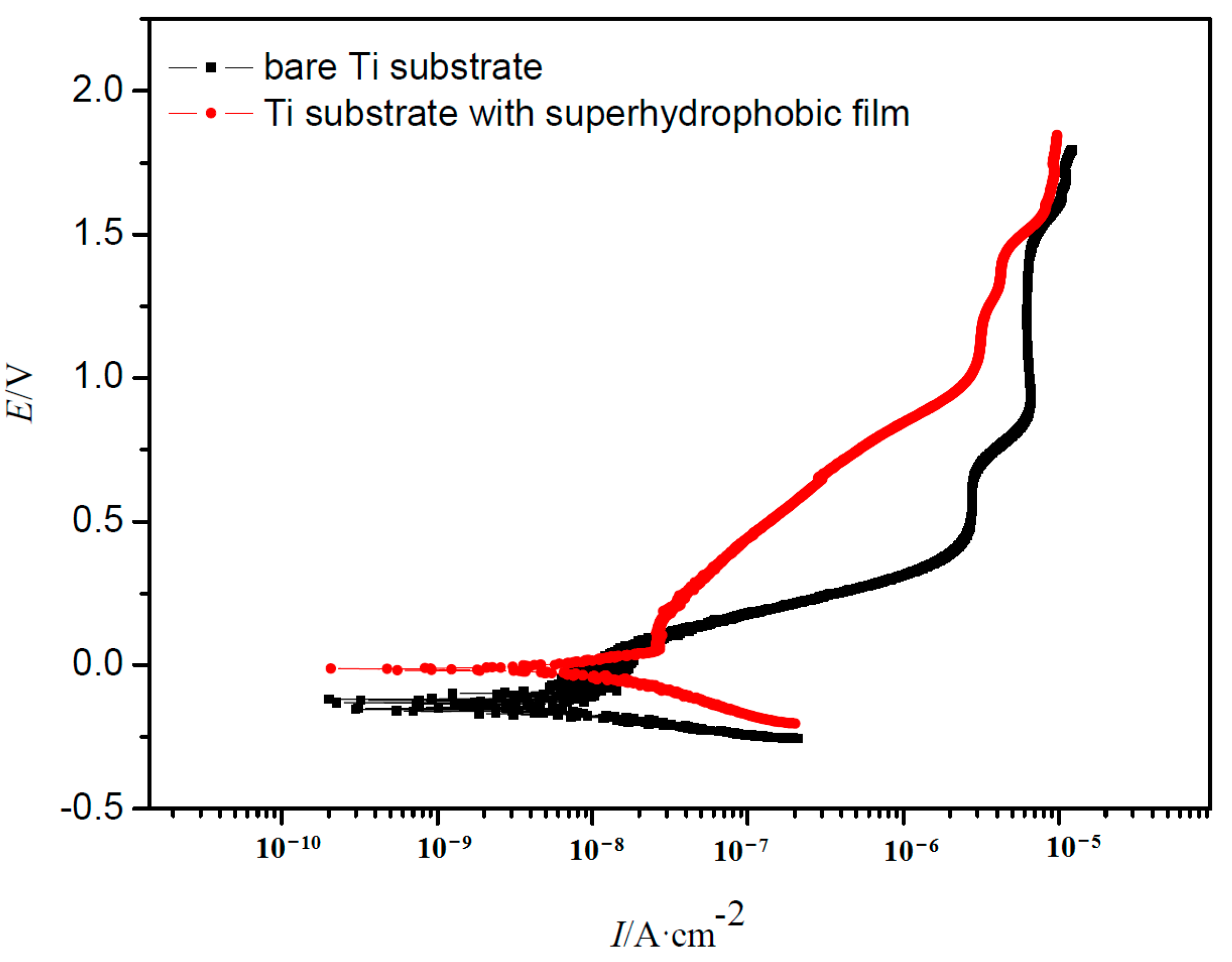
3.3. Electrochemical Test
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, X.; Shi, F.; Niu, J.; Jiang, Y.; Wang, Z. Superhydrophobic surfaces: From structural control to functional application. J. Mater. Chem. 2008, 18, 621–633. [Google Scholar] [CrossRef]
- Roach, P.; Shirtcliffe, N.J.; Newton, M.I. Progess in superhydrophobic surface development. Soft Matter 2008, 4, 224–240. [Google Scholar] [CrossRef]
- Satyaprasad, A.; Jain, V.; Nema, S.K. Deposition of superhydrophobic nanostructured Teflon-like coating using expanding plasma arc. Appl. Surf. Sci. 2007, 253, 5462–5466. [Google Scholar] [CrossRef]
- Liu, K.S.; Yao, X.; Jiang, L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 2010, 39, 3240–3255. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Gao, N.; Barthlott, W. Mimicking natural superhydrophobic surfaces and grasping the wetting process: A review on recent progress in preparing superhydrophobic surfaces. Adv. Colloid Interface Sci. 2011, 169, 80–105. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Li, S.; Li, Y. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Cassie, A.B.D.; Baxter, S. Wettability of porous surfaces. Trans. Faraday Soc. 1944, 40, 546–551. [Google Scholar] [CrossRef]
- Wenzel, R.N. Resistance of solid surfaces to wetting by water. Ind. Eng. Chem. 1936, 28, 988–994. [Google Scholar] [CrossRef]
- Ruan, M.; Li, W.; Wang, B.; Luo, Q.; Ma, F.; Yu, Z. Optimal conditions for the preparation of superhydrophobic surfaces on al substrates using a simple etching approach. Appl. Surf. Sci. 2012, 258, 7031–7035. [Google Scholar] [CrossRef]
- Yuan, Z.; Bin, J.; Wang, X.; Peng, C.; Wang, M.; Xing, S.; Xiao, J.; Zeng, J.; Xiao, X.; Fu, X.; et al. Fabrication of superhydrophobic surface with hierarchical multi-scale structure on copper foil. Surf. Coat. Technol. 2014, 254, 151–156. [Google Scholar] [CrossRef]
- Tsujii, K.; Yamamoto, T.; Onda, T.; Shibuchi, S. Super oil-repellent surfaces. Angew. Chem. Int. Ed. Engl. 1997, 36, 1011–1012. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, D.; Kang, Z. One-step electrodeposition process to fabricate corrosion-resistant superhydrophobic surface on magnesium alloy. ACS Appl. Mater. Interfaces 2015, 7, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, X. An expanding horizon: Facile fabrication of highly superhydrophobic coatings. Mater. Lett. 2017, 186, 357–360. [Google Scholar] [CrossRef]
- Latthe, S.S.; Imai, H.; Ganesan, V.; Rao, A.V. Superhydrophobic silica films by sol–gel co-precursor method. Appl. Surf. Sci. 2009, 256, 217–222. [Google Scholar] [CrossRef]
- Zhang, D.W.; Wang, L.T.; Qian, H.C.; Li, X.G. Superhydrophobic surfaces for corrosion protection: A review of recent progresses and future directions. J. Coat. Technol. Res. 2016, 13, 11–29. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.C.; Zhang, Y.J.; Iv, Q.; Xu, T.G.; Liu, T. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]
- Xu, W.; Song, J.; Sun, J.; Lu, Y.; Yu, Z. Rapid fabrication of large-area, corrosion-resistant superhydrophobic Mg alloy surfaces. ACS Appl. Mater. Interfaces 2011, 3, 4404–4414. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Chen, S.; Cheng, S.; Tian, J.; Chang, X.; Yin, Y. Corrosion behavior of super-hydrophobic surface on copper in seawater. Electrochim. Acta 2007, 52, 8003–8007. [Google Scholar] [CrossRef]
- Liu, H.; Szunerits, S.; Xu, W.; Boukherroub, R. Preparation of superhydrophobic coatings on zinc as effective corrosion barriers. ACS Appl. Mater. Interfaces 2009, 1, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Qian, H.C.; Wang, L.T.; Li, X.G. Comparison of barrier properties for a superhydrophobic epoxy coating under different simulated corrosion environments. Corros. Sci. 2016, 103, 230–241. [Google Scholar] [CrossRef]
- Han, I.; Vagaska, B.; Seo, H.J.; Kang, J.K.; Kwon, B.J.; Lee, M.H.; Park, J.C. Promoted cell and material interaction on atmospheric pressure plasma treated titanium. Appl. Surf. Sci. 2012, 258, 4718–4723. [Google Scholar] [CrossRef]
- Joung, Y.S.; Buie, C.R. A hybrid method employing breakdown anodization and electrophoretic deposition for superhydrophilic surfaces. J. Phys. Chem. B 2013, 117, 1714–1723. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Xu, W.J.; Song, J.L.; Liu, X.; Xing, Y.J.; Sun, J. Preparation of superhydrophobic titanium surfaces via electrochemical etching and fluorosilane modification. Appl. Surf. Sci. 2012, 263, 297–301. [Google Scholar] [CrossRef]
- Vanithakumari, S.C.; George, R.P.; Mudali, U.K. Enhancement of corrosion performance of titanium by micro-nano texturing. Corrosion 2013, 69, 804–812. [Google Scholar] [CrossRef]
- Liang, J.S.; Liu, K.Y.; Wang, D.Z. Hollow organosilica nanospheres prepared through surface hydrophobic layer protected selective etching. Appl. Surf. Sci. 2015, 338, 126–136. [Google Scholar] [CrossRef]
- Doshi, D.A.; Shah, P.B.; Singh, S.; Branson, E.D.; Malanoski, A.P.; Watkins, E.B.; Majewski, J.; Swol, V.F.; Brinker, C.J. Investigating the interface of superhydrophobic surfaces in contact with water. Langmuir 2005, 21, 7805–7811. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, A.J.; Gelling, V.J.; Woods, M.E.; Schmelz, J.R.; Enderson, B.P. The role of crosslinkers in epoxy–amine crosslinked silicon sol–gel barrier protection coatings. Thin Solid Films 2008, 517, 538–543. [Google Scholar] [CrossRef]
- Qian, M.; Mcintosh, A.S.; Tan, X.H.; Zeng, X.T.; Wijesinghe, S.L. Two-part epoxy-siloxane hybrid corrosion protection coatings for carbon steel. Thin Solid Films 2009, 517, 5237–5242. [Google Scholar] [CrossRef]
- Xue, C.R.; Dong, L.H.; Liu, T.; Zhang, F.; Yin, B.; Yin, Y.S. Preparation and anticorrosion performance of superhydrophobic TiO2 nanotube arrays on pure Ti. Corros. Sci. Prot. Technol. 2012, 24, 37–40. [Google Scholar]
- Zhang, F.; Chen, S.; Dong, L.; Lei, Y.; Liu, T.; Yin, Y. Preparation of superhydrophobic films on titanium as effective corrosion barriers. Appl. Surf. Sci. 2011, 257, 2587–2591. [Google Scholar] [CrossRef]
- Ou, J.F.; Liu, M.Z.; Li, W.; Wang, F.J.; Xue, M.S.; Li, C.Q. Corrosion behavior of superhydrophobic surfaces of Ti alloys in NaCl solutions. Appl. Surf. Sci. 2012, 258, 4724–4728. [Google Scholar] [CrossRef]
- Sagert, J.; Sun, C.; Waite, J.H. Chemical subtleties of Mussel and Polychaete holdfasts. In Biological Adhesives; Smith, A.M., Callow, J.A., Eds.; Springer: Berlin, Germany, 2006; pp. 125–143. [Google Scholar]







| Element | CK | NK | OK | FK | AuK | AgK | TiK |
|---|---|---|---|---|---|---|---|
| wt % | 01.15 | 05.69 | 02.94 | 00.50 | 00.17 | 15.70 | 73.85 |
| at % | 03.72 | 15.73 | 07.11 | 00.85 | 00.18 | 05.09 | 67.32 |
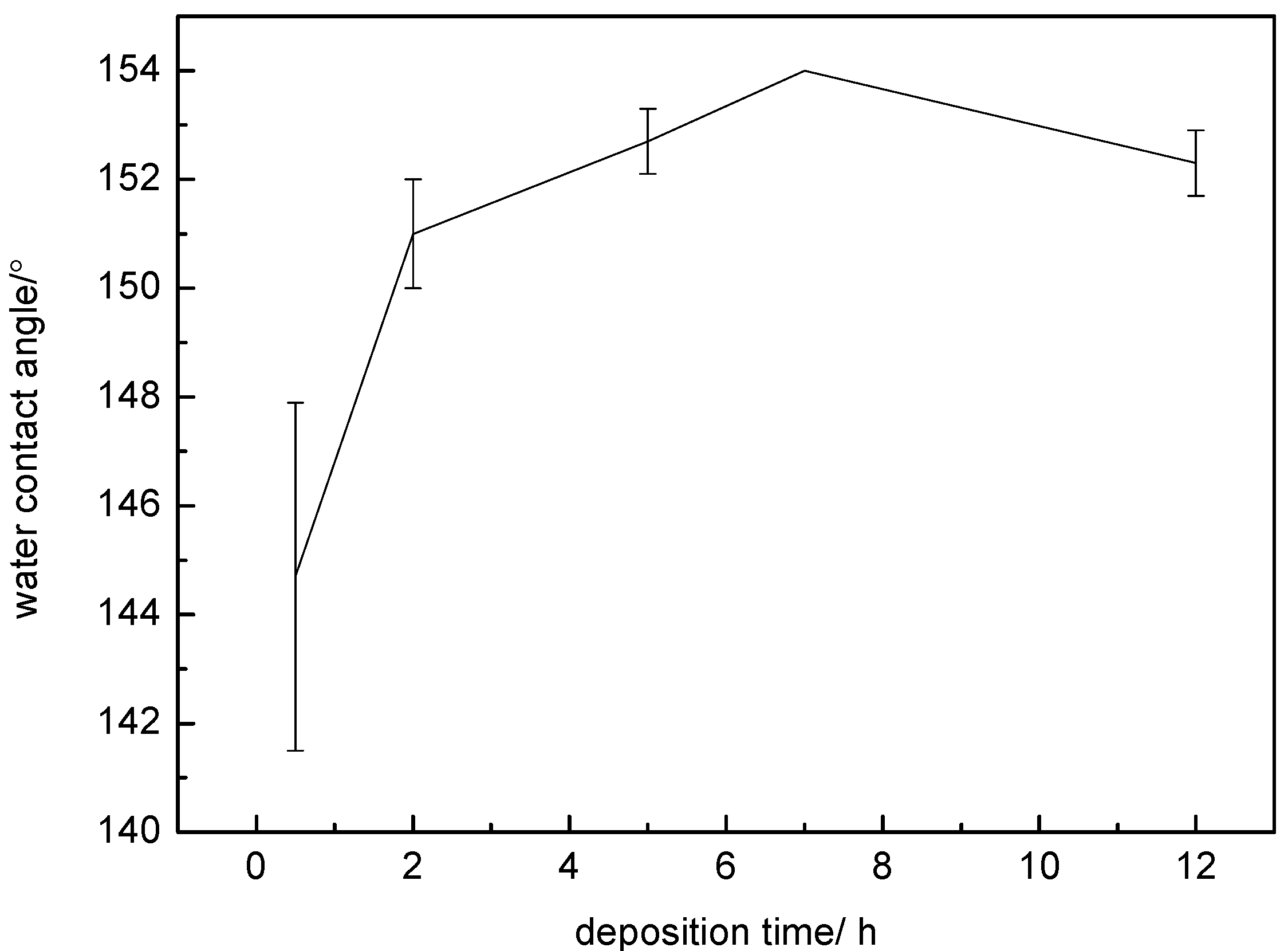
| Time/h | CA/° | Average CA/° | σ | ||
|---|---|---|---|---|---|
| 0.5 | 147 | 141 | 146 | 144.7 | 3.2 |
| 2 | 151 | 150 | 152 | 151 | 1.0 |
| 5 | 152 | 153 | 153 | 152.7 | 0.6 |
| 7 | 154 | 154 | 154 | 154 | 0 |
| 12 | 153 | 152 | 152 | 152.3 | 0.6 |
| Samples | Bare Ti | Ti with Film |
|---|---|---|
| Ecorr/V(vs. SCE) | −0.135 | −0.0126 |
| Ip/(nA/cm2) | 8.02 | 1.22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Tang, W.; Huang, L.; Zhang, D.; Du, C.; Yu, G.; Chen, M.; Chowwanonthapunya, T. Preparation of Superhydrophobic Film on Ti Substrate and Its Anticorrosion Property. Materials 2017, 10, 628. https://doi.org/10.3390/ma10060628
Zhu M, Tang W, Huang L, Zhang D, Du C, Yu G, Chen M, Chowwanonthapunya T. Preparation of Superhydrophobic Film on Ti Substrate and Its Anticorrosion Property. Materials. 2017; 10(6):628. https://doi.org/10.3390/ma10060628
Chicago/Turabian StyleZhu, Min, Wenchuan Tang, Luyao Huang, Dawei Zhang, Cuiwei Du, Gaohong Yu, Ming Chen, and Thee Chowwanonthapunya. 2017. "Preparation of Superhydrophobic Film on Ti Substrate and Its Anticorrosion Property" Materials 10, no. 6: 628. https://doi.org/10.3390/ma10060628





